Horst Kessler

was born in Suhl/Thuringia on April 4, 1940. He studied chemistry in Leipzig in 1958 and moved to Tübingen in August 1961 via Berlin. After finishing his diploma thesis in 1963 under the guidance of E. Müller, he earned his Ph.D. in 1966, investigating the copper-salt-catalyzed reactions of diazomethane with aromatic compounds and continued to work on his habilitation (1969) about the detection of intramolecular mobility by NMR spectroscopy. In 1971 he became full professor at the Johann Wolfgang von Goethe University in Frankfurt/Main, where he stayed until 1989. Then he moved to Munich for a chair at the Technical University. His publication list contains almost 500 contributions. Horst Kessler has received several awards, including the Otto Bayer Award (1986), the Max Bergmann Medal for peptide-chemistry (1988), the Emil Fischer Medal of the German Chemical Society (1997), the Max Planck Research Award (2001), and the Vincent Du Vignaud Award of the American Peptide Society (2002). He is a member of the Bavarian Academy of Science and the Leopoldina in Halle. Visiting professorships have brought him to the U.S., Canada, Japan, and Israel. He also is a member of the editorial boards of 18 scientific journals, including Angewandte Chemie (Head of the Kuratorium), Journal of Medicinal Chemistry, and ChemBioChem.
Scientific Sketch
The main research areas of Horst Kessler are rational drug design and combinatorial synthesis of inhibitors of protein-protein interaction based on 3D structures and dynamics of the target, the ligand, or their complexes. Furthermore, his group develops new NMR techniques for the elucidation of the structure and dynamics of biomolecules and their application to peptides, peptidomimetics, proteins, and their complexes. Attention is drawn also to the synthesis of peptides, sugars, and their mime-tics, as well as the application of molecular dynamics in explicit monophasic and biphasic solvents.
The first determinations of many rotation and inversion barriers in small organic molecules belong to the highlights of his research (Angew. Chem. Int. Ed. 1970, 9, 219). Further investigations made it possible to determine barriers of ion-pair recombination to covalent species directly (Angew. Chem. Int. Ed. 1977, 16, 256; Chem. Ber. 1978, 111,3200).
The Kessler group developed several NMR techniques to determine peptide and protein structures and dynamics (COLOC, J. Magn. Reson. 1984, 54, 331), first pulsed ROESY (J. Magn. Reson. 1986, 70, 106), many delayed and selective excitation techniques (J. Magn. Reson. 1986, 70, 106), the first heteronuclear 3D NMR spectra (Angew. Chem. Int. Ed. 1990, 29, 546), the MEXICO technique for the measurement of exchange rates in proteins (J. Am. Chem. Soc. 1993, 115, 1 1620), and new hetero-editing techniques of NOEs (J. Biomol. NMR 1999, 15, 177).
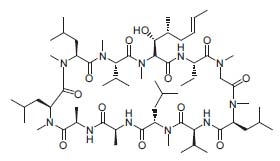
The structure of many bioactive molecules was determined, cyclosporine A (Fig. 1) probably being the first 3D structure elucidated by NMR (Helv. Chim.Acta 1985, 68, 661 and 682).
The structure parameters deriving from NMR spectroscopy of proteins and peptides are used by the Molecular Dynamics (MD) group for structure calculations. In addition, new methods for the consideration of explicit solvents in MD calculations are developed and applied for studies of flexible molecules in liquid/liquid inter-phases (J. Am. Chem. Soc. 1991, 113, 9566 and J. Phys. Chem. 1994, 98, 23). Furthermore, simulations and conformational analyses, especially of small cyclic peptides, and docking studies for the investigation of ligand-protein and protein-membrane interactions are performed.
Figure 1. Cyclosporine A.
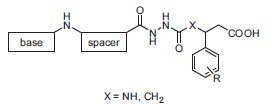
Kessler also focuses his interests on the development of selective and superactive RGD mimics (RGD: amino acid sequence Arg-Gly-Asp) from linear peptides to non-peptidic drug candidates via “spatial screening” and combinatorial synthesis (Fig. 2, J. Med. Chem. 2001,44, 1938).
Figure 2. Aza-RGD mimetics: combinatorial approach.
Rote Grütze
Red Gritz
Starting materials:
fresh (or, if not available frozen) red currants, strawberries, raspberries,
sour cherries
juice of the fruits
lemon juice
vanilla pudding mixture
vanilla sugar
custard sauce
I egg yolk
double cream
vanilla pod
Mix the fruits with their own juice (do not use water!) and bring the mixture to boil for a short time. Use vanilla pudding mixture to thicken the juice and add one splash of lemon juice. Do not use sugar (or at least only small amounts) to sweeten; use vanilla sugar instead. Add some fresh fruits after cooling down. Be careful not to overboil the mass.
Serve with rich custard sauce, which should be rounded off with an egg yolk, real vanilla, and double cream.
«Often, when we invited some friends over, my wife prepared red fruit pudding following her own recipe. Not only the children enjoyed this meal, and she got enthusiastic compliments.
The red color alluded to the GDR background, and the fruit pudding was mentioned several times in our home guestbook.
One day an English-speaking guest wanted to know the English name for this delicious meal, and my wife answered with “red gritz” - a name which became legendary.»
Horst Kessler