Leo Armand Paquette

was born in 1934 in Worcester, Massachusetts, and received his B.S. from Holy Cross College in 1956 and his Ph.D. from MIT in 1959. In the same year, he began his career at the Upjohn Company, where he worked until 1963. From there, Paquette joined the faculty of The Ohio State University, Columbus, where he was promoted to associate professor in 1966 and full professor in 1969. He held the title of Kimberly Professor from 1981 to 1987 and was promoted to Distinguished University Professor in 1987. He has held visiting faculty positions at several universities in the United States, France, and Germany. His work has given rise to more than 1 150 research publications, 38 book chapters, 17 edited book volumes, one encyclopedia, and 40 patents to his credit.
Paquette’s honors include an Alfred P. Sloan Fellow, the Outstanding Young Man of America, a Morley Medal from the Cleveland ACS Section, a Guggenheim Fellow, an ACS Award for Creative Work in Synthetic Organic Chemistry, election to the National Academy of Sciences in 1984, an Arthur C. Cope Senior Scholar Award, a Senior Humboldt Award, Fellow of the Japanese Society for the Promotion of Science, the Ernest Guenther Award of the ACS, the Distinguished International Award of the National Science Council of the Republic of China, the First France/Belgium Award for Research Excellence, and a Centenary Lectureship of the Royal Chemical Society. Paquette has held memberships on various boards, including the Medicinal Chemistry Study Section of the National Institutes of Health, the executive committee of the organic division of the American Chemical Society, National Science Foundation Chemistry Advisory Committee, the Board on Chemical Sciences and Technology of the National Research Council, and the Ohio Council on Research and Economic Development, and editorial boards of ten scientific journals and annual reviews.
Scientific Sketch
Paquette’s research interests include the synthesis of structurally unique natural products, the development of new synthetic pathways -particularly enantioselective and asymmetric transformations – the identification of new methods involving biochemical probes and sensors, and investigation of the use of organometallic reagents for the expedient preparation of organic compounds. His current work is aimed at the synthesis of spongistatin 1 (Fig. 1) and sanglifehrin (Fig. 2) typifies Paquette’s synthetic activity.
Figure 1. Spongistatin 1, a powerful toxic metabolite of red alga.
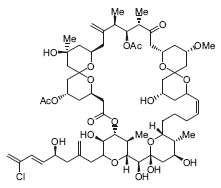
Figure 2. Sanglifehrin, an immunosupressant.
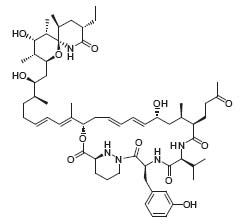
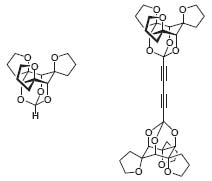
Paquette’s discovery of a general approach to the construction of inositol-based, metal-ion-ligating systems has led to many exciting applications of this chemistry in bio-organic contexts. The ability of these molecules to chelate metal ions selectively is expected to satisfy the increasing demand of modern medicine for sophisticated detection methods (Fig. 3, Org. Lett. 2000, 2, 139).
Figure 3. Inositol-based, metal-ion-liganding molecules.
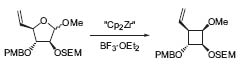
Paquette’s group is also concerned with defining new aspects of organometallic applications. The zirconocene-promoted enantiospeci-fic ring contraction of carbohydrate precursors represents an example of Paquette’s involvement with this complex structure chemistry (Fig. 4, Org. Lett. 2002, 4, 1927).
Figure 4. Enantiospecific zirconocene-mediated ring contraction.
Paquette’s Favorite Lasagna
Starting materials (serves 4):
500 g lasagna
700 g ground beef
1 package Swiss cheese
1 200-g package shredded cheddar cheese
1 200-g package mozzarella cheese
finely grated parmesan cheese
1 bottle (1.25 kg) Ragu sauce (traditional or meat)
Cook the lasagna. Fry the ground beef and mix in the Ragu sauce. In a large 35 × 25 cm pan, spread half of the Ragu-ground beef mixture. Cover with a layer of lasagna and top with a sprinkle of salt. Place Swiss cheese over the entire area. Layer another row of lasagna and cover with cheddar cheese. Place a third row of lasagna on top, sprinkle with parmesan and mozzarella cheese, and end with the second half of the Ragu-ground beef mixture. Bake in an oven for 30 minutes. Serve with garlic bread.
«The Paquette Research Group enjoys two social get-togethers a year. The first is in mid-summer, and the second occurs just prior to Christmas. On both occasions, a large amount and variety of food is served. Notwithstanding, the students invariably insist that my wife’s lasagna be served. Its absence from the menu brings on deep disappointment. Indeed, large amounts of the lasagna are consumed on both occasions.»
Leo A. Paquette