Manfred T. Reetz

was born in 1943 in Hirschberg, Germany, and obtained a B.Sc. degree from Washington University (St. Louis) in 1965 and an M.Sc. degree in chemistry from the University of Michigan in 1967. In 1969 he received his Ph.D. degree from the University of Göttingen under the guidance of U. Schöllkopf. Following postdoctoral training under R. W. Hoffmann at the University of Marburg, he obtained his habili-tation there in 1976, spent two years as associate professor at the University of Bonn, and became full professor in Marburg in 1980. In 1991 he moved to Mülheim/Ruhr and two years later became director of the Max-Planck-Institut für Kohlenforschung.
Among his numerous awards are the Jacobus van’t Hoff Preis (Netherlands, 1977), the Chemistry Prize of the Academy of Sciences Göttingen (1978), the Otto-Bayer-Preis (1986), the Leibniz-Preis der Deutschen Forschungsgemeinschaft (1989), the Fluka Prize “Reagent of the Year 1997”, and the Nagoya Gold Medal of Organic Chemistry (2000). Since 1997 he has been an elected member of the German Academy of Scientists Leopoldina Halle, and a member of the North-Rhine-Westphalian Academy of Sciences Düsseldorf since 2001. He has been an Honorary Professor of the Ruhr University Bochum since 1992 and has held many appointments during his scientific career. He was a member of the Executive Board of the German Chemical Society (GDCh) and its Vice-President in 1995, a member of the election commission of several German chemistry awards (August Wilhelm von Hofmann Medal, Carl Duisberg Prize, Karl-Heinz Beckurts Prize, Emil Fischer Medal, Adolf von Baeyer Medal, Alfried Krupp Prize), a member of the editorial boards of several highly reputed journals such as Topics in Organometallic Chemistry, Russian Journal of Organic Chemistry, and Advanced Synthesis and Catalysis, and a member of the Board of Trustees of Angewandte Chemie.
Scientific Sketch
The primary interest of Reetz’s research is the development of chemo- and stereoselective methodologies in organic chemistry. Catalytic processes are the main focus, but the research efforts are interdisciplinary.
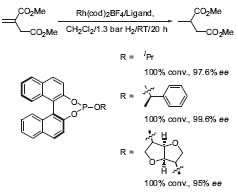
The first reseach area is the development of novel types of chiral ligands for asymmetric transition metal catalysis. New monophosphite-ligands are highly effective for asymmetric transformations, especially for enantioselective hydrogenations (Fig. 1, Angew. Chem. Int. Ed. 2000, 39, 3889).
Figure 1. Enantioselective reduction of itaconic acid dimethyl ester.
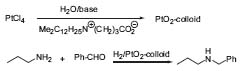
The second area of interest is the research on new water-soluble colloid metal oxides as catalysts for reductive aminations, for example. Transition metal salts such as PtCl4 are hydrolyzed under basic conditions in the presence of a water-soluble surfactant such as Me2RN+ (CH2)3CO2 – , leading to the formation of the corresponding surfactant-stabilized metal oxides. These colloids show reaction rates in the reductive amination of propyl amine with benzaldehyde 5 times higher than those of commercially available catalysts (Fig. 2, J. Am. Chem. Soc. 1999, 121,7933).
Figure 2. Synthesis and application of water-soluable PtO2 colloids.
Another important research topic deals with the kinetic resolution of chiral compounds using enzymes: catalysts (enzymes) are improved by combinatorial and evolution-based methods. (Fig. 3, Angew. Chem. Int. Ed. 2001, 40, 3589).
Figure 3. Kinetic resolution of racemic esters with an enzyme.
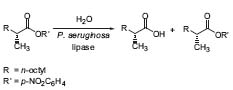
The wild-type enzyme provides a selectivity of 1.1:1 (E = 1) in favor of the S-acid. Manipulation of the amino acids of the enzyme leads to a selectivity of 11:1 (E = 11). Directed evolution leads to a mutant enzyme with an E-value of >51.
Herb Sauce Frankfurt Style
Starting materials (serves 4):
1 package herbs “Herb
Sauce Frankfurt Style”
(“Frankfurter Grüne Soße“)
or:
parsley
chervil
sorrel
cress
salad burnet
borage
chives
250 mL soured milk
1 tbsp mayonnaise
1 tsp mustard
pepper
salt
sugar
lemon juice
4 hardboiled eggs
Following a procedure by Dr. med. Elisabeth Reetz Verified by Professor Dr. Manfred T. Reetz
Wash and chop the herbs. Add soured milk, mayonnaise, and mustard. Season with pepper, salt, sugar, and lemon juice. Shell the eggs and cut them into small pieces to be added to the sauce.
To be served with freshly boiled potatoes.
«The author does not claim the privilege of having invented the procedure to prepare “Frankfurter Grüne Soße”. Indeed, this is an old Hessian dish, which is said to have been known and appreciated by Johann W. Goethe, who spent his youth in Frankfurt. Whether this is true or not is a subject of discussion among the historians, as the term “Frankfurter Grüne Soße” (or “Grüne Soße”) does not occur in the Goethe archives in Weimar. Anyway, being not only delicious but also very healthy, it could have contributed to Goethe’s long life (the great German writer died in 1832 at the age of 82).»
Manfred Reetz