Martin Suhm

was born 1962 in Gengenbach/Black Forest (Germany), went to school in Portugal, and studied chemistry at the University of Karlsruhe, finishing in 1985 with a diploma thesis on nuclear magnetic relaxation investigations of benzene/hexafluorobenzene interactions. After a research year with R. O. Watts at the Australian National University in Canberra (quantum Monte Carlo methods for water clusters), he joined the group of M. Quack at ETH Zürich, where he completed a Ph.D. thesis in 1990 on the far infrared spectroscopy and dynamics of hydrogen fluoride dimers. During research stays with D. NesbittatJILA in Boulder/Colorado (1991, 1992) and back at ETH, larger clusters of hydrogen fluoride were investigated both spectroscopically and theoretically, leading to a detailed understanding of the unusual clustering tendency of this molecule, which serves as a simple prototype for hydrogen bonding. After habilitation (1995), awarded with a Latsis University prize, an ADUC habilitation prize), and a Dozentenstipendium (Fonds der Chemischen Industrie, 1997), he was appointed full professor at the University of Göttingen in 1997.
Scientific Sketch
Whether a gardener is enchanted by the scent of a rose, whether a drug blocks a specific enzyme in the human body, whether genetic information is read from our DNA, or whether a thundercloud forms, it always has to do with specific interactions between molecules, which are mediated via hydrogen bonds and other attractive forces as well as repulsive contacts. In short, it has to do with molecular sociology. By studying simple model systems, Suhm and co-workers try to get to the bottom of such interaction mechanisms.
A particularly elementary example is the interaction between hydrogen chloride (HCl) and water. Given sufficient water molecules, they can dissociate HCl into protons and chloride ions. This leads to hydrochloric acid, e.g., in the stomach. With only one or two water molecules per HCl, the HCl stays intact. Recently, the Suhm group has succeeded in observing the vibration of an intact HCl molecule with one and two water molecules (Fig. 1, Phys. Chem. Chem. Phys. 2002, 4, 3933), whereas in the presence of many water molecules, dissociated HCl is detected.
Figure 1. Schematic view of a supersonic jet expansion of HCl and water in helium and its vibrational spectrum.
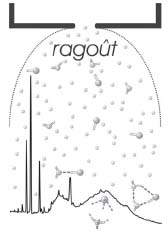
For this purpose, very cold molecular aggregates are generated in a giant supersonic jet expansion, and their vibrations are probed by a Fourier transform infrared spectrometer. The technique is called ragout-jet FTIR spectrosco-py (Faraday Disc. 2001, 118, 331).
In another application of this powerful technique, the ability of chiral molecules to distinguish between copies and mirror copies of themselves was investigated (Fig. 2, Phys. Chem. Chem. Phys. 2002,4,2721).
Figure 2. Glycidol dimer as one of the most elementary cases of a molecular handshake between chiral molecules.
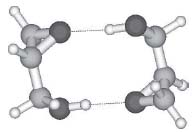
The situation resembles that of a handshake, which feels quite different depending on whether two right hands or a right and a left hand are employed. Such molecular recognition phenomena are omnipresent in biochemistry and the Suhm group tries to characterize them in detail, using simple prototype systems where one can focus on the essential features of the interaction.
An advantage of studying small molecular aggregates is that they are accessible to high-level quantum mechanical treatments. Still, the complexity is enormous and one often has to search through maps of the interaction energy in hundreds of dimensions to find favorable structures. It is the combination of spectros-copic detection and theoretical modeling which proves to be most fruitful for the understanding of molecular sociology, the scientific study of molecular behavior in groups.
Fish Soufflé Clausius-Clapeyron
Starting materials (serves 2):
1 small onion
40 g butter
250 g fish filets (e.g., cod or angler fish)
100 mL white wine
salt, pepper
1 bunch of dill
30 g flour
250 mL milk
grated lemon peel
4 yolks
5 egg whites
bread crumbs
Heat a small chopped onion in a pan with one half of the butter and braise the fish filets with covered lid for 10 minutes after having added the white wine. Then take the filets out of the pan, chop them, and season with salt, pepper, and plenty of dill. The liquid is concentrated (preferably in a rotary evaporator) to 1/10 of its volume. In another pan the rest of the butter is melted, the flour is added, and the mixture is braised shortly. Add the milk and the concentrated fish brew and heat to the boiling point under permanent stirring. Season with salt and pepper and some grated lemon peel and keep simmering until a thick protein-glykolipid mass is obtained. After cooling down to 40 °C, the yolks and the chopped fish are added. The egg whites and a pinch of salt are whipped with 5 mmol air until stiff. The fish-béchamel-mass is carefully mixed with the egg whites and filled into a greased ovenproof dish whose bottom has been covered with a thin layer of bread crumbs. The domino Maillard reaction is initiated at the surface by putting the dish into the preheated oven (200 °C). After 5 minutes the temperature can be reduced to 180 °C for another 40 minutes. According to Gay-Lussac’s Law for the air and the Clausius-Clapeyron Equation for the steam, the bubble size increases and the soufflé gains the double or trifold volume and possibly becomes more stable. Nevertheless the soufflé should be served immediately after preparation because Gay-Lussac’s Law and the Clausius-Clapeyron Equation are still valid while cooling down.
To be served with a salad.
«The soufflé should be served immediately after preparation because Gay-ussac’s Law and the Clausius-Clapeyron Equation are still valid while cooling down.»
Martin Suhm