Horst Kunz

was born in 1940 in Frankenhausen (Saxony) and studied chemistry at the Humboldt University, Berlin, and at the University of Mainz. He completed his Ph.D., under the supervision of L. Horner, about the synthesis of cyclic organophosphorus compounds in 1969. His habilitation, completed in 1977, dealt with ester analogues of acetyl-choline and their application in protecting group chemistry. He was appointed as associate professor for organic chemistry in 1979 and a full professor of bioorganic chemistry in 1988 at the University of Mainz. He received the Max Bergmann Medal in 1992 and the Emil Fischer Medal in 2000. In 1998, he was elected corresponding member of the “Sächsische Akademie der Wissenschaften zu Leipzig” (Saxony Academy of Science). In 2001, he delivered the Adolf Windaus Lecture and received the Adolf Windaus Medal of the Georg-August-Universität Göttingen. He is a member of the editorial boards of Advanced Synthesis, Catalysis Organic Chemistry and Current Opinion in Chemical Biology.
Scientific Sketch
Horst Kunz’s research focuses on stereoselect-ive reactions and on the synthesis and development of methods in alkaloid, peptide, carbohydrate, and glycopeptide chemistry as well as in combinatorial synthesis.
The carbohydrate moieties of glycoproteins play key roles in biological selection processes. For investigations of these biological recognition phenomena, glycopeptides of an exactly specified structure are required. The allylic protecting principle was elaborated to a novel allylic anchoring in the solid-phase synthesis of peptides and glycopeptides (Angew. Chem. Int. Ed. 1995, 34, 803). The efficiency of the strategy has been demonstrated, for instance, in the synthesis of glycosylated peptide T. Recently, in syntheses of partial sequences of the tandem repeat of the tumor-associated polymeric epithelial mucin MUC-1, the advantages of an allylic anchor (HYCRON), which incorporates a polar, flexible oligoethyleneglycol spacer linked to the aminomethyl polystyrene, have been demonstrated (Angew. Chem Int. Ed. 2001, 40, 366).
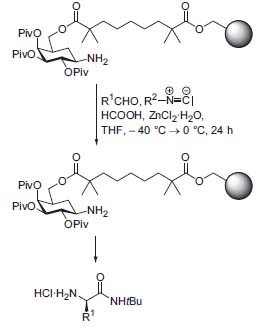
Carbohydrates are inexpensive natural products in which numerous functional groups and stereogenic centers are combined in one molecule. By directed regio- and stereoselective formation of derivatives, they can be converted into efficient chiral auxiliaries for controlling asymmetric syntheses. For example, Lewis acid-catalyzed Diels-Alder reactions of carbohydrate-linked dienophiles furnish the corresponding cycloadducts in high diastereoselectivity. The process has been combined with subsequent trapping reactions of the intermediates by electrophiles, resulting in the stereoselective syntheses of α-functionalized ÿ-branched carboxylic acid derivatives. Glycosylamines offer the possibility of versatile stereoselectve applications: in the presence of Lewis acids, the corresponding aldimines permit high-yielding syntheses of enantiomerically pure a-amino acids by Strecker and Ugi reactions, controlled by steric and stereoelectronic effects and by the formation of complexes (Fig. 1, Angew. Chem Int. Ed. 2000, 40, 1431). They can be used with equal efficiency for asymmetric syntheses of chiral homoallylamines and for asymmetric Mannich syntheses of ÿ-amino acids and chiral heterocycles, for example, alkaloids.
Figure 1. Stereoselective combinatorial Ugi-synthesis on solid phase.
Arzgebirg’sche Schusterkließ
Shoemaker’s dumplings from the Erzgebirge1 Somewhere else, probably misunderstood as “potato pancakes”
Starting materials (serves 4):
1.5 kg potatoes
1 onion
2 eggs
2 tbsp meal
1 tsp salt
white pepper
white pepper
Wash the potatoes, peel them, wash them again, and grate them with a fine grater2 into a bowl. Add the grated onion. This will examine the sentimentality of the respective cook.
Whisk eggs into the mixture, add flour and salt, and stir the ingredients into the potato batter. Add pepper in accordance with your own gusto. Pungence is an individual matter, not only in the case of taste but also in the case of thoughts and judgment. But it should not be exaggerated.
Finally, heat 3-4 tbsp oil or the corresponding amount of grease in a large pan. Best are heavy griddles, enameled inside, with the form of a small tub, like the ones that were heatable pretty ho-mogenously on a coalfire in former times (in this case use 6 tbsp oil).
Put 2–3 tbsp of the batter into the hot oil and spread it flat to “Schusterkließ,” i.e., into rounded rectangles.
While enjoying the resulting image of a large spot plate fry the pancake for about 4–5 minutes until the edges get golden brown. Turn over the “Schusterkließ” and also fry them justly and wisely for another four minutes from the other side. In the same manner, the rest of the batter is processed, while adding the appropriate amount of oil or grease, respectively.
In my personal opinion, “Schusterkließ” taste best when coming directly out of the pan, and one enjoys them together with a fresh and cool beer.
Indeed, “Schusterkließ” contain all that makes you strong, all the power carbohydrates provide. Those, who like it “sieße” (sweet), strew them with sugar, although, thus, disturbing the elegance of the helical α-glycopyranosides by mixing them with β-fructofura-nosides. Furthermore, they taste good with blueberry jam or apple purée.
«‘Schusterkließ’ are boosting resources for knights during their battle at the borderline of chemical knowledge.»
Horst Kunz
1 The Erzgebirge (Engl.: Ore Mountains) forms the border between Czechia and Germany. It is famous for its carved Christmas articles, e.g., nutcrackers.
2 This instrument is comparable to curious intimate questions from competitive colleagues who are used to being ahead in their knowledge.