Donald Hilvert

was born in Cincinnati, Ohio, in 1956. After receiving a B.A. degree from Brown University in 1978, he spent a year as a predoctoral fellow at the Swiss Federal Institute of Technology (ETH) in Zürich, Switzerland. He subsequently obtained a Ph.D. degree in 1983 from Columbia University under the supervision of R. Breslow. Following postdoctoral work at Rockefeller University with the late E.T. Kaiser, he joined the faculty of the Scripps Research Institute in LaJolla, California, as an assistant professor. He was subsequently promoted to associate professor in 1989 and full professor in 1994. In 1995, he was named the Janet and W. Keith Kellogg II Professor of Chemistry and an affiliate of the Skaggs Institute for Chemical Biology at Scripps. Since October 1997, he has been Professor of Chemistry at the ETH-Zürich in the Laboratorium für Organische Chemie.
Professor Hilvert”s research program focuses on understanding how enzymes work and on mimicking the properties of these remarkable catalysts in the laboratory. These efforts have been recognized by a number of awards, including an Alfred P. Sloan Research Fellowship (1991 –1993), the Arthur C. Cope Scholar Award (1992) from the American Chemical Society, and the Pfizer Award in Enzyme Chemistry (1994).
Scientific Sketch
The Hilvert group is developing strategies for creating protein molecules with tailored catalytic activities and specificities. The goal of this work is to understand, at the molecular level, the origins of the enormous rates and selectivi-ties that enzymes achieve. Successful enzyme engineering may also provide researchers of the future with useful catalysts for a wide range of applications in chemistry and biology.
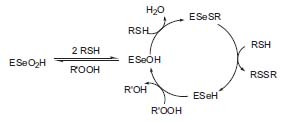
In one approach, Hilvert and his colleagues are redesigning existing protein molecules using re-combinant techniques, site-selective chemical modification, and total synthesis. For example, by chemically converting serines and cysteines into selenocysteines, they have prepared artificial selenoenzymes with novel redox and hy-drolytic properties (Fig. 1, Biochemistry 1995, 34, 6616). The selenium group is also a valuable probe of structure-function relationships, which can be characterized via kinetic and structural techniques such as high-field nuclear magnetic resonance spectroscopy and X-ray crystallography.
Figure 1. Redox reaction catalyzed by seleno-subtilisin.
In a complementary approach, they are exploiting the diversity and specificity of the mammalian immune system to produce monoclonal antibodies capable of catalysis (Fig. 2, Annu. Rev. Biochem. 2000, 69, 751).
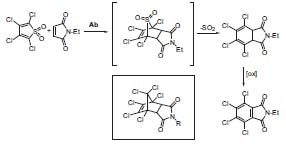
With suitably designed transition state analogues, they have successfully prepared antibody catalysts for a variety of important chemical transformations, including carbon-carbon-bond forming reactions and proton transfers. Because the properties of these molecules are defined by the structure of the transition state analogue, this strategy provides a versatile route to selective protein catalysts and sheds light on the fundamental principles of catalysis.
Figure 2. Antibody-catalyzed Diels-Alder reaction and respective transition state analogue (box).
Finally, they are applying the tools of molecular biology and genetics to the problem of protein design (Angew. Chem. Int. Ed. 2001, 40, 33 10). Specifically, they are exploring Darwinian evolution in the laboratory as a means to study and improve their first-generation antibodies and to create new proteins on a human rather than geological time scale. To date, the Hilvert group have successfully applied this approach to studies of catalytic mechanism, topological redesign of an enzyme, and de novo protein design.
Farfalle with Artichoke Cream Alessandro
Starting materials:
Medium sized pasta, e.g., farfalle or maccheroni – to serve 4–6
1 can (400 g) artichoke hearts in water, drained
1 container (250 g) mascar-pone cheese
150 g grated Parmesan cheese
60 mL olive oil
salt and pepper, and Tabasco or chili powder (optional)
Starting materials for elaborate meal:
4–6 servings farfalle Alessandro (see above)
1 head endive, leaves separated and washed
1 can (250 g) hearts of palm, drained and sliced into bite-
sized pieces
1 chicken breasts, poached
2 and sliced into bite-sized
pieces
snipped chives
Prepare, drain, and reserve pasta. In the cooking pot, coarsely shred the artichokes with a potato masher or other implement. Add all other ingredients, stir, mix in the reserved pasta, and reheat as desired. Serves 4–6.
With a few additions, this dish becomes elegant fare for entertaining. My wife arranges a striking array of shades of white against colored plates.
Keep prepared pasta warm. Fan several endive leaves across the top of each plate. Over the leaves” lower portion, heap some pasta, scatter the hearts of palm and chicken, and sprinkle the chives. If needed, adjust the endive back into its fan. Serves 6.
«This preparation is delicious and very simple. It is unusual enough to merit interest, yet, apart from the boiling of pasta, it requires only combining rather than cooking.
Where food is concerned, I consider myself a specialist: I wash up, leaving production to my family. Fortunately, though I have little instinct for cooking, even our 13-year-old son Alex and his younger brother Beto can produce an excellent meal. It was Alex who helped create the following dish, now a family favorite named after him.»
Donald Hilvert