Edwin Vedejs

was born on January 31, 1941 in Riga, Latvia. He went to the University of Michigan to finish his B.S. in 1962. After his Ph.D. under the guidance of H. Muxfeldt at the University of Wisconsin, Madison, USA, in 1966, he became Postdoctoral Fellow in the group of E. J. Corey at Harvard University. In 1967 he entered the faculty of the University of Wisconsin again to become assistant and later full professor. Accepting the chair as Moses Gomberg Professor of Chemistry he moved to the University of Michigan, Ann Arbor, USA, in 1999. His research interests include synthesis, natural products chemistry, heteroelement chemistry, asymmetric synthesis, and mechanistic chemistry.
Apart from a number of grants, Vedejs has received numerous awards, among those, the Alexander von Humboldt Senior Scientist Award (1984), the Pharmacia & Upjohn Teaching Award (1996), and the Paul Walden Medal of the Riga Technical University (1997). Over the years he has been a member of the editorial or advisory boards of a number of scientific journals and series, among those, Journal of the American Chemical Society, Journal of Organic Chemistry, Organic Syntheses, Chemistry of Heterocyclic Compounds. He is Chair Elect of the ACS Organic Division for the years 2002–2004.
Scientific Sketch
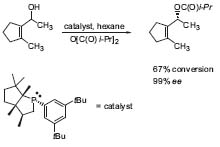
Edwin Vedejs’ work is designed to develop an understanding of conceptual, stereochemical, mechanistic, and preparative aspects of organic synthesis. Targets for total synthesis can be natural products derived from nitrogen-containing heterocycles, but they can also be complex unnatural heteroelement (boron, nitrogen, phosphorus, etc.) containing molecules that have been designed to serve as catalysts for enantio-selective reactions. He uses total synthesis as a challenging testing ground for the fundamental issues that are under study in his group, e.g., the kinetic resolution of allylic alcohols with a chiral phosphine catalyst (Fig. 1, Org. Lett. 2001, 3, 535). This methodology allows us to synthesize enantiopure allylic alcohols by selective acylation of one enantiomer.
Figure 1. Kinetic resolution of allylic alcohols using a phosphine catalyst.
Recently, some analogues of the higly cytotoxic diazonamide A have been synthesized in Vedejs’ group using efficient synthetic methods. The key steps are a Suzuki-cross-coupling followed by a Dieckmann-type cyclization (Fig. 2, Org. Lett. 2001, 3, 2551). During the past year, the structure of diazonamide was revised, resulting in replacement of the acetal by an aminal (nitrogen in place of one oxygen). The synthesis of the aminal series is under study.
Figure 2. Synthesis of the macrolide core of diazonamide A.
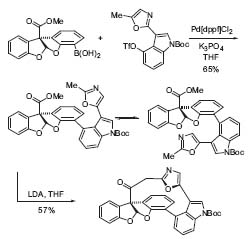
Coupling the aryl triflate derived from indole with a boronic acid yields the two atropisomers in a 65% yield. By treating the atropisomeric mixture with LDA, the macrocylic ketone is formed in a 57% yield.
A totally different field of research is crystallization-induced asymmetric transformation: stereogenic heteroelements can be generated in good diastereomeric ratios: the principal requirement is that interconversion (epimerization) of diastereomers must take place faster than crystallization.
Pat Anderson-Vedejs’ Wisconsin Linzer Cake
Starting materials (serves 4):
250 mL unblanched almonds
375 mL sifted flour
½ tsp cinnamon
1/8 tsp cloves
¼ tsp salt
170 mL butter
2 egg yolks, beaten
1 tsp grated lemon rind
250 mL raspberry jam
icing sugar
Chop almonds through medium blade of food chopper to make a coarse powder. Sift together flour, spices, and salt. Blend butter and sugar until creamy; then add egg yolk, chopped almonds, and lemon rind and beat until fluffy. Stir in dry ingredients. Knead until very well mixed. Press 3/4 of dough against sides and bottom of an 20-cm pie pan and cover with a layer of jam. Form remaining dough into eight 20-cm strips 1.3 cm thick. Make a lattice over jam with four strips criss-crossed in each direction. Bake at 180 °C (moderate heat) in oven 20–30 minutes. Cool, cut into about 24 wedges, and sprinkle lightly with powdered sugar.
«The recipe that I would like to submit for your “inspection” is actually my wife’s recipe for what is no doubt based on a European (Austrian?) original. My wife remembered serving this to Prof. Tietze and his wife during our months in Göttingen back in 1984, so I thought this would be the best connection that a non-cooking chemist like myself can manage (you don’t want to test my recipes). Since this is all in American weights and measures, it may be an interesting challenge for you to find the conversions, as well as a linguistic test. Perhaps after all the conversions, the product will not be quite identical to that original European version. Who knows how many translations were already involved.»
Edwin Vedejs