Ekkehard Winterfeldt

was born on May 1 3, 1932 in Danzig and grew up in Schleswig after the Second World War. In 1952 he began his studies in chemistry at the University of Hamburg, changing in the same year to the Technical University of Brunswick, where he received his Ph.D. in organic synthesis in 1958 in the research group of F. Bohlmann, with a thesis about the synthesis of hydroxy sparteins. Together with Bohlmann, he went to the Technical University of Berlin, where he made his ha-bilitation in the years 1959–1962. In 1967 he was appointed associate professor, and three years later he moved to the Technical University of Hanover to become full professor and director of the Institute of Organic Chemistry. In October 2000 he became professor emeritus. In 1990 he was visiting professor at the University of California at Irvine. He has been a member of the Brunswick Scientific Society since 1983, of the Göttingen Academy of Sciences since 1984, and of the German Academy of Scientists Leopoldina, Halle, since 1996. During his scientific career, Professor Winterfeldt has received numerous awards. Among those is the Emil Fischer Medal of the Gesellschaft Deutscher Chemiker (GDCh, 1990), the Adolf Windaus Medal of the University of Göttingen (1993), the Richard Kuhn Medal of the GDCh (1995), and the Hans Herloff Inhoffen Medal (1998). In 1991 he was awarded a Doctor honoris causa by the University of Liège (Belgium). He was elected President of the GDCh for the years 1996–1997 and a member of the senate of the Deutsche Forschungsgemeinschaft (DFG) for the period 1995–2002. He is editor of a number of scientific journals, among those the Journal of the Chemical Society, Perkin Transactions 1, Chemical Communications, Tetrahedron, and Tetrahedron Letters.
Scientific Sketch
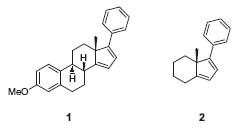
Ekkehard Winterfeldt’s recent research focused on enantioselective cycloadditions and their application in the synthesis of biologically active marine natural products. His aim was to mimic nature’s route to pure enantiomers. With his Diels-Alder/retro-Diels-Alder sequence, he developed a very useful tool to build up pure enantiomers in an efficient manner (Chem. Rev. 1993, 93, 827; Angew. Chem. 1995, 107, 489; J. Chem. Soc., Chem. Commun. 1996, 887). Using this strategy the enantiomerically pure steroid 1 and its simpler hydrindan analogue 2 were prepared (Fig. 1).
Figure 1. Steroidal structures built up by a Diels-Alder/retro-Diels-Alder sequence.
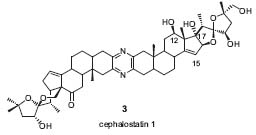
The face-, endo-, and regioselective cycloadditions of the enantiopure cyclopentadienes provide a selective approach to synthesize building blocks of several marine natural products such as epi-agelorin A (Tetrahedron 1998, 54, 7273) and cephalostatin analogues (Eur. J. Org. Chem. 1998, 281 1; Helv. Chim. Acta 2000, 83, 1854). The cephalostatins and ritterazines, e.g., cephalostatin 1 (3) (Fig. 2), are marine natural products isolated from the worm Cephalodiscus gilchristi and from the tunicate Ritterella tokioka. The availability from their natural sources is still limited. The outstanding cytostatic activity together with the new and interesting structure justify their total synthesis as well as the revelation of structure-activity relationships.
Figure 2. Structure of cephalostatin 1.
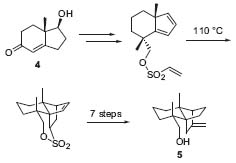
In vitro cancer-cell-line screening provides evidence for an increased tumor-inhibiting activity of Winterfeldt’s 17-O-functionalized analogues. Furthermore, Winterfeldt achieved the total synthesis of myltaylenol (5), a sesquiterpenoid alcohol bearing four chiral carbon atoms, starting from the easily available Hajos-Wiechert ke-tone (4), using an intramolecular Diels-Alder reaction (Fig. 3, Chem. Eur.J. 1998, 1480).
Figure 3. Total synthesis of myltaylenol.
One-pot Fish Soup
Starting materials:
fresh self-caught fish
assortment of vegetables
salt
pepper
parsley
dill
cream
Being a northern German coastal inhabitant, I contribute this procedure of how to prepare a fish soup. Procedure - not a recipe, because I prefer freehand cooking, without exact quantities or times - just following my feelings.
Although this fish soup is not one of my favorite dishes, it is linked to a nice little story. In his young years, our son Thomas was an enthusiastic fisher, but his prey was seldom noble or of sublime growth. Since we liked to give him the feeling of having contributed vitally to the family’s nutrition and welfare, we proceeded as follows:
The fish - independent of the species - was cut and then hung by means of a fine sieve into water, in which different sorts of vegetables had been cut. The hole was cooked for a while. Then the sieve was taken out and the rest of the fish was offered to the cats without Thomas’ knowledge. Salt and pepper were added, as well as parsley and lots of dill. After boiling shortly, the soup was enriched with large amounts of cream. Even the smallest fish provided a decent meal when applying this procedure.
Ekkehard Winterfeldt