Yoshinori Yamamoto

was born in Kobe/Japan on November 21, 1942. During the years 1962–1970 he studied chemistry at Osaka University, where he received his Ph.D. in organic chemistry under the guidance of I. Moritani. He was appointed instructor at Osaka University in 1970. While working as an instructor, he went to H. C. Brown’s group at Purdue University as a postdoctoral associate (1970–1972). In 1977 he was appointed associate professor at Kyoto University. In 1986 he moved to Tohoku University, Sendai, to become professor of organic chemistry. He also holds a professorship at IMRAM (Institute of Multidisciplinary Resdearch for Advanced Materials), Tohoku University, and a visiting professorship at Kyushu University. He is a recipient of the Chemical Society of Japan Award for Young Chemists (1976) and the Chemical Society of Japan Award (1995) and also received the Dortmunder-Gambrinus Award from the University of Dortmund (1999) and the Humbold Research Award (2002). For his research on boron neutron capture therapy, he received the Hiroshi Hatanaka Lectureship from the International Society of Neutron Capture Therapy (1996). He is the regional editor of Tetrahedron Letters and he was the President of the International Society of Heterocyclic Chemistry (2000–2001).
Scientific Sketch
The scientific interests of Yoshinori Yamamoto are divided into three categories.
The first covers the development of new synthetic methods with Lewis acid and transition metal catalysts.
Shortly after the opening of the “Lewis acid age,” he introduced a new concept in organo-copper chemistry in 1977 (J. Am. Chem. Soc. 1978, 100, 3240); a Lewis acid such as BF3·OEt2 dramatically increases the reaction rate and changes the selectivities of a conjugate addition reaction of an organocopper reagent (Angew. Chem. Int. Ed. 1986, 25, 947).
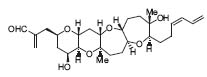
Beginning about 10 years ago, his contribution to the research field of transition metal-catalyzed, especially palladium-catalyzed, organic synthesis became prominent. He developed the palladium-catalyzed addition of pronucleophiles to C-C multiple bonds of unsaturated compounds such as allenes, alkynes, enynes, and methylenecyclopropanes (Chem. Soc. Rev. 1999, 28, 199). By applying the above mentioned Lewis acid-allylstannane methodology, hemibrevetoxin B was synthesized (Fig. 1).
Figure 1. Hemibrevetoxin B.
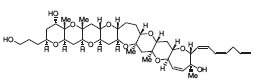
Furthermore, the ring skeleton of gambierol has been constructed by the Lewis acid-promoted one-shot synthesis of two ring segments (Fig. 2, J. Am. Chem. Soc. 2001, 123, 6702).
Figure 2. Gambierol.
The third field of research is an organochemical approach to Boron Neutron Capture Therapy (BNCT) in cancer treatment. He has a keen interest in this interdisciplinary research between organic chemistry and biochemistry and has developed several very important boron carriers (Fig. 3).
Figure 3. Drugs in BNCT: glycoside and Gd-157.
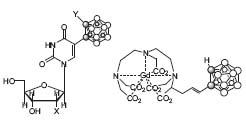
Some of the compounds were tested in vivo and exhibited better therapeutic results compared to the compounds utilized clinically until now.
Tofu Tempura
Starting materials (serves 6):
350 g tofu
flour or cornstarch
oil
125 mL of dashi stock or chicken broth
60 mL light-colored soy souce
15 mL mirin (Japanese sweet rice wine for cooking)
1 tsp cornstarch
water
Simple to make, nutritious, and tasty
The tofu, made from soybean and very popular in Japan (perhaps also available in Europe and the US), is drained. Usually, it is sold in a pack containing water. The tofu is placed on a cutting board and tilted to one side, or placed between two sheets of paper towels, in order to remove the water from the surface of the tofu.
The tofu (13 × 10 × 7 cm; about 350 g) is cut into fourths and coated with cornstarch or all-purpose flour.
Deep-frying oil is heated around 175 °C. The temperature control of the oil is important. Tofu, covered by flour (or cornstarch), is put into the heated oil and fried until the surface color changes to lightly brown. Tofu tempura is picked up and drained with paper towels.
The tempura sauce is prepared. Although usually it is commercially available, the preparative procedure is as follows:
(i) dashi stock or chicken broth,
(ii) light-colored soy sauce,
(iii) mirin, which is a Japanese sweet rice wine for cooking,
(iv) 1 tsp cornstarch plus 1 tsp water.
The four starting materials (substrates) are mixed in the order of (i)-(iv), and a very nice sauce is prepared. Tempura sauce ingredients are put into a small sauce pan and heated until sauce thickens. Tofu tempura is placed in an individual bowl and the sauce is poured over.
Scallion, ginger root, and/or bonito flakes are sprinkled, if you like. Even without these additives, tofu tempura is tasty. Of course, together with this tofu tempura, drinking beer (or wine) is very enjoyable, and hopefully fruitful to arouse one’s vitality for doing research and administrative work, ... on the next day.
«I am living alone in Sendai. Fortunately or unfortunately, my wife lives in Kobe and the distance between Sendai and Kobe is about 1000 km! Additionally, my three kids live separately: one in Brussels and the other two in Tokyo. So, all the family members of Yamamotos are living separately. Usually, I take a supper with my students at the university cafeteria, but on Saturday and Sunday I have to manage a dinner/supper by myself. The easiest way to do this task is to go to a restaurant, of course, or to go to shops in the basement floors of department stores since there are so many different types of dishes. However, sometimes, I cook by myself at home; the most important criterion in this case is whether or not the procedure is simple and the product is nutritious and tasty.»
Yoshinori Yamamoto