Alan Roy Katritzky

was born in London on August 18, 1928. He started his studies at the University of Oxford, finishing up with a D. Phil. in 1954. After some years as postdoctoral fellow and college lecturer, he became lecturer at the University of Cambridge in 1958 and two years later became Director of Studies of the newly founded Churchill College. From 1963 to 1980 he was Professor of Chemistry at the University of East Anglia and was Dean of the School of Chemical Science for 1 1 years. He was appointed Kenan Professor at the University of Florida, where he is at present. He became Director of the Center for Heterocyclic Compounds in 1985.
During his long and extremely productive scientific career (Katritzky is author or co-author of some 1800 publications), he obtained numerous awards. He is a “Cavaliere Ufficiale” of the Order of Merit of the Italian Republic (since 1975), Fellow of the Royal Society (since 1980), Foreign Member of the Polish Academy of Sciences (since 1991), Inaugural Fellow of the International Society of Heterocyclic Chemistry (since 1995), and Fellow of the American Association for the Advancement of Science (since 2000).
His numerous awards include the RSC Award in Heterocyclic Chemistry (1982), the Golden Tiger Award of the Exxon Corp. (1990), the Senior Humboldt Award, the ACS Florida Prize (1995), the Award of the International Society of Heterocyclic Chemistry (1993), the Kametani Prize (1999), the ACS Cope Senior Scholar Award (2001), and the Gold Medal of the Partnership for Peace Foundation, Moscow (2001). He is a Doctor honoris causa of 10 universities in 8 countries. He has edited some 20 scientific journals and series, among those, Advances in Heterocyclic Chemistry since its beginning in 1963. ARKAT-USA, the Alan and Linde Katritzky not-for profit foundation for the support of research and education, particularly in second- and third-world countries, was formed in 1999 and has established ARKIVOC, a free electronic journal.
Scientific Sketch
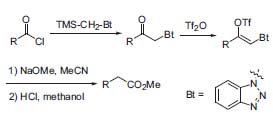
Katritzky’s group has advanced knowledge in many areas of heterocyclic chemistry, including N-oxides, heteroaromatic tautomerism, confor-mational analysis of heterocycles, mechanism of electrophilic and nucleophilic substitute reactions, pyrylium chemistry, and quantitative structure-property relationships (CODESSA program). Recently he has concentrated on synthetic methodology. Benzotriazole has been established as a versatile reagent for all kinds of organic transformations. The Arnd-Eistert reaction, a homologization of carboxylic acids has been improved using a benzotriazole-derivative instead of a diazomethyl ketone. This method is superior, because no unstable diazo-compound is needed (Fig. 1, Org. Lett. 2000, 2, 3789).
Figure 1. Benzotriazole-mediated homologa-tion of carboxylic acids.
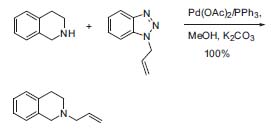
Another interesting discovery is the use of benzotriazole as a leaving group in the palladium-catalyzed allylic amination (Fig. 2):
Figure 2. Palladium-catalyzed allylic amination.
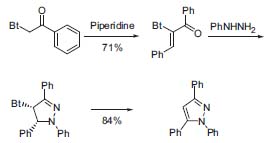
Some important advances in formation of heterocycles have been made recently in Katritzky’s group, including regioselective formations of pyrrazoles using benzotriazole-substituted a-cetophenones (Fig 3, J. Org. Chem. 2001, 66, 6787).
Figure 3. Regioselective synthesis of pyrrazoles.
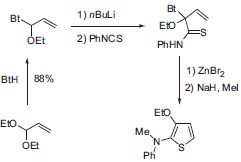
Also aminothiophenes, biologically interesting molecules, can be built up with a benzotriazole approach: starting from acrolein diethyl acetal, 1,2-disubstituded aminothiophenes can be synthesized in five steps (Fig. 4, J. Org. Chem. 2001, 66, 2850).
Figure 4. Synthesis of aminothiophenes.
This Sauerkraut Salad is easy to make and keeps fresh for several days.
Starting materials (serves 4):
500 g sauerkraut
1 tbsp vegetable oil
250 mL diced pineapple
1 apple, diced
1 onion, chopped
1 tsp syrup or sugar
Drain liquid off Sauerkraut, but first rinse, if too salty. Add oil, vinegar, sugar or syrup, apple, onion, and pineapple and mix.
Garnish with finely chopped herbs to taste.
«Being always under constant pressure means that something prepared quickly can be useful!»
Alan R. Katritzky