Victor Snieckus

was born in Lithuania in 1937 and spent his childhood in Germany during World War II. In 1959 he received a Bachelor’s degree at the University of Alberta, where he was strongly influenced by R. Sandin. After graduate work at the University of California, Berkeley (M.Sc. with D. S. Noyce), and Oregon (Ph.D. with V. Boekelheide), he returned to Canada for a postdoctoral year with O. E. Edwards at the National Research Council and then joined the faculty at the University of Waterloo in 1966. He held the Monsanto/Natural Sciences and Engineering Research Council of Canada (NSERC) Industrial Research Chair in Chemical Synthesis and Biomolecule Design from 1992 to 1998. He has held visiting professorships at the Rand Afrikaans University in Johannesburg, South Africa (1991), at the University of Zurich, Switzerland (1994), at the University of Innsbruck, Austria (1995), and at the Australian National University, Canberra (2001 and 2002). Due to his relevant scientific work, he was honored with several awards, including the Alfred Bader Award in Organic Chemistry (1993), the Humboldt Research Award (1996), the Arthur C. Cope Scholar Award of the American Chemical Society (2001), the International Society for Heterocyclic Chemistry Award (2001), and the Gedimino Order of the Republic of Lithuania (2002).
Victor Snieckus was and is a member of severeal editorial boards, e.g., the Journal of Organic Chemistry (1984–1989) and Progress in Heterocyclic Chemistry (since 1988).
Scientific Sketch
A major thrust of Snieckus’ group is concerned with the development of new DOM (Directed ortho metallation) strategies and tactics, referred to as “die neue Aromatische Chemie” (See-bach), for the regiospecific and controlled construction of polysubstituted aromatics and he-teroaromatics. Snieckus’ group enjoys the challenge of discovering new directed metalation groups (DMGs, Fig. 1) and of measuring their limitations with respect to established processes. Current active areas include the discovery of new DMGs, the development of new industrially convenient conditions for metallation, and the systematic investigation of the scope of combined use of DMGs to obtain specific sequences of synthetic value, including “walk-around-the-ring” procedures. In addition, synthetic utility for heterocyclic ring construction and annelation is also a continuing general aim.
Figure 1. Directed ortho-metalation (DOM).
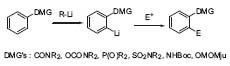
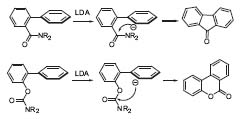
The directed remote metallation (DreM), developed in Snieckus’ group (Chem. Rev. 1990, 90, 879), means capturing the anionic species in an intramolecular way. The metallating group serves also as an electrophile; interesting products, e.g., fluorenones and dibenzopyranones are easily available using this method (Fig. 2, J. Org. Chem. 1991, 56, 1683).
Figure 2. Directed remote metalation (DreM).
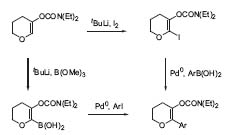
Another field of research is the combination of metallation and cross-coupling reactions. By choosing halide, tin, or boron electrophiles, the products can undergo palladium-catalyzed cross-coupling reactions with aryl halides (Fig. 3, J.Org.Chem. 1998,63, 1514).
Figure 3. Combined metalation/cross-coupling reactions.
Cold Beetroot Soup
Starting materials:
2 cooked beetroots
2 fresh cucumbers
2 hard boiled eggs
100 g sour cream
1 L sour milk or butter milk
250 mL boiled water
8 sprigs fresh dill
250 mL scallion greens
salt
potatoes
Crush egg yolks with finely chopped scallion greens and salt. Add finely chopped cucumbers, finely chopped egg whites, sour cream, and sour milk or butter milk. Peel and coarsely grate the beet, combine with the rest, add 250 mL of boiled but chilled water, and mix well.
Serve, in individual bowls sprinkled with dill, with hot potatoes.