Ei-ichi Negishi

grew up in Japan and received his baccalaureate degree in 1958 from the University of Tokyo. He worked as a research chemist at the Japanese chemical company Teijin, Ltd. He came to the U.S. as a Fulbright scholar in 1960 and earned a Ph.D. in organic chemistry in 1963 from the University of Pennsylvania. Negishi resumed his post at Teijin, but returned to the United States in 1966 for postdoctoral work in organoborane chemistry in H. C. Brown’s laboratories at Purdue University. After holding a series of academic positions at Purdue and Syracuse, Negishi became a chemistry professor at Purdue in 1979. In 1999 he became the inaugural H. C. Brown Distinguished Professor. Negishi’s research has earned him numerous awards and honors, including the J. S. Guggenheim Memorial Foundation Fellowship (1987), the A. R. Day Award (1996), the Chemical Society of Japan Award (1997), the Organometallic Chemistry Award from the American Chemical Society and the Herbert N. McCoy Award (both given in 1998), the Alexander von Humboldt Award, Germany (1998–2001), and the Sir Edward Frankland Prize Lectureship from the Royal Society of Chemistry (2000). He has given lectures throughout the world. Beside several patents and a few dozen essays, he has published about 300 scientific papers, some of which belong to the most cited papers of synthetic organic chemists.
Scientific Sketch
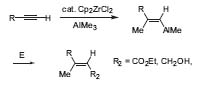
In his early years, Ei-ichi Negishi began developing new organometallic reactions. One of his first chemical breakthroughs was the development of the zirconium-catalyzed carboalumi-nation of terminal alkynes. With this method, trisubstiuted double bonds can be generated in a stereoselective fashion. (Fig. 1, J. Am. Chem. Soc. 1978, 1 00, 2252).
Figure 1. Zirconium-catalyzed carboalumination of terminal alkynes.
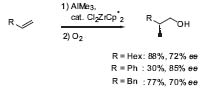
This methodology has been extended to terminal alkenes. By using a chiral zirconium complex, these double bonds can be aluminated and afterwards oxidized in an enantioselective manner (Fig. 2, J. Am. Chem. Soc. 1995, 117, 10771).
Figure 2. Enantioselective carboalumination of terminal alkenes.
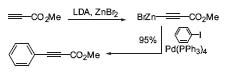
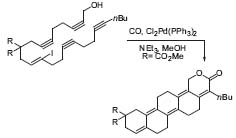
Negishi discovered the nickel-catalyzed cross-coupling reaction of organoaluminum compounds. This was improved to one of the first palladium-catalyzed carbon-carbon bond-forming reactions, which led to the subsequent developments of the Stille and Suzuki reactions. One of Negishi’s latest improvements to this cross-coupling reaction deals with coupling of electron-deficient alkynylzinc-compounds with aryl halides (Fig. 3). This is not practical with the Sonogashira coupling.
Figure 3. Palladium-catalyzed cross-coupling reaction of alkynylzinc compounds.
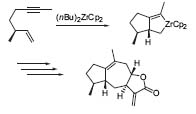
Another important field of research is the cycli-zation of enynes using a zirconium catalyst. This method was used to synthesize (±)-7-epi-β-bulnesene (Fig. 4; J. Org. Chem. 1997, 62, 1922).
Figure 4. The eneyne-cyclization reaction.
Another example of the application of transition metal catalysis is the so-called “zipper” reaction, a palladium-catalyzed cyclic cascade carbopalladation. An oligoyne is carbopalladated subsequently, and a pentacycle is formed in one reaction starting from an acyclic precursor (Fig. 5, J. Am. Chem. Soc. 1994, 116, 7923).
Figure 5. The “zipper” reaction.
Goma-ae with White Sesame, Goma-yogoshi with Black Sesame by Sumire and Ei-ichi Negishi
Starting materials (serves 4-5):
5 tbsp sesame, well roasted and ground
1.5 tbsp soy sauce
1 pinch salt or as needed
1.5 tbsp sugar or an amount to your taste
boiled vegetable of your choice, boiled to your taste, squeeze or apply any other methods to remove an excess of water. Your vegetable items should not be soggy. Examples of commonly used vegetables include spinach, any other soft green leaf vegetables, string beans, green asparagus, etc.
Experimental procedure
1. Mix well the first four “S”-items, i.e., sesame, soy sauce, salt, and sugar.
2. Pour the product mixture onto the boiled and partially dehydrated vegetables.
You may or may not mix the whole thing. It depends. For example, you normally mix if green beans are used. On the other hand, boiled spinach is squeezed and nicely cut into ca. 3 cm long layered bunch. Just pour the sauce.
It is simple and versatile. Above all, it is healthy.
Good luck,
Ei-ichi
«According to the ancient oriental tradition, Man is reborn after a cycle of 60 years. The history of mankind has indicated that the second 60-year cycle is generally, at least somewhat, more difficult than the first. With this in mind, I wish to present a token supply of roasted white and black sesame along with one of the simplest possible recipies. Sesame has long been considered, and perhaps even is proven, to be a food item for longevity.
So regardless of whether or not you like my recipe, I recommend that you use it frequently.»
Ei-ichi Negishi