Practice Test 3
Click here to download and print the PDF version of this exercise.
AP® Chemistry Exam
SECTION I: Multiple-Choice Questions
DO NOT OPEN THIS BOOKLET UNTIL YOU ARE TOLD TO DO SO.
At a Glance
Total Time
1 hour and 30 minutes
Number of Questions
60
Percent of Total Grade
50%
Writing Instrument
Pencil required
Instructions
Section I of this examination contains 60 multiple-choice questions. Fill in only the ovals for numbers 1 through 60 on your answer sheet.
CALCULATORS MAY NOT BE USED IN THIS PART OF THE EXAMINATION.
Indicate all of your answers to the multiple-choice questions on the answer sheet. No credit will be given for anything written in this exam booklet, but you may use the booklet for notes or scratch work. After you have decided which of the suggested answers is best, completely fill in the corresponding oval on the answer sheet. Give only one answer to each question. If you change an answer, be sure that the previous mark is erased completely. Here is a sample question and answer.
Sample Question
Chicago is a
(A) state
(B) city
(C) country
(D) continent
Sample Answer

Use your time effectively, working as quickly as you can without losing accuracy. Do not spend too much time on any one question. Go on to other questions and come back to the ones you have not answered if you have time. It is not expected that everyone will know the answers to all the multiple-choice questions.
About Guessing
Many candidates wonder whether or not to guess the answers to questions about which they are not certain. Multiple-choice scores are based on the number of questions answered correctly. Points are not deducted for incorrect answers, and no points are awarded for unanswered questions. Because points are not deducted for incorrect answers, you are encouraged to answer all multiple-choice questions. On any questions you do not know the answer to, you should eliminate as many choices as you can, and then select the best answer among the remaining choices.
CHEMISTRY
SECTION I
Time—1 hour and 30 minutes
INFORMATION IN THE TABLE BELOW AND ON THE FOLLOWING PAGES MAY BE USEFUL IN ANSWERING THE QUESTIONS IN THIS SECTION OF THE EXAMINATION.

ADVANCED PLACEMENT CHEMISTRY EQUATIONS AND CONSTANTS
Throughout the test the following symbols have the definitions specified unless otherwise noted.


| Acid |
Lewis Diagram | Ka |
| HOBr |
| 2.0 × 10–9 |
| HOCl |
| 3.0 × 10–8 |
1. The Ka values and Lewis structures for two different oxoacids are listed above. Which of the following statements correctly identifies the stronger acid and offers up the best justification?
(A) HOBr, because bromine is larger than chlorine and strongly repels the –OH group
(B) HOBr, because bromine’s electron cloud is more polarizable than chlorine’s
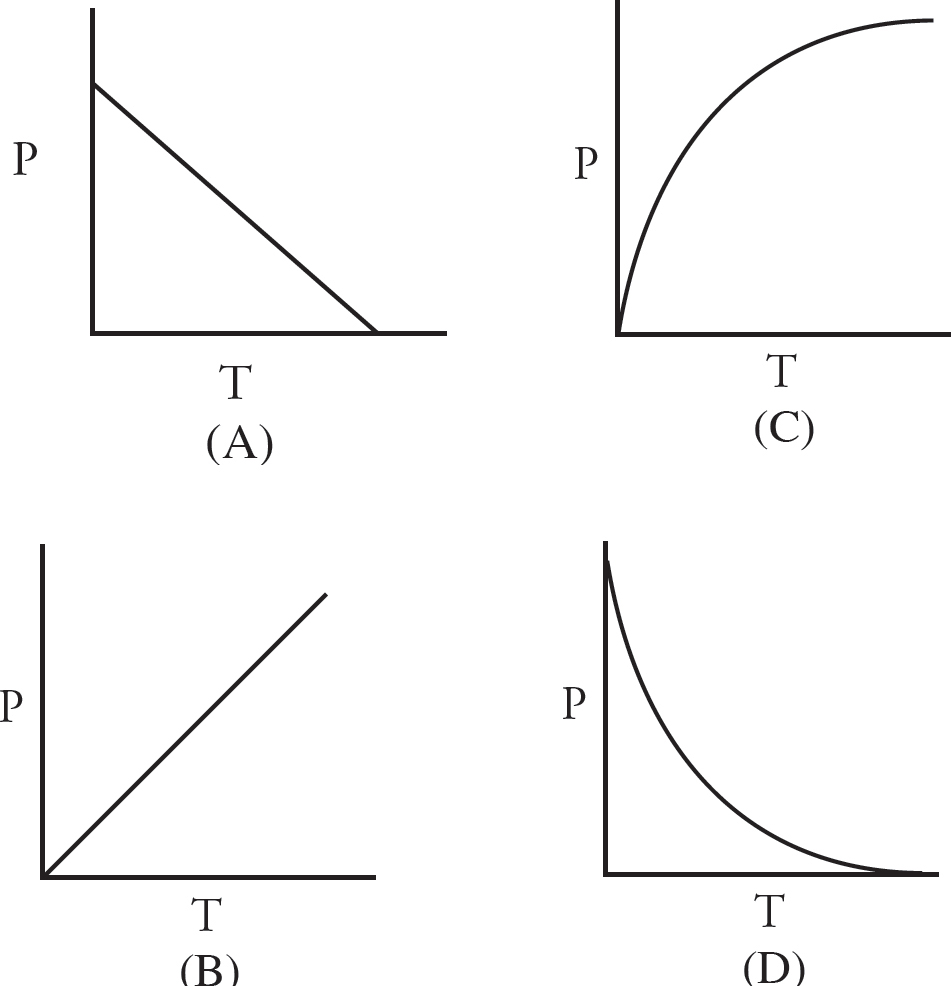
(C) HOCl, because the OCl– ion has a stronger attraction for protons than the OBr– ion
(D) HOCl, because chlorine is more electronegative than bromine and weakens the H-O bond more effectively
2. Approximately how many grams of oxygen are present in a 10.0 g sample of NaOH?
(A) 2.00 g
(B) 4.00 g
(C) 6.00 g
(D) 8.00 g
2Li (s) + 2H2O (l) → 2Li+ (aq) + H2 (g) + 2OH− (aq)
3. A 3.50 g sample of lithium metal is dropped into a beaker of water, causing the above reaction to occur. How many liters of hydrogen gas are produced if this reaction takes place at STP?
(A) 5.60 L
(B) 11.2 L
(C) 22.4 L
(D) 28.0 L
| Metal Initial Temperature | 98.0oC |
| Water Initial Temperature | 26.0oC |
| Water + Metal Final Temperature | 28.0oC |
| Mass Metal | 10.0 g |
| Mass Water | 100. g |
4. A piece of an unknown metal is heated to a high temperature, and then dropped into a cup of water. Given the data above and assuming no heat is lost to the environment, what is the approximate specific heat of the metal? (Water has a specific heat of 4.2 J/goC.)
(A) 0.60 J/goC
(B) 0.80 J/goC
(C) 1.00 J/goC
(D) 1.20 J/goC
Fe2O3 (s) + 3H2 (g) ⇌ 3H2O (l) + 2Fe (s)
5. What would the equilibrium constant expression, Kc, be for the above reaction?
(A) Kc = 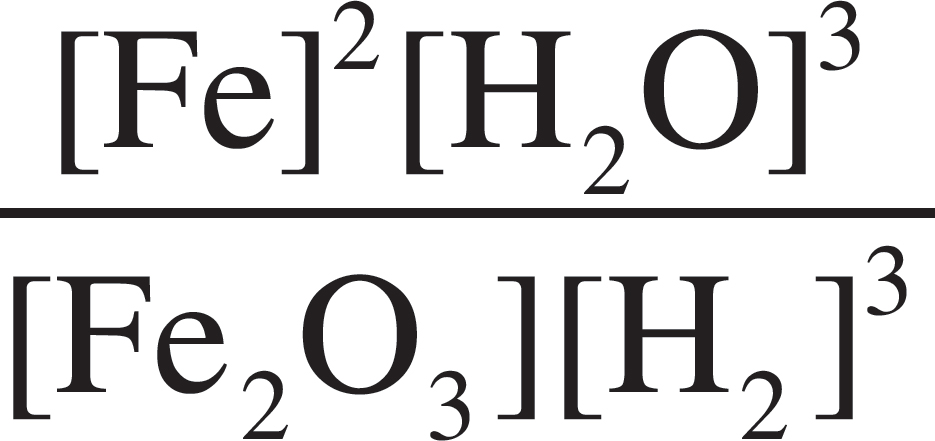
(B) Kc = 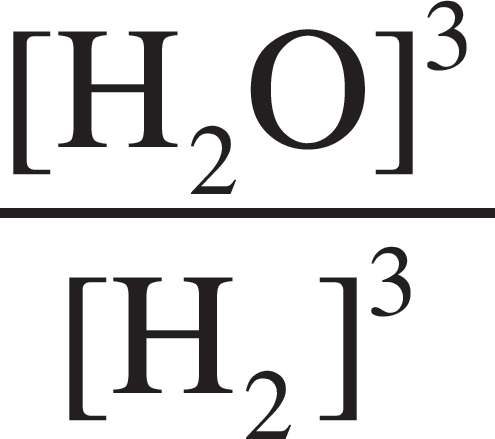
(C) Kc = 
(D) Kc = 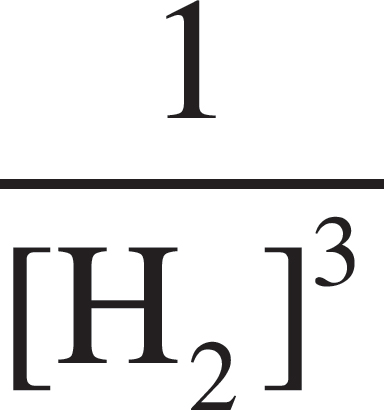
Questions 6-10 refer to the following standard reduction potentials.
| Half Reaction | Reduction Potential |
| O2 (g) + 4H+ (aq) + 4e– → 2H2O (l) | 1.23 V |
| Ag+ (aq) + e– → Ag (s) | 0.80 V |
| Cu2+ (aq) + 2e– → Cu (s) | 0.34 V |
| Fe2+ (aq) + 2e– → Fe (s) | –0.44 V |
| 2H2O (l) + 2e– → H2 (g) + 2OH– | –0.83 V |
| Al3+ (aq) + 3e– → Al (s) | –1.66 V |
6. What would the cell potential be for a galvanic cell constructed of Ag/Ag+ and Fe/Fe2+ half-cells?
(A) 0.36 V
(B) 1.16 V
(C) 1.24 V
(D) 2.04 V
7. What would the net ionic reaction be for the galvanic cell described in question #6?
(A) 2Ag+ (aq) + Fe (s) → 2Ag (s) + Fe2+ (aq)
(B) Fe2+ (aq) + 2Ag (s) → Fe (s) + 2Ag+ (aq)
(C) Ag+ (aq) + Fe2+ (aq) → Ag (s) + Fe (s)
(D) 2Ag (s) + Fe (s) → 2Ag+ (aq) + Fe2+ (aq)
8. A 2.0 A current is applied to beakers containing identical volumes of each of the following 1.0 M solutions. In beaker will the greatest mass of metal plate out?
(A) AgNO3 (aq)
(B) CuSO4 (aq)
(C) FeCl2 (aq)
(D) AlBr3 (aq)
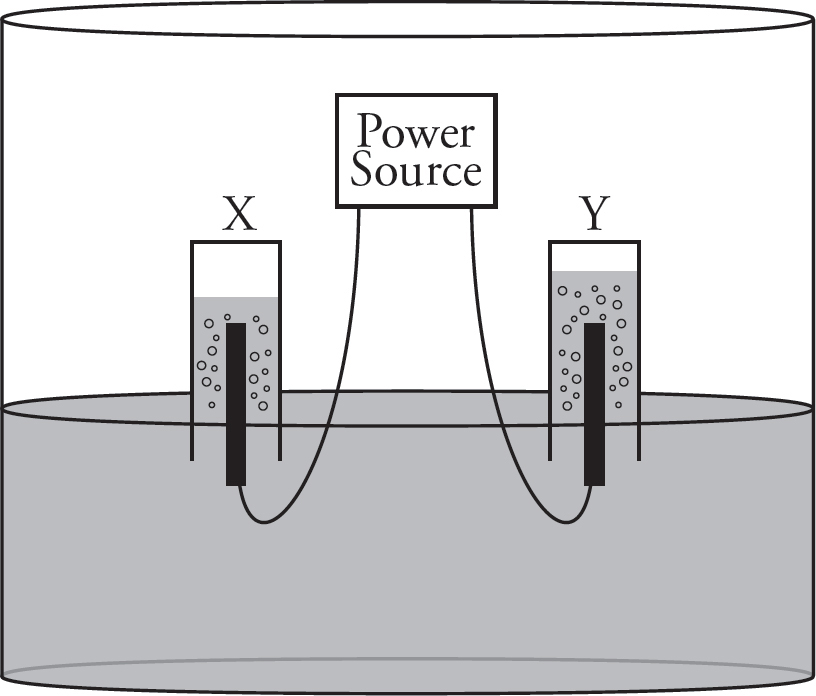
9. A current is run through a solution of pure water, and gases are collected as shown in the above diagram. Which of the following correctly identifies the cathode and anode during the hydrolysis, along with identifying the gas present at each?
| Electrode X Identity | Gas collected at Electrode X | |
| (A) | Cathode | Hydrogen |
| (B) | Anode | Hydrogen |
| (C) | Cathode | Oxygen |
| (D) | Anode | Oxygen |
10. Which of the following would NOT cause a reaction to occur?
(A) Adding some aluminum pellets to a solution containing Fe2+ ions
(B) Placing a copper strip into a solution containing Ag+ ions
(C) Adding powdered iron to a solution containing Cu2+ ions
(D) Dropping a silver coin into a solution containing Al3+ ions
Use the following information to answer questions 11-13.

|
Peak 1 |
Peak 2 |
Peak 3 |
Peak 4 |
Peak 5 |
|
2300 eV |
450 eV |
150 eV |
30 eV |
5.0 eV |
The photoelectron spectrum for a neutral aluminum atom is located above.
11. The amount of energy necessary to remove an electron from the 2p subshell is closest to which value?
(A) 450 eV
(B) 150 eV
(C) 30 eV
(D) 5.0 eV
12. On a spectrum of an aluminum ion:
(A) All peaks would be identical.
(B) The peak furthest to the right be twice as tall.
(C) The two peaks furthest to the right would be missing.
(D) All peaks would be half as tall.
13. A different aluminum atom is exposed to incoming radiation with an energy of 200 eV. Ejected electrons that were originally in which orbital would have the lowest kinetic energy?
(A) 1s
(B) 2s
(C) 2p
(D) 3p
14. A current of 4.0 A is run through an electrolytic cell for a total of 2.0 minutes. How many coulombs of charge passed through the cell during its operation?
(A) 2.0 C
(B) 8.0 C
(C) 30 C
(D) 480 C
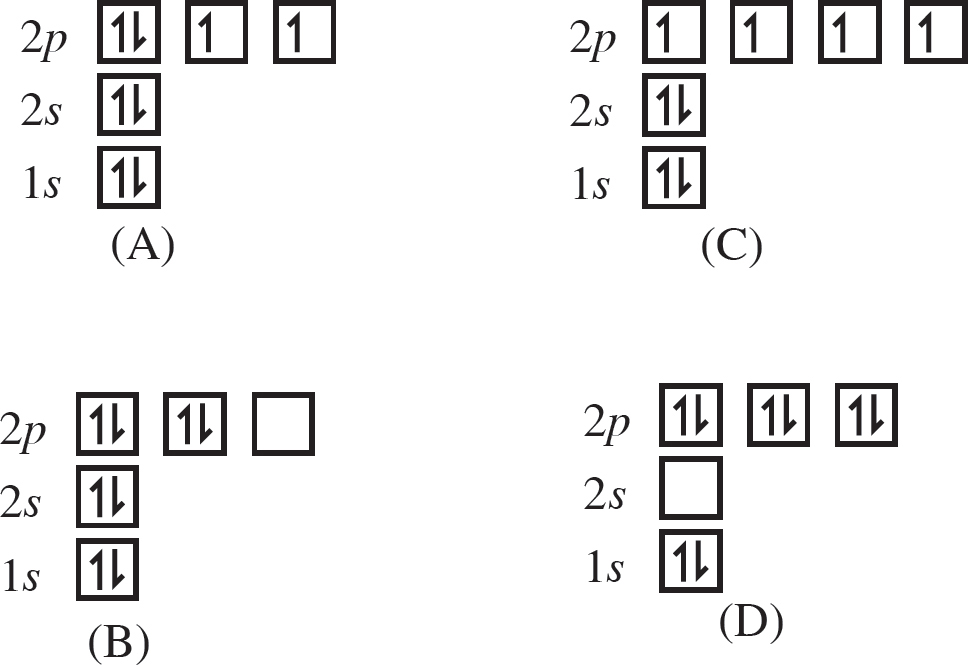
15. Which of the following orbital diagrams shows the correct electron configuration for an oxygen atom?
CxHy (g) + O2 (g) → CO2 (g) + H2O (g)
16. A hydrocarbon of an unknown formula consisting of only hydrogen and carbon is combusted in excess oxygen, producing 44.0 g of carbon dioxide and 18.0 g of water via the above unbalanced reaction. Which of the following is a potential formula for the hydrocarbon?
(A) CH4
(B) C2H3
(C) C2H4
(D) C3H5
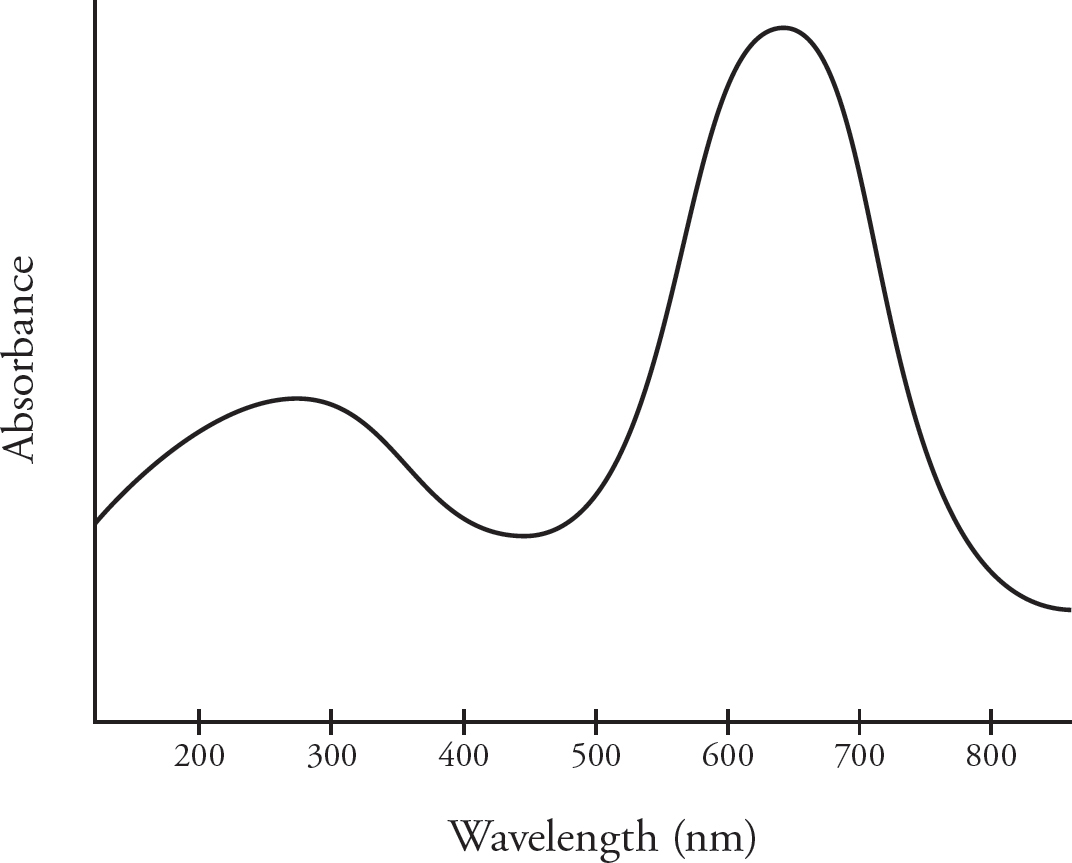
17. Cu2+ ions appear blue in solution, and the NO3– ion is colorless. The absorbance of a Cu(NO3)2 solution is measured at various wavelengths using a spectrophotometer. To determine the concentration of a Cu(NO3)2 solution of unknown concentration, what wavelength of light would produce the best results?
(A) 350 nm
(B) 500 nm
(C) 640 nm
(D) 800 nm
Use the following information to answer questions 18-20.

A solution of potassium dichromate, K2Cr2O7, is poured over a piece of solid tin, causing the oxidation-reduction reaction above to occur.
18. Which substance is oxidized, and which is reduced?
| Oxidized | Reduced | |
| (A) | Cr2O7 | Sn |
| (B) | H+ | Cr2O7 |
| (C) | Sn | Cr2O7 |
| (D) | Cr2O7 | H+ |
19. How many moles of electrons are transferred in the balanced equation?
(A) 2
(B) 3
(C) 4
(D) 6
20. How many grams of tin will react with 100. mL of 0.033 M K2Cr2O7?
(A) 0.40 g
(B) 1.2 g
(C) 2.4 g
(D) 3.6 g
| Salt | ∆Hsoln (kJ/mol) |
|
LiClO4 |
–26.55 |
|
NaClO4 |
13.88 |
|
KClO4 |
41.38 |
21. The enthalpy of solution for three different perchlorate compounds is listed in the table above. Based on the data, which of the following statements is correct?
(A) As cation size increases, the rate at which the lattice energy is changing exceeds the rate at which the hydration energy is changing.
(B) The smaller the cation, the smaller the magnitude of the lattice energy will be.
(C) As cation size increases, the magnitude of the temperature change that occurs in the surrounding solution consistently increases.
(D) Of the three salts, only lithium perchlorate is fully soluble in water, which is demonstrated by its negative enthalpy of solution.
Au+ (aq) + 2CN− (aq) ⇌ Au(CN)2 − (aq) ΔH° = 242 kJ/mol
22. The above system is at equilibrium. Which of the following would cause an immediate increase in the rate of the reverse reaction?
(A) Adding some water
(B) Adding some AuNO3 (aq)
(C) Increasing the pressure
(D) Increasing the temperature

23. A silver/copper galvanic cell is set up, and the above reaction occurs. If all other variables were to remain the same, what is one way to increase the cell potential?
(A) Increase the mass of the copper electrode
(B) Increase the mass of the silver electrode
(C) Increase [Ag+]
(D) Increase [Cu2+]
Use the following information to answer questions 24-27.
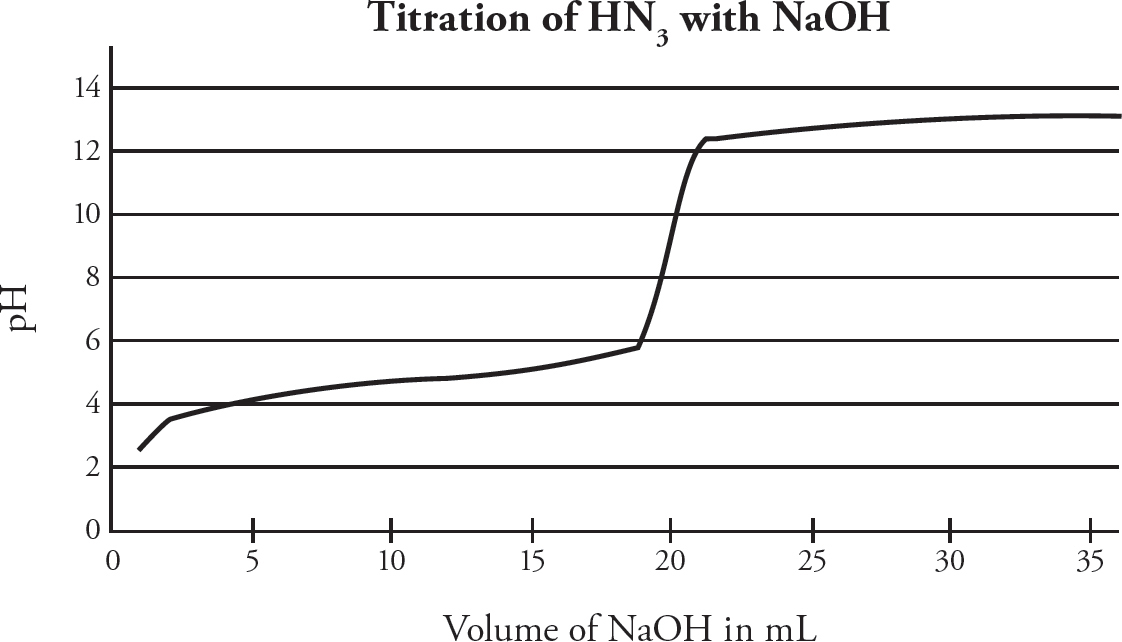
HN3 (aq) + OH– (aq) → N3– (aq) + H2O (l)
25.0 mL of hydrazoic acid, HN3 (Ka = 1.9 × 10–5), is titrated with 0.50 M NaOH. The pH is measured during the titration, producing the above graph. The equivalence point is reached at a volume of 20.0 mL of NaOH added.
24. What is the concentration of the HN3?
(A) 0.40 M
(B) 0.50 M
(C) 0.60 M
(D) 0.80 M
25. At what volume of NaOH added would [N3–] = [HN3]?
(A) 5.0 mL
(B) 10.0 mL
(C) 20.0 mL
(D) 25.0 mL
26. Which indicator would be the best to use to determine the endpoint of the titration?
| Indicator | pKa | |
| (A) | Methyl Violet | 0.80 |
| (B) | Congo Red | 4.0 |
| (C) | Thymol Blue | 8.9 |
| (D) | Indigo Carmine | 12.2 |
27. If the hydrazoic acid were replaced with hydrocyanic acid (Ka = 6.2 × 10–10) of identical concentration, which of the following changes would occur?
(A) Less NaOH would be required to reach the titration endpoint.
(B) The initial pH would change as soon as any NaOH is added will be greater.
(C) The pH of the solution at the 30.0 mL mark would be higher.
(D) The pH at the equivalence point would be decreased.
|
Ion | Ionization energy (kJ/mol) |
|
Al3+ |
11500 |
|
Mg2+ |
7732 |
|
Na+ |
4562 |
28. The next ionization energy values for different ions of three period 3 metals are listed above. The reason that the aluminum ion has the greatest next ionization energy is:
(A) Aluminum metal is less chemically active than magnesium or sodium.
(B) The radius of the aluminum ion is the smallest.
(C) The aluminum ion has the greatest number of unpaired electrons.
(D) The aluminum ion experiences the smallest amount of shielding.
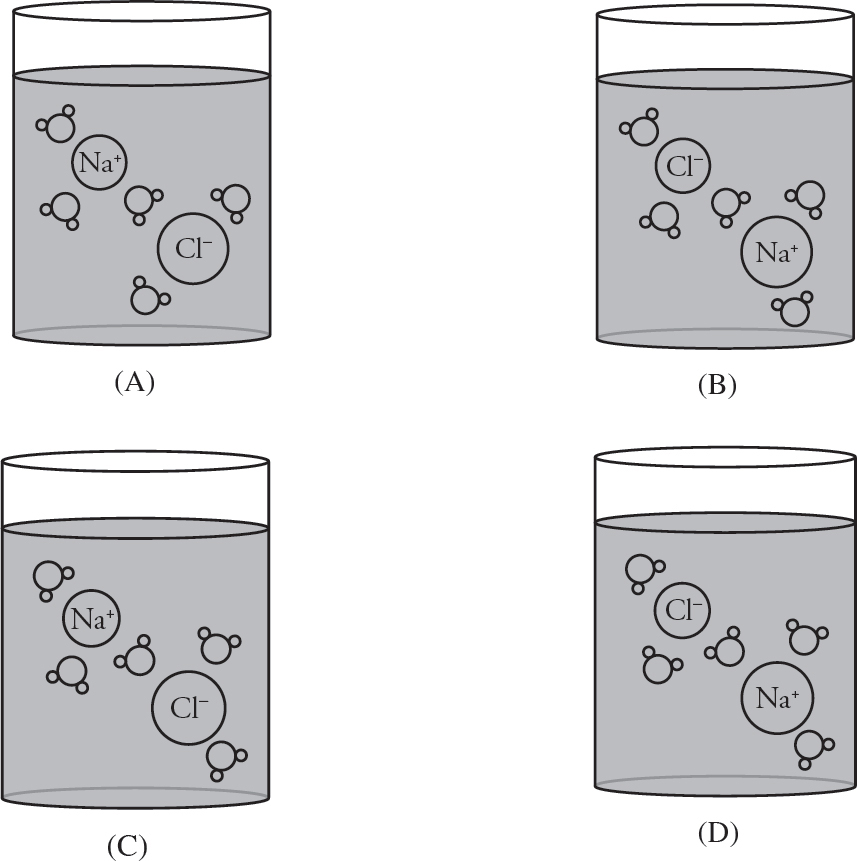
NaCl (s) → Na+ (aq) + Cl– (aq)
29. A sample of sodium chloride, NaCl, is dissolved fully in water. Which diagram below is an accurate representation of how the solution would look on the particulate level?
30. Which of the following expressions would accurately give the magnitude for the density of a sample of helium gas at STP?
(A) 
(B) 
(C) 
(D) (4.0)(273)(0.0821)

31. Given the enthalpy changes for the above reactions, what would be the enthalpy change for the following reaction?
N2 (g) + 2O2 (g) → 2NO2 (g)
(A) x + 2y + 2z
(B) 4x – y – z
(C) x + y – 4z
(D) 2x + y – z

32. Using the above information, determine the equilibrium constant for 2CO (g) + 2H2O (g) ⇌ 2CO2 (g) + 2H2 (g).
(A) –1.0 × 10–4
(B) 2.0 × 103
(C) 1.0 × 104
(D) 4.0 × 104
Use the following information to answer questions 33-37.
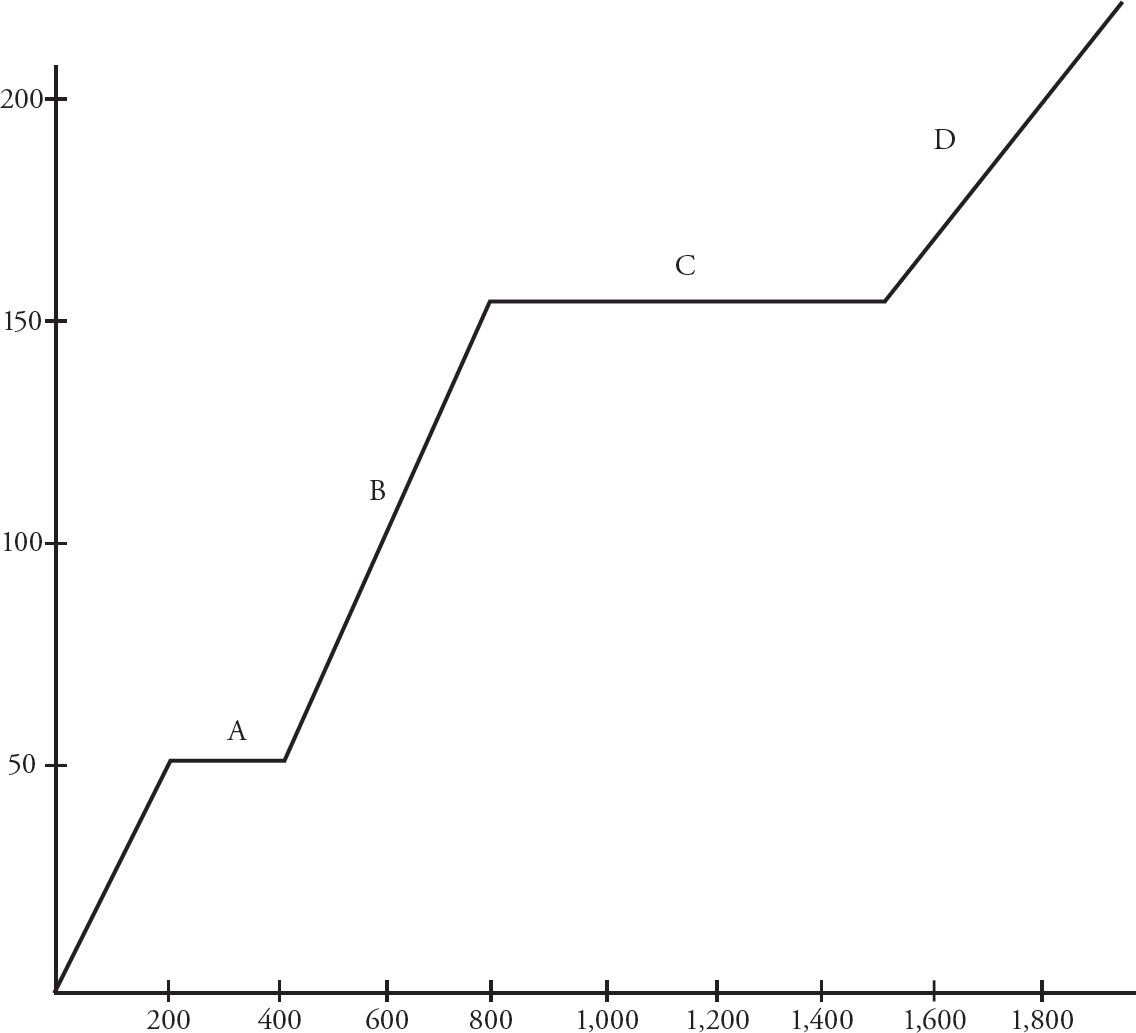
A 2.0 g sample of a solid covalent substance is placed inside an enclosed chamber, and heat is added at a constant rate. The temperature of the substance is tracked as heat is added, producing the above graph.
33. At which point on the graph will the substance exist in both its solid and liquid phase simultaneously?
(A) Point A
(B) Point B
(C) Point C
(D) Point D
34. At which point(s) on the graph will intermolecular forces be the weakest?”
(A) Point A only
(B) Points B and D
(C) Point D only
(D) Intermolecular forces are present at all times.
35. Which of the following statements is true, according to the graph?
(A) The intramolecular forces are strongest during phase A.
(B) The heat of vaporization exceeds the heat of fusion.
(C) The strength of the intermolecular forces is unaffected by the addition of energy.
(D) The average molecular speed increases throughout the heating process.
36. How much energy would be required to melt a separate 1.0 g sample of the substance?
(A) 100 J
(B) 200 J
(C) 300 J
(D) 400 J
37. What is the approximate specific heat of the substance in its liquid phase?
(A) 2.0 J/goC
(B) 4.0 J/goC
(C) 6.0 J/goC
(D) 8.0 J/goC
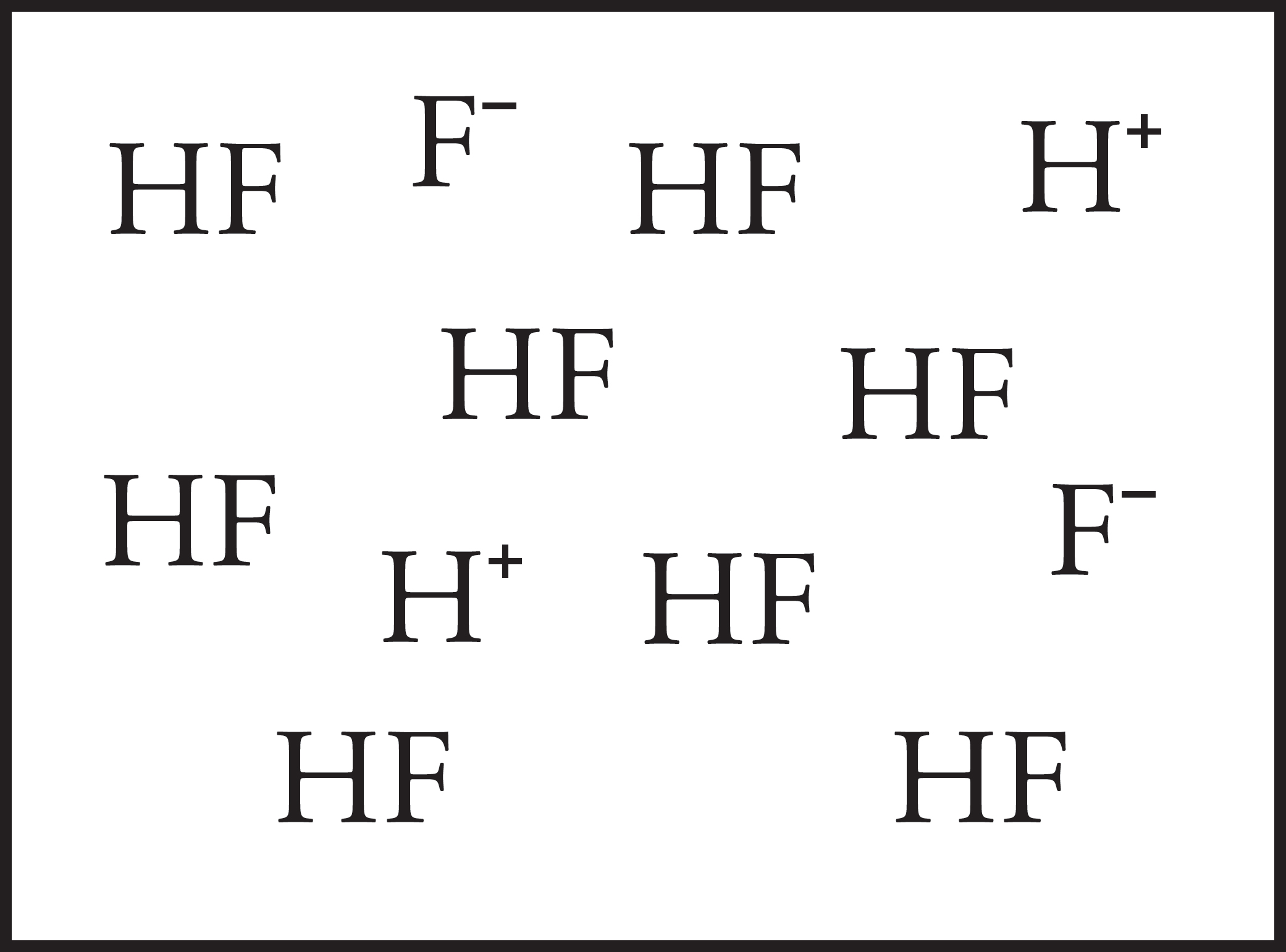
HF (aq) ⇌ H+ (aq) + F− (aq)
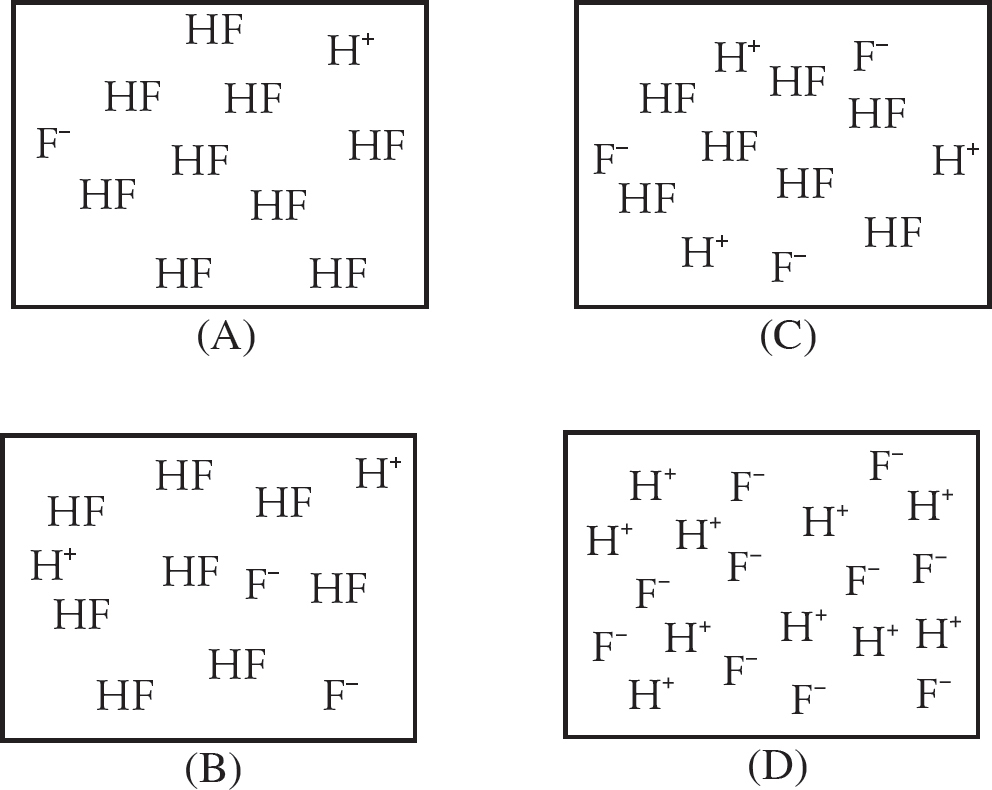
38. HF, is a weak acid with a Ka value of 7.2 × 10–4. The box above represents a particulate-level view of a HF solution of known concentration. If some water is added, which of the following options would represent a particulate representation of the diluted solution?

Pb2+ (aq) + Zn (s) → Pb (s) + Zn2+ (aq)
| [Pb2+] | [Zn2+] | Mass Pb electrode | Mass Zn electrode | |
| Battery X | 1.0 M | 1.0 M | 10. g | 10. g |
| Battery Y | 2.0 M | 2.0 M | 20. g | 20. g |
39. Two batteries are constructed via the above diagram. The concentration of the solutions and the mass of the electrodes are identified in the data table below the diagram. How would the voltage and the battery life for the two batteries compare?
| Voltage | Battery Life | |
| (A) | X < Y | X < Y |
| (B) | X < Y | X = Y |
| (C) | X = Y | X = Y |
| (D) | X = Y | X < Y |
40. Which of the four substances listed below would have the highest melting point?
(A) KCl
(B) CaBr2
(C) MgO
(D) C6H12O6
Use the following information to answer questions 41-43.
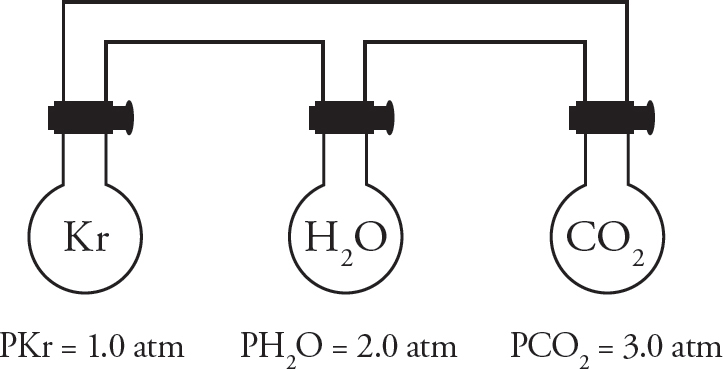
Three identical 1.0 L containers filled with different gases are connected using glass tubes. The stopcocks at the top of each flask are initially closed and all three containers are held at 125°C.
41. Which gas particles are moving the fastest?
(A) Kr
(B) H2O
(C) CO2
(D) All gas particles are moving at the same speed
42. Assuming the volume of the connecting tubes is negligible and that temperature remains constant, what would the total pressure of the system be after all three stopcocks are opened and the gases fully mix?
(A) 2.0 atm
(B) 4.0 atm
(C) 6.0 atm
(D) 8.0 atm
43. After the stopcocks have been opened, the entire system is heated. Choose the graph below that correctly shows how the pressure would change as the temperature is increased.
44. A 1.0 M solution of which of the following would have the highest conductivity?
(A) C2H5OH (aq)
(B) NH4Cl (aq)
(C) PF3 (aq)
(D) Na2CO3 (aq)
|
Halogen |
Boiling Point (oC) |
|
F2 |
–188 |
|
Cl2 |
–35 |
|
Br2 |
59 |
|
I2 |
184 |
45. The boiling point trend observed in the halogens is due to which of the following trends increasing going down the group?
(A) Molar mass
(B) First ionization energy
(C) Electron cloud polarizability
(D) Electronegativity

| Bond | Enthalpy (kJ/mol) |
| C-H | 410 |
| C=C | 720 |
| O=O | 500 |
| C=O | 800 |
| H-O | 470 |
46. Given the bond enthalpy values in the data table above, determine the enthalpy change for the reaction above it.
(A) –1220 kJ/mol
(B) – 400 kJ/mol
(C) 400 kJ/mol
(D) 1220 kJ/mol
2NO2 (g) ⇌ 2NO (g) + O2 (g)
47. At 0oC, the partial pressure of each gas above in an equilibrium system are PNO2 = 0.60 atm, PNO = 0.30 atm, and PO2 = 0.20 atm. What is Kp for this reaction at 0oC?
(A) 0.05
(B) 0.10
(C) 10.
(D) 20.
Use the following information to answer questions 48-50.

A Lewis diagram of the acetamide molecule, CH3CONH2, is drawn above. Note the bond angles are not drawn to scale.
48. Identify the bond angles on the diagram from greatest to smallest.
(A) X > Y > Z
(B) X > Z > Y
(C) Z > X > Y
(D) Y > X > Z
49. How many pi bonds are present in an acetamide molecule?
(A) 0
(B) 1
(C) 2
(D) 3
50. What is the strongest type of intermolecular force present between acetamide molecules in an aqueous solution?
(A) London dispersion forces
(B) Hydrogen bonding
(C) Ionic bonding
(D) Covalent bonding
51. Chlorous acid, HClO2, has a Ka value of 1.0 × 10–2. What would the pH of a solution of 1.0 M sodium chlorite, NaClO2, be?
(A) 4
(B) 6
(C) 8
(D) 10
SO2 (g) + O3 (g) → SO3 (g) + O2 (g)
|
Trial |
[SO2](M) |
[O3](M) |
Initial Rate (M/s) |
|
1 |
0.10 |
0.10 |
0.075 |
|
2 |
0.10 |
0.20 |
0.075 |
|
3 |
0.20 |
0.10 |
0.300 |
52. The above reaction is run three separate times, varying the initial concentration of both reactants as shown. The initial rate is shown for each reaction. What is the rate law for the reaction?
(A) Rate = k[SO2][O3]
(B) Rate = k[SO2]
(C) Rate = k[SO2]2
(D) Rate = k[O3]2
Use the following information to answer questions 53-56.
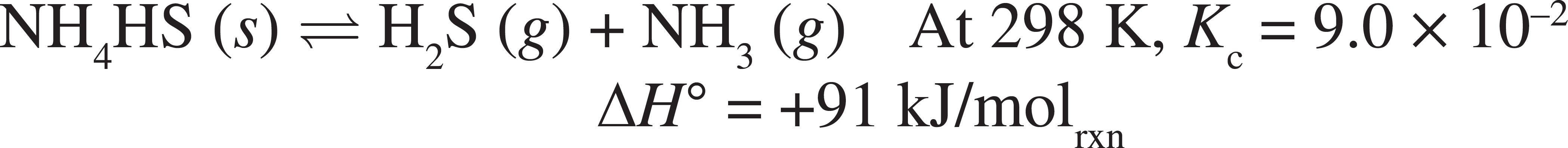
Some solid NH4HS is placed in a sealed and evacuated 250 mL flask at 298 K, and the above decomposition reaction is allowed to reach equilibrium.
53. Calculate the concentration of the NH3 (g) at equilibrium.
(A) 9.0 × 10–2 M
(B) 3.0 × 10–1 M
(C) 1.0 × 102 M
(D) 9.0 × 102 M
54. If some additional NH3 (g) is injected into the flask, which of the following options correctly predicts the reaction shift and what would happen to the concentration of NH4HS?
| Shift | [NH4HS] | |
| (A) | Left | Increase |
| (B) | Left | No Change |
| (C) | Right | Decrease |
| (D) | None | No Change |
55. If the temperature of the flask were to be increased to 313 K, which of the following identifies what would happen to the amount of NH4HS present at equilibrium and correctly explains why?
(A) Increase because both reaction rates have been permanently increased
(B) Decrease because the value for K has increased
(C) Decrease because the reaction is becoming more disordered
(D) Remain the same because it is a solid and unaffected by reaction shifts
56. If the reaction were to be repeated at 298 K using the same initial amount of NH4HS, but in a 500 mL flask, how would that affect the concentration of NH3 at equilibrium compared to the trial in the 250 mL flask?
(A) It would be unchanged.
(B) It would decrease by a factor of four.
(C) It would decrease by a factor of two.
(D) It would increase by a factor of two.

57. A semiconductor with a silicon lattice is doped by the addition of another element, symbolized by the letter X in the above diagram. Which of the following statements best describes the possible identity of the element as well as the type of doping that is occurring?
(A) Boron, which has less valence electrons than silicon and creates positive “holes” in the lattice
(B) Phosphorus, which has extra valence electrons and creates a mobile negative charge in the lattice
(C) Aluminum, which has metalloid properties that lend to increased conductivity when added to a semiconductor
(D) Carbon, which has an equal number of valence electrons as silicon and strengths the conducting lattice
58. What is the oxidation state on the sulfur atom in the compound S2Cl2?
(A) –2
(B) –1
(C) 0
(D) +1
59. Which of the following diagrams correctly demonstrates the permanent dipole attractions between two molecules of oxygen difluoride, OF2? Note that the dashed lines are representative of attractive intermolecular forces.

60. Which of the following reactions would have the most negative value for ΔS°?
(A) 2NH3 (g) → N2 (g) + 3H2 (g)
(B) H2O (1) → H2O (g)
(C) CaO (s) + CO2 (g) → CaCO3 (s)
(D) MgCl2 (s) + H2O (1) → MgO (s) + 2HCl (g)
END OF SECTION I
Section II
INFORMATION IN THE TABLE BELOW AND ON THE FOLLOWING PAGES MAY BE USEFUL IN ANSWERING THE QUESTIONS IN THIS SECTION OF THE EXAMINATION.

ADVANCED PLACEMENT CHEMISTRY EQUATIONS AND CONSTANTS
Throughout the test the following symbols have the definitions specified unless otherwise noted.


CHEMISTRY
Section II
7 Questions
(Total time—105 minutes)
YOU MAY USE YOUR CALCULATOR FOR THIS SECTION
Directions: Questions 1-3 are long free-response questions that require about 23 minutes each to answer and are worth 10 points each. Questions 4-7 are short free-response questions that require about 9 minutes each to answer and are worth 4 points each.
Write your response in the space provided following each question. Examples and equations may be included in your responses where appropriate. For calculations, clearly show the method used and the steps involved in arriving at your answers. You must show your work to receive credit for your answer. Pay attention to significant figures.
CaCl2 (aq) + (NH4)2C2O4 (aq) → CaC2O4 (s) + 2NH4Cl (aq)
1. Most vitamin tablets consist of not only the active ingredient, but also other ingredients that are there to bind the vitamin together or coat it for easier swallowing. A calcium tablet with a mass of 1.49 g is crushed, and then dissolved in some concentrated HCl, creating aqueous calcium chloride. The tablet solution is then mixed with excess ammonium oxalate in a basic environment, causing calcium oxalate to precipitate.
(a) Describe a method to fully separate the precipitate from the solution and accurately measure its mass.
(b) The final mass of the CaC2O4 is determined to be 1.64 g.
(i) How many moles of calcium are present in the precipitate?
(ii) What is the mass percent of calcium in the original vitamin tablet?
(c) CaC2O4 has a Ksp value of 1.9 × 10–9 and dissociates in solution as follows.
CaC2O4 (s) ⇌ Ca2+ (aq) + C2O42– (aq)
(i) What is the concentration of Ca2+ ions and C2O42– ions in a saturated solution of CaC2O4?
(ii) If some of the water evaporated from a saturated solution of CaC2O4, how would that effect the concentration of the ions you determined in part (c)(i)? Justify your answer.
The results do not match the accepted value of calcium in the tablet provided by the manufacturer. Upon reviewing the reaction, it was determined that the final product was not, in fact, pure calcium oxalate, but was instead a hydrate of calcium oxalate.
(d) Would not accounting for the water of hydration cause the calcium value in the tablet to be artificially high or artificially low? Why?
(e) A 5.00 g sample of the calcium oxalate hydrate is heated several times, resulting in a final mass for the anhydrous salt of 4.38 g.
(i) How many moles of water were present in the sample?
(ii) What is the hydration coefficient?
NaF (s)  Na+ (aq) + F– (aq)
Na+ (aq) + F– (aq)
2. Sodium fluoride is the active ingredient in toothpaste that helps prevent tooth decay. One of the important aspects of sodium fluoride is that it is completely soluble in water, and will dissociate fully.
(a) What are the forces that exist between sodium fluoride and water molecules that allow for this dissociation to occur?
(b) Which ion has the larger size- Na+ or F–? Justify your answer.
(c) The below beaker is filled with water molecules. In the beaker, correctly draw the location of the Na+ and F– ions in a dissolved solution of sodium fluoride. Make sure each ion is labeled clearly.
(d) 5.00 g of NaF is dissolved into 50.0 mL of water, and the temperature of the water decreases by 0.52°C. The specific heat and density of solution are identical to that of pure water; 4.18 J/g°C and 1.0 g/mL, respectively.
(i) How much energy is lost by the solution during the dissolution process?
(ii) What is the enthalpy of solution, in kJ/mol, for the dissolution of sodium fluoride?
(iii) For the dissolution of sodium fluoride, which value has a greater magnitude—the lattice energy of sodium fluoride, or the hydration energy between sodium fluoride and water? How do you know?
(e) How would you expect the magnitude of the hydration energy for sodium fluoride compare to that of potassium chloride, KCl? Justify your answer.

3. Lactic acid is an acid found in many dairy-related products.
(a) Write the equilibrium constant expression, Ka, for lactic acid.
(b) (i) The pH of an 0.50 M solution of lactic acid is found to be 2.08. Calculate the percent dissociation of the 0.50 M lactic acid solution.
(ii) If the 0.50 M solution from part (i) were to be diluted, how would that affect the percent dissociation, if at all? Justify your answer.
(c) Lactic acid is the active ingredient in sour cream which makes it taste sour. A sour cream sample with a volume of 500. mL is titrated, and the lactic acid present in it is found to have concentration of 0.113 M. If the sour cream sample has a density of 1.0 g/mL, what is the percent by mass of lactic acid present in the sour cream?
(d) To control the pH level of lactic acid containing substances, sodium lactate, NaC3H5O3, is often used.
(i) How many moles of sodium lactate would need to be added to 100. mL of 0.10 M lactic acid to create a buffer with a pH of 4.0?
(ii) Adding a small amount of which of the following chemicals to a solution of lactic acid would also create a buffer solution? Justify your answer using a chemical reaction.

4. Many foods are dyed various colors to make them more aesthetically pleasing. The dyes are often complex organic compounds, including on that is commercially called Brilliant Blue FCF, which can be abbreviated as BB. When BB reacts with the active ingredient in bleach, the hypochlorite ion (ClO–), the color will fade over time. Thus, the rate of reaction can be determined by using a colorimeter to measure the rate at which the BB fades.
To determine the reaction order for both BB and ClO– in this reaction, three trials are run at 25°C and the rate of reaction is measured in each.
| Trial | [BB] (M) | [ClO–] (M) | Rate (M/s) |
| 1 | 1.5 × 10–3 | 1.5 × 10–3 | 3.3 × 10–3 |
| 2 | 2.0 × 10–3 | 1.5 × 10–3 | 4.4 × 10–3 |
| 3 | 3.0 × 10–3 | 2.5 × 10–3 | 1.1 × 10–2 |
(a) What is the reaction order with respect to:
(i) BB
(ii) ClO–
(b) Calculate the rate constant for the reaction between BB and ClO– at 25°C. Include units.

5. Three different liquids are placed in three sealed flasks of identical volume as shown above. All of the flasks are held at a constant temperature of 25°C, and the vapor pressure of each gas is listed below the flask.
(a) Which liquid has the strongest intermolecular forces? Justify your answer on a particular level.
(b) If the temperature of each of the flasks were to be increased, what effect would that have on the vapor pressure, if any? Why?
(c) (i) Are the gases present in each flask least likely to behave ideally at very low or very high temperatures? Explain your reasoning.
(ii) Which substance would show the greatest deviation from ideal behaviors under the conditions you chose in (c)(i)? Justify your answer.
6. Use the below diagram of the chlorate ion, ClO3–, to answer the following questions as needed.

(a) What is the oxidation state on the chlorine atom in the chlorate ion? Show any necessary calculations.
(b) Calculate the formal charge on each atom in the chlorate ion. Note that each oxygen atom should be clearly labeled to match the subscripts in the diagram.
(c) Is the Cl-Ox bond shorter than, longer than, or the same length as the Cl-Oy bond? Justify your answer.
(d) Chlorine atoms can expand their octet, but oxygen atoms cannot. Why?
7. The Br-Br bond in a bromine molecule has a bond energy of 193 kJ/mol.
(a) (i) How much energy, in Joules, is required to break the bond in a single Br molecule?
(ii) What wavelength of light is necessary to break a single Br-Br bond?
(b) Is the wavelength of light necessary to break a F-F bond shorter than, longer than, or the same as the wavelength of light necessary to break the Br-Br bond? Justify your answer.
STOP
END OF EXAM
_______________________

