2
Functional Foods from Cyanobacteria: An Emerging Source for Functional Food Products of Pharmaceutical Importance
Neha Panjiar1, Shashank Mishra1,2*, Ajar Nath Yadav3, and Priyanka Verma4
1 Department of Bio‐Engineering, Birla Institute of Technology, Mesra, Ranchi, Jharkhand, India
2 Biotech Park, Lucknow, Uttar Pradesh, India
3 Department of Biotechnology, Akal College of Agriculture, Eternal University, Baru Sahib, India
4 Department of Microbiology, Akal College of Basic Science, Eternal University, Baru Sahib, India
*Corresponding author e‐mail: smishra83@gmail.com
Introduction
Cyanobacteria: A Successful Evolutionary Phylum
Cyanobacteria have emerged as one of the most successful prokaryotic phyla sustained during the course of evolution. They are considered as one of the primitive life forms found on our planet (Dixit and Suseela 2013). Evidence has suggested that eukaryotic photosynthesis in chloroplasts has a cyanobacterial origin, leading to the evolution of aerobic respiration 2.22–2.45 billion years ago (Gothalwal and Bajpai 2012; Schirrmeister et al. 2011). Although the autotrophic mode of nutrition is dominant, a few cynaobacterial species can grow in anaerobic and dark conditions, including Nostoc and Oscillatoria (Uzair et al. 2012). Certain species of cyanobacteria like Nostoc can also fix atmospheric nitrogen (Gothalwal and Bajpai 2012; Yadav et al. 2011). Furthermore, they are also among the few phyla in which complex multicellular structures developed from simple unicellular structures during evolution (Schirrmeister et al. 2011). They exhibit broad morphological diversity, ranging from gram‐negative unicellular to multicellular filamentous and colonial forms (Singh et al. 2011). They inhabit various niches, which may be attributed to their evolution towards multicellularity as well as their tolerance of environmental stress conditions (Schirrmeister et al. 2011). The various ecological habitats occupied by cyanobacteria include soil, rock, deserts as well as glaciers, freshwater bodies like rivers, lakes, cold springs, hot springs and brackish or marine ecosystems with saline water (Nair and Bhimba 2013).
Cyanobacteria, being one of the major constituents of phytoplankton, provide ample opportunity for exploitation as a producer of secondary metabolites. The morphological, genetic, and ecological diversity of cyanobacteria leads to the production of a wide range of compounds with applications in the food, feed, pharmaceutical, and neutraceutical industries (Tan 2007). Stability of the products of cyanobacterial origin over a wide range of pH and temperature and solubility in water are some of the desirable properties which make these compounds important for research and human welfare. Active cyanobacterial species producing secondary metabolite belong to the order Oscillatoriales (49%), followed by Nostocales (26%), Chroococcales (16%), Pleurocapsales (6%), and Stigonematales (4%) (Gerwick et al. 2008).
Cyanobacteria: A Potential Source of Functional Foods
Conventional food or food ingredients which provide the required amounts of essential nutritional compounds, such as carbohydrates, proteins, fats, vitamins, and minerals, are termed functional foods (El‐Sohaimy 2012). They may confer additional health benefits. Functional foods may contain bioactive compounds, which are natural chemicals derived from plants, animals or micro‐organisms and are beneficial for human health.
Cyanobacteria have been exploited as a potential food supplement since ancient times. The history of commercial use of Spirulina maxima as a food dates back to 1521, when it was harvested from Lake Texcoco and sold in dried form in the markets of today’s Mexico City (Farrar 1966). It was also here that the first commercial production of Spirulina started in the 1970s. People of the Kanembu tribe living near Lake Chad in Africa have used Spirulina as a protein supplement since 1940 (Abdulqader et al. 2000). Evidence also exists for the use of cyanobacteria other than Spirulina, for example Nostoc commune in Asia, N. flagelliforme in China, N. punctiforme in China, Mongolia and South America, as food and feed supplements (Facciola 1998; Soeder 1980; Takenaka et al. 1998). N. punctiforme is still being marketed in South America as “Lakeplum” (Trainor 1978). Although the market is dominated by the filamentous cyanobacteria, unicellular forms have also created their own niche in the market, like Aphanotheca sacrum, which is being consumed in Japan (Fujishiro et al. 2004).
The global population explosion has resulted in the need to look for alternative sustainable sources of food apart from conventional agricultural products. This has promoted interest in the use of functional foods to meet the nutritional demands of the growing human population. Natural products have been used as food supplements for thousands of years. Approximately 80% of the world population depends on traditional methods for primary healthcare, as estimated by the World Health Organization (Dixit and Suseela 2013). Micro‐organisms, especially cyanobacteria, are an untapped resource as their secondary metabolites have nutritional or therapeutic values. Commercial exploitation of cyanobacteria since the establishment of human civilization is owing to its different properties which make it a suitable source of functional foods.
Many characteristics make cyanobacteria an attractive alternative for sustainable food production:
- high nutrient content of cyanobacteria, especially Spirulina, which is the most commericalized cultivated cyanobacterial species
- worldwide distribution
- need for only small amounts of water for growth; salt water can also be used
- need for smaller amounts of land; unfertile land unsuitable for other types of crops can also be used
- easily digestible
- product stability over a wide pH and temperature range.
According to some statistics, 1 kg of Spirulina may replace 1000 kg of assorted fruits and vegetables in terms of nutritional value. Dried Spirulina is sold in the United States as a health food, with annual sales of approximately $40 million (Singh et al. 2005).
Realizing the present requirement for extensive research on functional foods from cyanobacteria, many companies have initiated industrial‐scale production of cyanobacteria. Culturing, harvesting, processing (drying), and packaging are the crucial steps involved. Currently, more than 70 countries have commercialized products of nutritional importance obtained from cyanobacteria (Gantar and Svircev 2008). Some of the companies are listed in Table 2.1. The demand for large‐scale production of cynaobacterial products is increasing, but there are constraints associated with their production which need to be resolved (Figure 2.1). Development of efficient technologies is required to reduce the production cost along with maintenance and improvement of product quality. Potential cyanobacterial strain selection should be focused and research oriented to conserve the valuable germplasm (Emeka et al. 2012). Limitations in terms of water requirement should be considered for possible substitutes like waste water or sea water (Emeka et al. 2012). Photobioreactors have also been designed to cultivate and scale up cyanobacterial cultures in optimum conditions (Campo et al. 2007).
Table 2.1 Companies marketing functional food products of cyanobacterial origin.
| Country | Companies |
| USA | Earthrise Farms, Cyanotech Corporation |
| Myanmar | Myanmar Microalgae Biotechnology Project, Myanmar Spirulina |
| China | Hainan DIC Microalgae, Nan Pao Resins |
| India | Ballarpur Industries, EID Parry |
| Taiwan | Nan Pao Resins |
| Thailand | Neotech Food, Siam Algae |
| Cuba | Genix |
| Chile | Solarium Biotechnology |
| Canada | Ocean Nutrition |
| Japan | Nippon Spirulina, Dainippon |
| Mexico | Spirulina Mexicana |
| Australia | Panmol |
| Mongolia | Inner Mongolia Biomedical Engineering |
| Germany | Blue Biotech |
| Israel | Koor Foods |
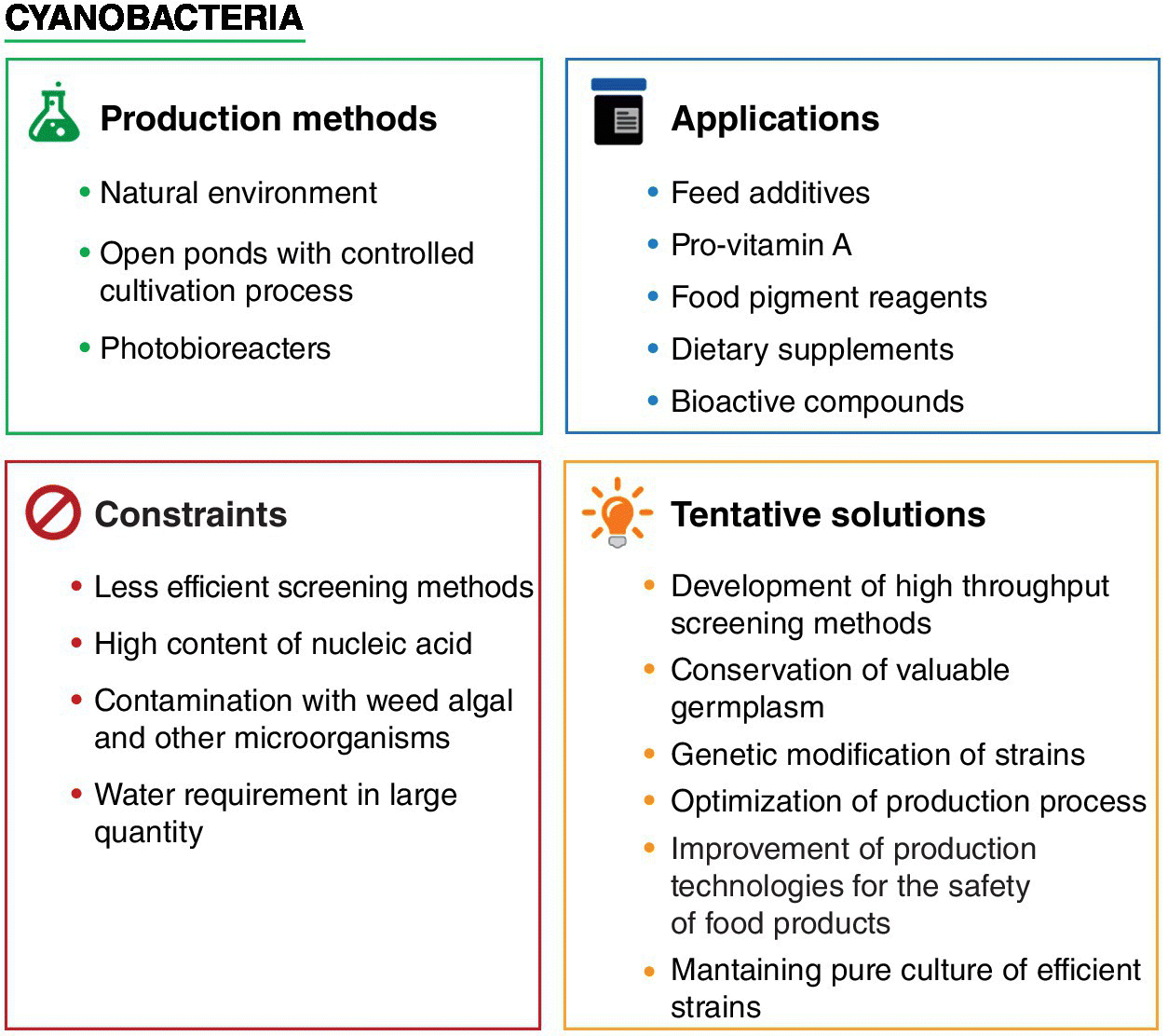
Figure 2.1 General overview of the applications, constraints associated with the production of the cynaobacterial food products and their tentative solutions.
Functional Food Ingredients of Cyanobacterial Origin
Carbohydrate and Fibers
Carbohydrates are the primary energy source used by the body. One gram of carbohydrate provides four calories of energy to the body. According to the World Health Organization, at least 55% of the body’s total energy requirement should be provided by a carbohydrate source (Nishida and Nocito 2007; www.fao.org/docrep/w8079E/w8079e08.htm). Although fulfillment of energy requirements is the main function of carbohydrates, they also play a significant role in the structure and function of cells, tissues, and organs. Intake of optimum daily amounts of carbohydrate helps prevent fat accumulation in the body. Table 2.2 represents the percentage of carbohydrate present in different cyanobacterial species. Amongst the different cyanobacterial species reviewed by Rajeshwari and Rajashekhar (2011), maximum carbohydrate content was found in Scytonema bohneri (28.4%) compared to the average percentage of 15–20% for Spirulina species. The protein percentage was less in other cyanobacterial species than Spirulina as estimated by Fatma et al. (1994), which make Spirulina a favorable candidate for functional food.
Table 2.2 Carbohydrate and protein content of common cyanobacterial strains.
| Cyanobacteria | Carbohydrate (%) | Protein (%) | References |
| Oscillatoria limnetica | 15.23±0.29 | 16.93±0.25 | Subramanian et al. (2014) |
| Oscillatoria tenuis | 11.05±0.31 | 11.82±0.11 | Subramanian et al. (2014) |
| Phormidium abronema | 8.26±0.19 | 22.71±0.24 | Subramanian et al. (2014) |
| Lyngbya cryptovaginata | 19.26±0.27 | 25.23±0.32 | Subramanian et al. (2014) |
| Tolypothrix tenuis | 16.34±0.32 | 12.21±0.22 | Subramanian et al. (2014) |
| Spirulina platensis LEB‐18 | 7.5 | 58 | Moreira et al. (2013) |
| Calothrix fusca | 19.5 | 1.6 | Rajeshwari and Rajashekhar (2011) |
| Gloeocapsa livida | 18.0 | 1.8 | |
| Lyngbya limnetica | 18.4 | 3.1 | |
| Scytonema bohneri | 28.4 | 0.7 | |
| Oscillatoria acuminata | 14 | 6.9 | |
| Oscillatoria calcuttensis | 9.6 | 2.2 | |
| Oscillatoria foreaui | 8 | 7 | |
| Spirulina pacifica | ‐ | 58.01 | Mišurcová et al. (2010) |
| Spirulina platensis | ‐ | 56.05 |
Dietary fiber, soluble and insoluble, is a type of complex carbohydrate. Even though it is not a nutrient, it is important as it is not fully digested and hence not assimilated in the body. It constitutes the roughage portion of the diet. Soluble fiber dissolves in water and is essential for lowering blood cholesterol as well as maintaining blood sugar level (El‐Sohaimy 2012). Insoluble fiber consumption helps in reducing the diet’s caloric value. Dietary fiber therefore helps to maintain a healthy digestive system and is of significant importance for obese and diabetic people (Bolton‐Smith and Woodward 1994; El‐Sohaimy 2012). Recent research also reveals its use for colon cancer prevention (Zeng et al. 2014). The Food and Agricultural Organization (FAO) of the United Nations has prescribed an average intake of 20–35 g of dietary fiber per day after 2 years of age (Dobbing 1989).
In recent years, prebiotics or the non‐digestible oligosaccharide carbohydrates have gained the attention of the scientific community as they help in the maintenance of a beneficial gut microbial population. Tremaroli and Bäckhed (2012) have shown that changes in the composition of gut microbiota can lead to alterations in metabolic capacity in obese people, thereby promoting adiposity and influencing physiological processes like satiety control from the brain and hormonal release from the gut. Fiber content of Spirulina is in the range of 5–8% dry weight (Shetty et al. 2006).
Proteins and Peptides
Proteins are structurally complex macromolecules with building blocks of amino acids. They are polypeptides composed of more than 50 amino acids, while peptides have fewer than 50 amino acids. Proteins and peptides have diverse biological activities in the human body ranging from catalysis in the form of enzymes, structural support as a major component of connective tissues, cell movement, being actively involved in body defense mechanisms, body regulation in the form of peptide hormones and growth regulators and in stress response. Deficiency or inadequate intake of calories can cause protein energy malnutrition (PEM), mainly kwashiorkor which accounts for 6 million deaths annually (http://fnic.nal.usda.gov/dietary‐guidance/dietary‐reference‐intakes/dri‐nutrient‐reports). Therefore an economical diet rich in protein is required worldwide and especially in underdeveloped and developing countries. The proportion of the disease burden is is expected to rise to 57% by 2020 (Gershwin and Belay 2008). Management of the global prevalence of these diseases from the nutritional point of view is of the utmost importance.
Micro‐organisms, being widely distributed in nature, can provide a global solution. However, high protein content bacteria are very scarce, like Cellulomonas with 80% of their dry weight as protein, but their high protein content is associated with high nucleic acid content, which is undesirable from a medical point of view (Litchfield 1983). Metabolism of nucleic acid results in uric acid formation and accumulation in the body, leading to pathological conditions like gout. According to the Protein Advisory Group (PAG) of the United Nations, the daily intake of nucleic acids for a healthy adult should not exceed 2 g (http://archive.unu.edu/unupress/food/8F051e/8F051E0d.htm). Cyanobacteria, particularly Spirulina, can surpass bacteria like Cellulomonas nutritionally by having a nucleic acid concentration of less than 5% and are thus advantageous as a future protein supplement (Shetty et al. 2006). As shown in Table 2.2, Spirulina has a higher protein content than other cyanobacterial species. Crude protein content of spirulina is 50–60%, which is greater than other regular protein foods like egg (47%), whole soyabean flour (36%), parmesan cheese (36%), wheat germ (27%), peanut (26%), chicken (24%), and fish (22%) (Ali and Saleh 2012). Studies by Fica et al. (1984) showed that in Romania, protein supplements from Spirulina helped patients with nutritional deficiency; the patients gained weight and their health improved. In China, it has been implemented as a baby food ingredient in baked barley sprouts (Shetty et al. 2006).
Lipids and Fatty Acids
Cyanobacteria contain significant quantities of lipid, with a composition similar to that of vegetable oils (Singh et al. 2002). Cyanobacterial lipids generally are esters of fatty acids and glycerol with a chain length of C14 to C22, which may be either saturated or unsaturated (Shetty et al. 2006). However, cyanobacteria lipids, compared to eukaryotes, usually lack sterols (Quinn and Williams 1983). Lipid composition of cyanobacterial species is generally affected by their growth phase, pH, temperature, illuminance, carbon dioxide concentration, and nitrogen deficiency (Kaiwan‐arporn et al. 2012; Liu et al. 2014; Loura et al. 1987; Sharathchandra and Rajashekhar 2011). Some of the filamentous cyanobacteria have been reported to have large quantities of polyunsaturated fatty acids (25–60% of the total fatty acid present) and essential fatty acids (Shetty et al. 2006). Lipid of Synechocystis aquatilis TISTR8612 is rich in essential fatty acids such as linoleic (C18:2) and linolenic (C18:3) acid, which has medical implications (Kaiwan‐arporn et al. 2012). Percentage of lipid on a dry weight basis is 2–3% in Spirulina, with linoleic (C18:2) and linolenic (C18:3) acid content of about 17.9% and 24.9% of total fatty acids, respectively (Shetty et al. 2006). The fatty acid composition and high amounts of PUFA make the Spirulina platensis lipids a special component (Moreira et al. 2013). PUFA gained interest as it has benefits in alleviating cardiovascular, inflammatory, autoimmune disorder, and diabetes and is good for joints (Riemersma 2001). Spirulina lipids also have the advantage of being cholesterol free which is medically suitable for people suffering from obesity, atherosclerosis, and diabetes. They have also been studied for their cholesterol‐reducing effects (Shetty et al. 2006).
Minerals and Vitamins
Cyanobateria are rich in minerals and vitamins. Levels of iron (15 mg/10 g of Spirulina), calcium (100 mg/10 g of Spirulina), magnesium (40 mg/10 g of Spirulina), potassium (160 mg/10 g of Spirulina) and trace elements are very high in Spirulina compared to other readily available foods (Shetty et al. 2006). This has medical implications as it is good for bones, teeth, and blood. Other minerals found in Spirulina (per 10 g) are zinc (300 μg), phosphorus (90 mg), copper (120 μg), sodium (60 mg), manganese (500 μg), and selenium (2 μg) (Shetty et al. 2006). Oscillatoria foreaui and Lyngbya limnetica also contain substantial amounts of minerals, such as 57.65±1.9 and 31.20±2.2 copper, 539.20±0.2 and 379.60±0.5 manganese, 6402.00±0.5 and 5455.70 ± 0.2 iron, 211.20±0.2 and 134.10±1.1 zinc, 9.37±1.2 and 2.20±0.7 nickel, 12,812.00±1.0 and 18,650.00±0.5 μg/mL magnesium respectively (Rajeshwari and Rajashekhar 2011).
Cyanobacteria can also surpass other foods as being the richest source of vitamin B12 (Shetty et al. 2006). It has been shown that intake of only 1 g of Spirulina is sufficient to meet the daily requirement for vitamin B12. It contains other vitamins also, including A, B1, B2, B3, B6, E, H, folacin, pantothenic acid, and inositol. Pantothenic acid is present in an amount equivalent to the recommended daily allowance. The quantity of β‐carotene (32 μg) present in Spirulina is more than the RDA (6 μg) and is 20 times more than that present in carrot (Shetty et al. 2006). In India, a large‐scale study on preschool children with vitamin A deficiency showed that they recovered with a Spirulina food supplement (Seshadri 1993). Many of these vitamins and minerals have strong antioxidant properties, which help in eliminate toxins and fight disease.
Bioactive Compounds
The oceanic cyanobacteria are an extraordinarily rich source of secondary metabolites (Cardellina and Moore 2010). Cyanobacterial secondary metabolites contain various bioactive molecules including cytotoxic (41%), antitumor (13%), antiviral (4%), and antimicrobial (12%), and other compounds (18%) include antimalarials, antimycotics, multidrug resistance reversers, herbicides, insecticides, algaecides, and immunosuppressive agents (Burja et al. 2001). These natural compounds not only serve directly as drugs, but also are being used as prototypes for the synthesis of new drugs. Therefore, cyanobacterial metabolites continue to be explored for their application in many biological areas and can be an exceptional source of compounds for drug discovery (Bajpai et al. 2010; Nunnery et al. 2010; Singh et al. 2005, 2011).
Anticancerous Activity
Statistics from the American Cancer Society showed that 7.6 million people died worldwide during 2007 from cancer and this will only increase in future, if not controlled properly (http://uk.reuters.com/article/2007/12/17/health‐cancer‐worlddc‐idUKN1633064920071218). Currently available drugs like vinca alkaloids and taxanes can become inactive due to the development of drug‐resistant properties by the tumor cells, which is a key reason for failure of chemotherapeutic treatment of cancers.
Screening of cyanobacterial extracts for new anticancer compounds was initiated by Moore (Oregon State University) and Gerwick (University of Hawaii) in the 1990s. Tubulin or actin filaments in eukaryotic cells are targeted by many cyanobacterial bioactive compounds, which make these compunds a potent source of anticancer agents (Jordan and Wilson 1998). The small anticancerous peptides dolastatin 10 and dolastatin 12 were isolated from Symploca sp. and Leptolyngbya sp. (Catassi et al. 2006; Kalemkerian et al. 1999). Curacin A showed antiproliferative property that has been isolated from Lyngbya majuscula (Nagle et al. 1995). It was also artificially synthesized because of its pharmacological importance (Muir et al. 2002).
Antiviral Activity
International spread of lethal viral diseases like HIV, AIDS, dengue, and avian influenza (H5N1 virus) has had serious consequences. The anti‐HIV highly active antiretroviral therapy (HAART) is effective in controlling the development of HIV infections but is toxic (Luescher‐Mattli 2003). Hence, novel drugs are now needed to fight these lethal diseases. Antiviral compounds isolated from cyanobacteria are usually found to have bioactivity by blocking viral absorption or penetration and inhibiting replication stages of progeny viruses after penetration into cells. Protection of human lymphoblastoid T‐cells from the cytopathic effect of HIV infection with an extract of Lyngbya lagerheimeii and Phormidium tenue was reported by Gustafson et al. (1989). A new class of HIV inhibitor called sulfonic acid, containing glycolipid, was isolated from an extract of cyanobacteria and the compounds were found to be active against the HIV virus. Cyanovirin‐N (CVN), a peptide isolated from cyanobacteria, inactivates strains of HIV virus and inhibits cell‐to‐cell and virus‐to‐cell fusion (Yang et al. 1997).
Antibacterial Activity
In recent years, multidrug‐resistant bacteria causing nosocomial infections such as methicillin‐resistant Staphylococcus aureus, vancomycin‐resistant Enterococci and Amp C β‐lactamase producing Enterobacteriaceae have posed therapeutic challenges and are of great concern worldwide (Reinert et al. 2007). In an effort to develop new antibiotics, scientists are screening cyanobacterial extracts for their antibacterial activity (Biondi et al. 2008; Kreitlow et al. 1999; Skulberg 2000) and have found them potentially active against various bacteria. Noscomin from Nostoc commune exhibited antibacterial activity against Bacillus cereus, Staphylococcus epidermidis, and Escherichia coli (Jaki et al. 2000). Nostocarboline from Nostoc was found to inhibit the growth of other cyanobacteria and green alga (Blom et al. 2006). Hirata et al. (2003) found that Nostocine A isolated from Nostoc spongiaeforme inhibited the growth of green algae more strongly than cyanobacteria. Asthana et al. (2009) isolated hapalindole (alkaloids) from Nostoc CCC537 and Fischerella sp. and found antimicrobial activity against Mycobacterium tuberculosis H37Rv, Staphylococcus aureus ATCC25923, Salmonella typhi MTCC3216, Pseudomonas aeruginosa ATCC27853, E. coli ATCC25992, and Enterobacter aerogenes MTCC2822.
Because of growing bacterial resistance against antibiotics, the search for new active substances with antibacterial activity is urgent and cyanobacteria will be potentially promising candidates.
Antiprotozoal Activity
According to a WHO estimate, more than 1 billion people throughout the world are suffering from tropical diseases caused by Plasmodium, Trypanosoma, Leishmania, Schistosoma, and others (Simmons et al. 2008). Failures in the treatment of these diseases, especially in cases of malaria (Lanzer and Rohrbach,2007) and leishmaniasis (Prioto et al. 2007), are due to development of resistance by these protozoa. On the other hand, progress in the advancement of drug discovery programs against these diseases is very slow (Sheifert et al. 2007). In an effort to encourage the development of effective and affordable treatment for these diseases, the Panamanian International Co‐operative Biodiversity Group is screening extracts from terrestrial and marine sources. In addition, the protease inhibitor nostocarboline (Barbaraus et al. 2008), an alkaloid isolated from Nostoc sp. 78‐12, was found to be active against Trypanosoma brucei, Trypanosoma cruzi, Leishmania donovani, and Plasmodium falciparum. Aerucyclamide (Portmann et al. 2008) isolated from Microcystis aeruginosa PCC 7806 was also found to be active against T. brucei, and the already known aerucyclamide B against P. falciparum. Clark et al. (2008) isolated six new acyl proline derivatives, tumonoic acids D–I, from the marine cyanobacterium Blennothrix cantharidosmum, among which tumonoic acid I displayed moderate activity in an antimalarial assay.
Protease Inhibition Activity
The discovery of new protease inhibitors may be of great pharmaceutical value. Jaspars and Lawton (1998) described some protease inhibitors of cyanobacterial origin, such as microginins, aeruginosins, and cyanopeptolins. Microginins are used in the treatment of high blood pressure. Serine protease inhibitors like cyanopeptolin are applied in the treatment of conditions such as asthma and viral infections.
Immunomodulatory Activity
Different products prepared from Spirulina influence immune systems in various ways such as increasing the phagocytic activity of macrophages, stimulating antibody and cytokine production, increasing accumulation of natural killer cells in tissues, and activating T‐ and B‐cells (Khan et al. 2005). The effect of Spirulina in mice was studied and increased phagocytic activity and enhanced antigen production in the test animals were reported (Hayashi et al. 1994). Qureshi and Ali (1996) reported increased phagocytic activity, increased antigen production, and increased natural killer cell‐mediated antitumor activity in chicken for the same cyanobacterium (Spirulina).
Summary and Future Prospects
Cyanobacteria are photosynthetic bacteria populating diverse habitats worldwide. They are the only prokaryotes capable of fixing carbon dioxide from the atmosphere through photosynthesis, with efficiency higher than vascular plants. Their cultivation is economical, simple and does not directly compete with agricultural crops for water and land. Recent research leading to technical improvements and increased consumer demand has resulted in market expansion for cyanobacterial species and their products. However, their biotechnological potential is still not explored completely and requires exhaustive research for industrial‐scale development of its approved functional food products. Functional foods are defined as foods providing health benefits apart from basic nutrition. Cyanobacteria provide an ultimate blend of nutrition in the right quantities as a single food. Although they are a promising source offering diverse functional foods, they are still underexplored as a natural resource.
Commercial exploitation of cyanobacteria is hampered by lack of knowledge of culture techniques, biochemical pathways leading to formation of diverse metabolites and cost‐effective production methods. Advances in molecular biology, biochemistry, instrumentation, and computational approaches have directed attention towards the biosynthesis of diverse metabolites from cyanobacteria. One vital factor determining their biosynthesis is the genetic make‐up of the producer organisms. Strain improvement through genetic modifications could be used to significantly improve the yield and quality of cyanobacterial bioactive compounds. The combined efforts of molecular biology and genetic engineering have led to the sequencing of over 126 genomes of cyanobacterial strains, thus paving the way for further metabolic engineering techniques to explore their unique biosynthetic information. This may also lead to the development of new strains with better growth rate, higher substrate tolerance and consistent production of biomass aimed at producing designer novel metabolites in the near future.
References
- Abdulqader, G., Barsanti, L. and Tredici, M.R. (2000) Harvest of Arthrospira platensis from Lake Kossorom (Chad) and its household usage among the Kanembu. J. Appl. Phycol., 12: 493–498.
- Ali, S.K. and Saleh, A.M. (2012) Spirulina – an overview. Int. J. Pharm. Pharmaceut. Sci., 4(3): 9–15.
- Asthana, R.K., Tripathi, M.K., Deepali, A. et al. (2009) Isolation and identification of a new antibacterial entity from the Antarctic cyanobacterium Nostoc CCC 537. J. Appl. Phycol., 21: 81–88.
- Bajpai, R., Sharma, N.K. and Rai A.K. (2010) Anticancerous/antiviral compounds from cyanobacteria. Algas, 43: 4–6.
- Barbaraus, D., Kaiser, M., Brun, R. and Gademann, K. (2008) Potent and selective antiplasmodial activity of the cyanobacterial alkaloid nostocarboline and its dimers. Bioorganic Medicinal Chem. Lett., 18: 4413–4415.
- Biondi, N., Tredici, M.R., Taton, A. et al. (2008) Cyanobacteria from benthic mats of Antarctic lakes as a new source of bioactivities. J. Appl. Microbiol., 105: 105–115.
- Blom, J.F., Brutsch, T., Barbaras, D. et al. (2006) Potent algicides based on the cyanobacterial alkaloid nostocarboline. Organic Lett., 8: 737–740.
- Bolton‐Smith, C. and Woodward, M. (1994) Dietary composition and fat to sugar ratios in relation to obesity. Int. J. Obesity, 18: 820–828.
- Burja, A.M., Banaigs, B., Abou‐Mansour, E., Burgess, G. and Wright P.C. (2001) Marine cyanobacteria: a prolific source of natural products. Tetrahedron, 57: 9347–9377.
- Campo, J., Garcia‐Gonzalez, M. and Miguel, G.G. (2007) Outdoor cultivation of microalgae for carotenoid production: current state and perspectives. Appl. Microbiol. Biotechnol., 74: 1163–1174.
- Cardellina, J.H. and Moore, B.S. (2010) Editorial: Richard E Moore (1933–2007). J. Nat. Products, 73: 301–302.
- Catassi, A., Cesario, A., Arzani, D. et al. (2006) Characterization of apoptosis induced by marine natural products in non small cell lung cancer A549 cells. Cell. Molec. Life Sci., 63: 2377–2386.
- Clark, B.R., Engene, N., Teasdale, M.E. et al. (2008) Natural products chemistry and taxonomy of the marine cyanobacterium. Blennothrix cantharidosmum. J. Nat. Products, 71: 1530–1537.
- Dixit, R.B. and Suseela, M.R. (2013) Cyanobacteria: potential candidates for drug discovery. Antonie van Leeuwenhoek, 103: 947–961.
- Dobbing, J. (1989) Dietary Starches and Sugars in Man: A Comparison. ILSI Human Nutrition Review Series. New York: Springer.
- El‐Sohaimy, S.A. (2012) Functional foods and nutraceuticals – modern approach to food science. World Appl. Sci. J., 20(5): 691–708.
- Emeka, U., Ndukwe, G.I., Mustapha, K.B. and Ayo, R.I. (2012) Constraints to large scale algae biomass production and utilization. J. Algal Biomass Util., 3(2): 14–32.
- Facciola, S. (1998) Cornucopia II: A Sourcebook of Edible Plants, 2nd edn. Vista: Kampong Publications.
- Farrar, W.V. (1966) Tecuitlatl: a glimpse of Aztec food technology. Nature, 21: 1341–1342.
- Fatma, T., Sarada, R. and Venkataraman, L.V. (1994) Evaluation of selected strains of Spirulina for their constituents. Phykos, 33: 89–97.
- Fica, V. (1984) Observations on the utilization of Spirulina as an adjuvant nutritive factor in treating diseases accompanied by a nutritional deficiency, Clinica 11 Medicala, Spitalui clinic Municipiului Bucuresti. Med. Interna, 36(3).
- Fujishiro, T., Ogawa, T., Matsuoka, M., Nagahama, K., Takeshima,Y. and Hagiwara, H. (2004) Establishment of a pure culture of the hitherto unicellular cyanobacterium Aphanothecasacrum, and phylogenetic position of the organism. Appl. Environ. Microbiol., 70: 3338–3345.
- Gantar, M. and Svircev, Z. (2008) Microalgae and cynobacteria : food for thought. J. Phycol., 44: 260–268.
- Gershwin, M.E. and Belay, A. (2008) Spirulina in Human Nutrition and Health. Boca Raton: CRC Press.
- Gerwick, W.H., Coates R.C., Engene, N. et al. (2008) Giant marine cyanobacteria produce exciting potential pharmaceuticals. Microbe, 3: 277–284.
- Gothalwal, R. and Bajpai, P. (2012) Cynobacteria a comprehensive review. Int. Res. J. Pharm., 3(2): 53–57.
- Gustafson, K.R., Cardellina, J.H., Fuller, R.W. et al. (1989) AIDS antiviral sulfolipids from cyanobacteria (blue‐green algae). J. Natl Cancer Inst., 81: 1254–1258.
- Hayashi, O., Katoh, T. and Okuwaki, Y. (1994) Enhancement of antibody production in mice by dietary Spirulina platensis. J. Nutr. Sci. Vitaminol., 40: 431–441.
- Hirata, K., Yoshitomi, S., Dwi, S. et al. (2003) Bioactivities of nostocine A produced by a freshwater cyanobacterium Nostoc spongiaeforme TISTR 8169. J. Biosci. Bioeng., 95: 512–517.
- Jaki, B., Orjala, J., Heilmann, J., Linden, A., Vogler, B. and Sticher, O. (2000) Novel extracellular diterpenoids with biological activity from the cyanobacterium Nostoc commune. J. Nat. Products, 63: 339–343.
- Jaspars, M. and Lawton, L.A. (1998) Cyanobacteria – a novel source for pharmaceuticals. Curr. Opin. Drug Discov. Dev., 1: 77–84.
- Jordan, M.A. and Wilson L. (1998) Microtubules and actin filaments: dynamic targets for cancer chemotherapy. Curr. Opin. Cell Biol., 10: 123–130.
- Kaiwan‐arporn, P., Hai, P.D., Thu, N.T. and Annachhatre, A.P. (2012) Cultivation of cyanobacteria for extraction of lipids. Biomass Bioenergy, 44: 142–149.
- Kalemkerian, G.P., Ou, X.L., Adil, M.R. et al. (1999) Activity of dolastatin 10 against small‐cell lung cancer in vitro and in vivo: induction of apoptosis and bcl‐2 modification. Cancer Chemother. Pharmacol., 43: 507–515.
- Khan, Z., Bhadouria, P. and Bisen, P.S. (2005) Nutritional and therapeutic potential of Spirulina. Curr. Pharmaceut. Biotechnol., 6: 373–379.
- Kreitlow, S., Mundt, S. and Lindequist, U. (1999) Cyanobacteria – a potential source of new biologically active substance. J. Biotechnol., 70: 61–73.
- Lanzer, M.R. and Rohrbach, P. (2007) Subcellular pH and Ca2+ in Plasmodium falciparum: implications for understanding drug resistance mechanisms. Curr. Sci., 92: 1561–1570.
- Litchfield, J.H. (1983) Single–cell proteins. Science, 219: 740–746.
- Liu, Y., Zhang, J., Nie, H. et al. (2014) Study on variation of lipids during different growth phases of living cyanobacteria using easy ambient sonic‐spray ionization mass spectrometry. Analyt. Chem., 86: 7096–7102.
- Loura, I.C.D., Dubacq, J.P. and Thomas, J.C. (1987) The effects of nitrogen deficiency on pigments and lipids of cyanobacteria. Plant Physiol., 83: 838–843.
- Luescher‐Mattli, M. (2003) Algae as a possible source of new antiviral agents. Curr. Med. Chem. Anti‐Infect. Agents, 2: 219–225.
- Mišurcová, L., Kráčmar, S., Klejdus, B. and Vacek, J.(2010) Nitrogen content, dietary fiber, and digestibility in algal food products. Czech J. Food Sci., 28(1): 27–35.
- Moore, R.E., Corbett, T.H., Patterson, G.M.L. and Valeriote, F.A. (1996) The search for new antitumor drug from blue green algae. Curr. Pharmaceut. Design, 2(3): 17–330.
- Moreira, L.M., Ribeiro, A.C., Duarte, F.A., Morais, M.G. and Soares, L.A.S. (2013) Spirulina platensis biomass cultivated in Southern Brazil as a source of essential minerals and other nutrients. Afr. J. Food Sci., 7(12): 451–455.
- Muir, J.C., Pattenden, G. and Ye, T. (2002) Total synthesis of (+)‐curacin A, a novel antimitotic metabolite from a cyanobacterium. J. Chem. Soc. Perkin Transactions, 1: 2243–2250.
- Nagle, D.G., Geralds, R.S., Yoo, H. and Gerwick, W.H. (1995) Absolute configuration of curacin A, a novel antimitotic agent from the tropical marine cyanobacterium Lyngbya majuscula. Tetrahedron Lett., 36: 1189–1192.
- Nair, S. and Bhimba, B.V.(2013) Bioactive potency of cynobacteria Oscillatoria spp. Int. J. Pharm. Pharmaceut. Sci., 5(2): 611–612.
- Nishida, C. and Nocito, F.M. (2007) FAO/WHO Scientific Update on carbohydrates in human nutrition: introduction. Eur. J. Clin. Nutr., 61(1): S1–S4.
- Nunnery, J.K., Mevers, E. and Gerwick, W.H. (2010) Biologically active secondary metabolites from marine cyanobacteria. Curr. Opin. Biotechnol., 21: 787–793.
- Portmann, C., Blom, J.F., Kaiser, M., Brun, R., Jüttner, F. and Gademann, K. (2008) Isolation of aerucyclamides C and D and structure revision of microcyclamide 7806A, heterocyclic ribosomal peptides from Microcystis aeruginosa PCC 7806 and their antiparasite evaluation. J. Nat. Products, 71: 1891–1896.
- Priotto, G., Kasparian, S., Ngouama, D. et al. (2007) Nifurtimox‐eflornithine combination therapy for second‐stage Trypanosoma brucei gambiense sleeping sickness: a randomized clinical trial in Congo. Clin. Infect. Dis., 45: 1435–1442.
- Qureshi, M. and Ali, R. (1996) Spirulina platensis exposure enhances macrophage phagocytic function in cats. Immunopharmacol. Immunotoxicol., 18: 457–463.
- Rajeshwari, K.R. and Rajashekhar, M. (2011) Biochemical composition of seven species of cyanobacteria isolated from different aquatic habitats of western ghats, southern India. Brazil. Arch. Biol. Technol., 54(5): 849–857.
- Reinert, R., Donald, E.L., Rosi, F.X., Watal, C. and Dowzicky, M. (2007) Antimicrobial susceptibility among organisms from the Asia/Pacific Rim, Europe and Latin and North America collected as part of TEST and the in vitro activity of tigecycline. J. Antimicrob. Chemother., 60: 1018–1029.
- Riemersma, R.A. (2001) The demise of the n‐6 to n‐3 fatty acid ratio. Eur. J. Lipid Sci. Technol., 103: 372–373.
- Sallal, A.K., Nimer, N.A. and Radwan, S.S. (1990) Lipid and fatty acid composition of freshwater cyanobacteria. J. Gen. Microbiol., 136: 2043–2048.
- Schirrmeister, B.E., Antonelli, A. and Bagheri, H.C.(2011) The origin of multicellularity in cyanobacteria. BMC Evolution. Biol., 11(45): 1–21.
- Seshadri, C.V. (1993) Large‐scale nutritional supplementation with Spirulina alga. All India Project, MCRC, Madras.
- Sharathchandra, K. and Rajashekhar, M. (2011) Total lipid and fatty acid composition in some freshwater cyanobacteria. J. Algal Biomass Util., 2(2): 83–97.
- Sheifert, K., Lunke, A., Croft, S.L. and Kayser, O. (2007) Antileishmanial structure activity relationships of synthetic phospholipids: in‐vitro and in‐vivo activities of selected derivatives. Antimicrob. Agents Chemother., 51: 4525–4528.
- Shetty, K., Paliyath, G., Pometto, A. and Robert, E.L. (2006) Food Biotechnology, 2nd edn. Boca Raton: CRC Press.
- Simmons, L.T., Engene, N., Ureña, L.D. et al. (2008) Viridamides A and B, lipodepsipeptides with antiprotozoal activity from marine cyanobacterium Oscillatoria nigroviridis. J. Nat. Products, 71: 1544–1550.
- Singh, R.K., Tiwari, S.P., Rai, A.K. and Mohapatra. T.M. (2011) Cyanobacteria: an emerging source for drug discovery. J. Antibiotics, 64: 401–412.
- Singh, S., Kate, B.N. and Banerjee, U.C. (2005) Bioactive compounds from cyanobacteria and microalgae: an overview. Crit. Rev. Biotechnol., 25: 73–95.
- Skulberg, O.M. (2000) Microalgae as a source of bioactive molecules: experience from cyanophyte research. J. Appl. Phycol., 12: 341–348.
- Soeder, C.J. (1980) The Scope of Microalgae for Food and Feed. New York: Elsevier/North‐Holland Biomedical Press, pp. 9–20.
- Subramanian, G., Anusha, M.B., Bai, A.L.G., Latha, R., Sasikala, J. and Chandrasakaran, R. (2014) Carbohydrate and protein content of five species of marine cyanobacteria from Pamban and Vadakadu (Rameswaram) coastal regions, Tamil Nadu, India. Int. J. Adv. Interdisciplinary Res., 1(6): 18–20.
- Takenaka, H., Yamaguchi, Y., Sakaki, S. et al. (1998) Safety evaluation of Nostoc flagelliforme (Nostocales, Cyanophyceae) as a potential food. Food Chem. Toxicol., 36: 1073–1077.
- Tan, L.T. (2007) Bioactive natural products from marine cyanobacteria for drug discovery. Phytochemistry, 68: 954–979.
- Trainor, F.R. (1978) Introductory Phycology. New York: Wiley and Sons.
- Tremaroli, V. and Bäckhed, F. (2012) Functional interactions between the gut microbiota and host metabolism. Nature, 489: 242–249.
- Uzair, B., Tabassum, S., Rasheed, M. and Rehman, S.F. (2012) Exploring marine cyanobacteria for lead compounds of pharmaceutical importance. Sci. World J., 179(82): 1–10.
- Yadav, S., Sinha, R.P., Tyagi, M.B. and Kumar, A. (2011) Cyanobacterial secondary metabolites. Int. J. Pharma Bio Sci., 2(2): 144–167.
- Yang, H., Lee, E. and Kim, H. (1997) Spirulina platensis inhibits anaphylactic reaction. Life Sci., 61: 1237–1244.
- Zeng, H., Lazarova, D.L. and Bordonaro, M. (2014) Mechanisms linking dietary fiber, gut microbiota and colon cancer prevention. World J. Gastrointest. Oncol., 6(2): 41–51.