Kyriacos C. Nicolaou

was born on July 5, 1946 in Cyprus where he grew up and went to school until the age of 18. In 1964, he went to England to spend two years learning English and preparing to enter university. He studied chemistry at the University of London (B.Sc. 1969, Bedford College; Ph.D. 1972, University College, with Professors F. Sondheimer and P. J. Garratt). He moved to the United States and joined – after postdoctoral appointments at Columbia University (1972–1973, Professor T. J. Katz) and Harvard University (1973–1976, Professor E. J. Corey) – the faculty at the University of Pennsylvania, where he rose through the ranks to become the Rhodes-Thompson Professor of Chemistry. Since 1989, he has held joint appointments at the University of California, San Diego, and The Scripps Research Institute, LaJolla (Darlene Shiley Professor of Chemistry, Chairman of the Department of Chemistry). In 1996, he was additionally appointed Aline W. and L. S. Skaggs Professor of Chemical Biology in The Skaggs Institute for Chemical Biology, The Scripps Research Institute. His work is published in more than 500 papers and 50 patents, and he has supervised the training of hundreds of Ph.D. students and postdoctoral researchers. He serves on the Scientific Advisory Board of numerous scientific journals and is an advisor to several biotechnology and pharmaceutical companies. Among K. C. Nicolaou’s numerous awards and honors are the Tetrahedron Prize, the Schering Prize (Germany), the Max Tishler Prize Lecture (Harvard), the Yamada Prize (Japan), the Janssen Prize (Belgium), the Nagoya Medal (Japan), the Centenary Medal (Royal Society UK), the Paul Karrer Medal (Switzerland), the Inhoffen Medal (Germany), the Nichols Medal (USA), the Linus Pauling Medal (USA), the Esselen Award (USA), the ACS Award for Creative Work in Synthetic Organic Chemistry (USA), the ACS Guenther Award in Natural Products Chemistry (USA), and several honorary degrees. He is a Fellow of the American Academy of Arts and Sciences, a member of the National Academy of Sciences, and a Foreign Member of the Academy of Athens.
Scirntific Sketch
Overall, Nicolaou’s programs are based on sophisticated synthetic organic chemistry and are directed towards the construction of novel molecular architectures of natural or designed origins. Emphasis is placed on both biomedical relevance and the advancement of organic synthesis as a science for its own sake.
Figure 1. Taxol™ and brevetoxin A.
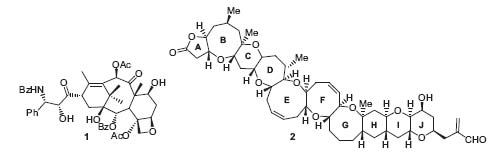
During the past few years he has developed many total syntheses of bioactive natural products and their derivatives, such as Taxol™ and brevetoxin A (Fig. 1, Nature 1994, 367, 260; Nature 1998, 392, 264), oligosaccharides (Angew. Chem. Int. Ed. 2001, 40, 1576), enediyne anticancer agents (Angew. Chem. Int. Ed. 1993, 32, 1377), DNA-interacting molecules, cholesterol-lowering compounds (Angew. Chem. Int. Ed. 1999, 38, 1669), epothilones (Angew. Chem. Int. Ed. 1998, 37, 2014), antibiotics (Angew. Chem. Int. Ed. 1999, 38, 2097), and the CP molecules (Angew. Chem. Int. Ed. 2002, 41, 2678). The design of bioactive molecules is often based on a combination of molecular modeling and biological studies (Proc. Natl. Acad. Sci. USA 2000, 97, 2904).
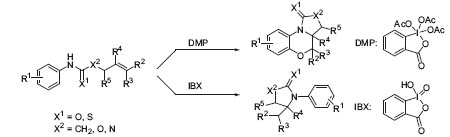
In addition to these target-oriented programs, the discovery and development of new synthetic technologies are pursued (Angew. Chem. Int. Ed. 2000, 39, 622, 625), including new reactions (Fig. 2) and combinatorial solid-phase synthesis for new molecular diversity, e.g., in the synthesis of epothilone and its derivatives to reveal structure activity relationships (Nature 1997, 387, 268), and the synthesis of a large library of benzopyrans (J. Am. Chem. Soc. 1999, 121, 9939, 9954, 9968) from which several biologically active ligands have emerged. Thus a modular highoutput system has been designed and developed. The system employs three technological innovations to achieve its high efficiency and reliability: (1) development of enabling technologies for solid-phase chemistry; (2) application of microreactors as the reaction units in solid-phase synthesis; and (3) use of radiofrequency tagging as the non-chemical tracking method.
Figure 2. The reaction of Dess-Martin periodinane and IBX with anilides and related compounds.
Fish & Chips
Starting materials (serves 4):
1. 3 kg Northern European Halibut (or Cod)
all-purpose flour (preferably special batter mix flour for fish & chips, pale yellow)
H2O
1.3 kg potatoes
vegetable oil
cider vinegar
salt
lemon juice
Cut filet of fish into pieces (1.5–2.5 cm thick, 4–6.5 cm wide, 15–20 cm long). Wash with H2O, dry with paper towel, and marinate in lemon juice for 0.5 to 1 hour.
Prepare batter by mixing flour with H2O, stirring until a homogenous, free-flowing slurry is formed (thin).
Roll fish pieces into dry flour to cover all around before dipping into batter slurry and deep-frying in hot oil until golden yellow-brown (approx. 10 minutes).
Clean potatoes, cut them into long strips, and deep-fry them in separate vegetable oil until they become golden yellow-brown chips (french fries).
Serve fish & chips with salt and vinegar (or lemon) while hot. Enjoy with Franken or Alsatian white wine.

«This little recipe has sentimental value to me, since I learned it long before I became a synthetic organic chemist – during my struggles for survival in England as a student and a fish & chips chef.
The accompanying photos will remind you of me then and now!»
Kyriacos C. Nicolaou