Peter Wipf

was born in Aarau, Switzerland. He received his chemistry diploma in 1984 and his Ph.D. in 1987 from the University of Zurich, Switzerland, under the guidance of H. Heimgartner. His postdoctoral work as a Swiss NSF Postdoctoral Fellow with R.E. Ireland led him to the University of Virginia in Charlottesville. In 1990 he joined the faculty at the University of Pittsburgh as an assistant professor, where he was promoted to the rank of associate professor in 1995 and full professor in 1997. Among the recent awards of Peter Wipf are the Arthur C. Cope Scholar Award (1998), the Novartis Research Award (2000), and the Japan Society for the Promotion of Science Fellow Award. He is a member of the Advisory Board of Molecules and the Journal of Organic Chemistry, a member-at-large of the Organic Division Executive Committee of the American Chemical Society, and a member of the Editorial Advisory Board of Chirality.
Scientific Sketch
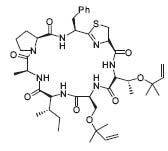
Peter Wipf s scientific interests focus on different subjects of organic chemistry, including the total synthesis of natural products, for example, the synthesis of trunkamide A (Fig. 1, J. Org. Chem. 2000, 65, 1037) and (±)–nisamycin (J. Org. Chem. 1999, 64, 5033).
Figure 1. Trunkamide A.
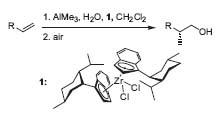
Dealing with organometallic chemistry, his group has a long tradition in the synthetic application of transition metals. In recent publications, he described the use of zirconocene catalysts for the asymmetric methylalumination of a-olefins (Fig. 2, Org. Lett. 2000, 2, 171 3), zirconium-zinc transmetalation, and in situ catalytic asymmetric addition to aldehydes (J. Org. Chem. 1998,63,6454).
Figure 2. Zirconocene-catalyzed enantioselective methylalumination.
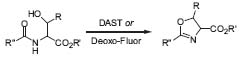
In the field of heterocyclic chemistry, Wipf and coworkers concentrate on the development of new stereoselective methods for the preparation of highly functionalized heterocycles, especially novel protocols for five-membered heter-ocycle synthesis, e.g., the use of diethylamino-sulfurtrifluoride (DAST) for the cyclodehy-drative conversion of β-hydroxy amides to ox-azolines (Fig. 3, Org. Lett. 2000, 2, 1 165).
Figure 3. Mild cyclization of β-hydroxy amides to oxazolines.
Combinatorial chemistry and solid phase synthesis are used for reagent and catalyst design in organic and heterocyclic chemistry as well as for structure-activity analysis of bioactive compounds. Special attention is drawn to collaborative drug development for cancer therapy as previously described (Pharm. Exp. Ther. 2000, 292, 530).
In cooperation with the group of Beratan, Wipf works in the field of computational prediction of macroscopic properties, such as the assignment of the relative and absolute stereochemistry of organic molecules and the prediction of chiroptical phemomena.
3Lemon-Kiwi Pie
Starting materials (serves 4):
170 mL boiling water
1 package JELL-O brand lemon-flavored gelatine
2 tsp grated lemon peel
2 tbsp lemon juice
125 mL cold water
4 ice cubes
225 g Cool Whip, thawed
1 ready-to-use Graham cracker crust (approx. 25 cm)
½ package vanilla-flavored JELL-O instant pudding & pie filling (75 g)
170mLmilk
1–2 kiwi fruit
shamrock candy sprinkles
Pour 170 mL of milk into large bowl. Beat 1/2 package. JELL-O instant pudding & pie powder and 225 g thawed Cool Whip whipped with wire wisk for 1 minute. The pudding should become very viscous. Spread it with a spoon across the bottom of the Graham cracker crust. Refrigerate for 20 minutes. In the meantime, stir boiling water into gelatin in large bowl at least 2 minutes or until completely dissolved. Stir in lemon gratings and juice. Mix cold water and ice and add to gelatin, stirring until slightly thickened. Remove any remaining ice. Stir in the remaining whipped topping with wire wisk until smooth. Refrigerated for 20–30 minutes or until mixture is very thick and will mound. Spoon into Graham crust on top of vanilla pudding layer. Cut 1–2 kiwis into slices and spread over the top. Decorate with shamrock candy sprinkles.
Refrigerate for 6 hours or overnight until firm.

Peter Wipf