Daniel H. Rich

was born on December 12, 1942 in Fairmont, Minnesota. He received a B.S. degree in chemistry from the University of Minnesota in 1964 and his Ph.D. in organic chemistry from Cornell University (Ithaca) in 1968 with Professor A. T. Blomquist. He held postdoctoral appointments at Cornell University with Nobel Laureate V. du Vigneaud and at Stanford University with W. S. Johnson. In 1968 and 1969 he was a research chemist in the pharmaceutical division of the Dow Chemical Company, before joining the faculty at the University of Wisconsin-Madison in 1970. Rich was promoted to the rank of full professor in 1981. His research has been described in more than 220 refereed publications and recognized by a long list of prestigious awards: the 1990 Vincent du Vigneaud Award in Peptide Chemistry, the 1992 American Chemical Society (ACS) Division of Medicinal Chemistry Award, the 1992 Research Achievement Award of the American Association of Pharmaceutical Scientists, the 1992 George Herbert Hitchings Award for Innovative Methods in the Design and Discovery of Drugs, the 1993 ACS Ralph F. Hirschmann Award in Peptide Chemistry, a WARF University Professorship at the University of Wisconsin in Madison in 1994, the E. Volwiler Research Achievement Award from the American Association of Colleges of Pharmacy in 1995, and an Arthur C. Cope Scholar Award from the ACS in 1999. In addition, D. Rich has been a Fellow of the American Association for the Advancement of Science since 1986 and was an Alexander von Humboldt Scholar in Germany in 1993. He was a member of the Bioorganic and Natural Products study section of the National Institutes of Health from 1981 to 1985 (Chairman for the final two years of his term), served as Associate Editor for the Journal of Medicinal Chemistry from 1988 to 1992, and chaired the Division of Medicinal Chemistry for the American Chemical Society in 1992. Currently he is Associate Editor of the new ACS journal, Organic Letters.
Scientific Sketch
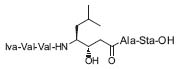
Daniel H. Rich is interested in the chemistry and bio-organic chemistry of natural products derived from peptides. His goals are to understand how a molecule stabilizes binding to the receptor molecule, to characterize the catalytic mechanisms of the affected enzymes, and to devise selective inhibitors of related, therapeu-tically important enzymes. His synthesis of analogues of pepstatin (Fig. 1) and the characterization of their mode of binding to pepsins by various methods have provided a general strategy for designing inhibitors of this class of enzyme that has been used to develop several therapeutic agents, including inhibitors of renin, HIV protease, and, most recently, β-secretase (J. Med. Chem. 1 986, 29, 2519; J. Med. Chem. 1983, 26,904).
Figure 1. Pepstatin.
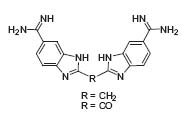
Today, the conformation of ligands bound to target proteins can be determined by X-ray and NMR methods, but what is needed are ways to identify possible lead structures based only on the knowledge of the enzyme’s ligand-binding site and to select from the many potential inhibitors those that are likely to exhibit the phar-macodynamic properties needed to obtain drugs. Rich’s research group is testing various approaches for designing or creating novel enzyme inhibitors. By use of computerized structure-generating programs, thousands of potential mimics of the inhibitor are grown in the active site of the enzyme. Some of these are synthesized by his students and tested to see how well they inhibit the target enzyme. This strategy is being applied to a variety of enzymes that have therapeutic potential. These include botu-linum toxin metalloproteases and various aspar-tic proteases including b-secretase, bleomycin hydrolase, and cathepsin L. All of these inhibitors are co-crystallized with the enzyme and analyzed by X-ray chrystallography if possible. The structure-generating program is then utilized to “create” novel non-peptide inhibitor candidates, which are synthesized and assayed. The goal is to find orally active inhibitors that might serve as lead compounds for further drug discovery. Botulinum toxin metalloprotease is one of the most selective peptidases known and needs to recognize at least 35 amino acids in a protein in order to cleave the substrate sequence. There are no known natural inhibitors of these enzymes and traditional approaches employed to inhibit related enzymes fail with this one. Recently the research group of Rich obtained the first non-peptide, low-molecular-weight inhibitor of BoNT/B peptidase (Fig. 2, J. Am. Chem. Soc. 2000, 122, 11268). They are actively trying to modify this lead using structure-based design and combinatorial chemistry in hopes of obtaining clinically useful lead structures.
Figure 2. BABIM inhibition of BoNT/B.
Ciappino – Italian-Style Seafood Stew with Tomatoes and Basil
Starting materials (serves 4):
60 mL olive oil
325 mL chopped onion
2 tbsp chopped garlic
4 tsp dried oregano
1½ tsp fennel seeds
625 mL crushed tomatoes
with added purée
625 mL bottled clam juice
250 mL dry wine (e.g., Coppola Chardonnay)
650 g cans chopped clams,
drained, liquid reserved; use
fresh if available
450 g uncooked large
shrimp, peeled, deveined
400 g can crabmeat, drained
125 mL chopped fresh basil
Cayenne pepper
In season, fresh scallops and mussels can be added.
Heat olive oil in heavy large pot over medium heat. Add onion, garlic, oregano, and fennel seeds and saute until onion is tender –about 8 minutes. Add tomatoes, clam juice, white wine, and the liquid reserved from clams. Increase heat and boil until slightly thickened, about 15 minutes.
Add clams, shrimp, and crabmeat. Reduce heat and simmer 2 minutes. Mix in fresh basil and simmer until shrimp are just opaque in center, about 2 minutes longer. Season stew to taste with cayenne, salt, and pepper.
«We often visit our daughter who lives in Anacortes, Washington, with her husband. The Pacific Northwest is a beautiful area on the coast, and Anacortes is a port city for the ferries leaving for the San Juan Islands and Victoria, Canada. The area has great seafood, and we like to take advantage of that anytime we can. This recipe is a simple but tasty way to take advantage of the ocean offerings. It is an Italian-style stew, called ciappino. We had it with a green salad, a crusty French bread, and a great Pinot Grigio.»
Daniel H. Rich