4
Functional Foods from Mushroom
Vinay Basavegowda Raghavendra1*, Chandrasekar Venkitasamy1, Zhongli Pan1,2, and Chandra Nayak3
1 Department of Biological and Agricultural Engineering, University of California, Davis, California, USA
2 Healthy Processed Foods Research Unit, USDA‐ARS‐WRRC, Albany, USA
3 Department of Studies in Biotechnology, Mangalore University, Mangalagangotri, Mangalore, Karnataka, India
*Corresponding author e‐mail: viragh79@gmail.com
Introduction
Food is used not only to satisfy hunger but also to provide nutrition. Nutritious foods prevent nutrition‐related diseases. People have become increasingly conscious about their health which has sparked a global market for natural foods consumed as dietary supplements (Siro et al. 2008). All natural foods are functional by virtue of their nutrients but the concept of functional food derives from the observation that some processed foods containing particular ingredients can provide beneficial effects for human health. Functional foods are foods with positive health benefits that exceed those attributable to the nutritional value of the food which promotes optimal health and helps in reducing the risk of many diseases. According to Hasler (1998), “Functional foods are the foods that provide health benefit beyond basic nutrition. Food choices can control our health, knowing that some food can provide specific health benefits.” Food products are only considered as functional foods if they provide a basic nutritional impact on the human organism, either improving physical conditions or decreasing the risk of diseases (Siro et al. 2008). “Functional foods may improve health, decrease the risk of some diseases, and could even be used for curing some fever. It was recognized that there is a demand for these products as different demographical studies revealed that the medical service of the aging population is rather expensive” (Ouwehand et al. 2002). According to a Canadian definition “The functional foods are similar in appearance to a conventional food consumed as a part of the usual diet that enhance physiological benefits which reduce the risk of chronic diseases beyond basic nutritional functions” (Miles and Chang 1997).
Design and development of functional foods is a scientific challenge relevant to functions that are sensitive to modulation by food components, which are pivotal to maintenance of well‐being and health, and that, when altered, may be linked to a change in the risk of a disease. Increasing demands for functional foods have been seen since the introduction of whole foods or food components with ability to prevent cancer, osteoporosis or cardiovascular disease, improve immunity, detoxification, physical performance, weight loss, cognitive function, and the ability to cope with stress, and inhibit inflammation, antioxidants and modulating the effects of hormones (Roberfroid 2000).
Historically, mushrooms were classified among the lower plants in the division of Thallophyta by Linnaeus based on anatomically uncomplicated structural bodies (lack of true roots, true stem, true leaves, true flowers, and true seeds). Modern studies have established that mushroom biota, together with other fungi, have features which are sufficiently distinctive to place them in a separate fungal kingdom, the Mycetaceae (Chang and Miles 2004). More than 14 000 species of mushrooms are included in the Basidiomycetes and Ascomycetes. Only 25% of mushroom species are edible, in various degrees, 50% are inedible and the remaining 25% are poisonous (Chang 1980).
The early civilizations of the Greeks, Egyptians, Romans, Chinese, and Mexicans probably knew about the therapeutic value of mushrooms and used them in different ways. The Greeks believed that mushroom provided health and strength for soldiers and praised mushroom as a delicacy, and the Romans thought mushrooms were the “food of the gods.” The Chinese treasured mushrooms as “food of life” and Mexicans used mushroom as a “gift of love” in religious ceremonies (Chang and Miles 2004). The Chinese were the first to cultivate Auricularia auricula‐judae in AD 600 and Lentinus edodes mushrooms between AD 1000 and 1100, followed by Agaricus bisporus cultivation in France in 1600 and Pleurotus bisporus cultivation in the USA in 1900 (Chang 1999).
In recent years, mushroom has gained a remarkable amount of interest for its delicious taste and nutritional and medicinal value (Crisan and Sands 1978). Mushrooms are not only a source of nutrients but also are a protein‐rich food cultivated all over the world as an important agricultural product.
Definition
A mushroom is the fleshy, spore‐bearing fruiting body of a fungus (basidiomycete) typically produced above ground on soil or on its food source. Hence the word “mushroom” is most often applied to those fungi (Basidiomycota, Agaricomycetes) that have a stem, cap, and gills on the underside of the cap. These gills produce microscopic spores that help the fungus spread across the ground or its occupant surface (Huang et al. 1985).
According to Chang and Buswell (1996), mushroom is a macro‐fungus with a distinctive fruiting body which can be either epigeous or hypogeous and large enough to be seen with the naked eye. Chang and Miles (2004) stated that “Mushrooms need not be Basidiomycetes, nor aerial, nor fleshy, nor edible, but also few Ascomycetes, which can grow underground having fleshy or non‐fleshy texture and need not be edible”.
Cultivation
Mushrooms have been cultivated for many decades and are now an important agricultural product worldwide. To date, more than 60 mushroom species have been cultivated in East Asian countries, France, and America. Nearly 30 species have been cultivated only in China. Some grow on fresh wood residues (e.g., Lentinula, Pleurotus, Flammulina, Auricularia, Pholiata, Tremella, Agrocybe, Ganiderma), some on slightly composted lignocellulosic materials (e.g., Volvariella, Stropharia, Coprinus), some on well‐composted materials or animal dung (e.g., Agaricus), while others grow on soil and humus (e.g., Lepiota, Leptista, Morchella, Gyromitra). The most popular species grown all over the world are L. edodes and Pleurotus spp. The production statistics of 10 major edible mushroom‐producing countries are shown in Table 4.1.
Table 4.1 Production statistics of 10 major edible mushroom‐producing countries in 2014 (FAO, IFAD, WFP 2014).
| Country | Production ($1000) | Production (metric tons) |
| China | 921 928 | 5 150 000 |
| Italy | 1 416 342 | 785 000 |
| USA | 700 864 | 388 450 |
| Netherlands | 553 907 | 307 000 |
| Poland | 396 936 | 220 000 |
| Spain | 263 421 | 146 000 |
| France | 210 329 | 116 574 |
| Iran | 158 188 | 87 675 |
| Canada | 147 949 | 82 000 |
| UK | 131 891 | 73 100 |
Nutritional Value
Moisture
Moisture content of mushrooms is close to 90% of their fresh weight. Some fleshy mushrooms contain moisture in a different range. Grifolia and Agaricus bisporus contain the highest moisture content of about 92.8–94.8% (Manzi et al. 2001), L. edodes has 81.8–90.0% (Mau et al. 2001b), and Flammulina velutipes has 87.2–89.1% (Yang et al. 2001). The moisture content varies from species to species based on the time of harvest, cropping conditions, temperature, relative humidity, and the postharvest period.
Protein
The crude protein content of edible mushrooms is less than that of animal meat but usually higher than several food products, including milk. In general, mushrooms are considerably higher in protein content, which is twice that of asparagus, cabbage and other vegetables and 12 times higher than oranges and apples, respectively. On dry weight basis, the protein content of mushroom ranges from 19% to 35% whereas the protein content of rice is 7.3%, wheat is 39.1%; and that of soybean and milk is higher than 25%. Edible mushroom contains total proteins of albumins, globulins, natural glutelins, prolamins, and prolamin‐like materials. Protein contents of some common edible mushrooms vary depending on species and stage of development. The protein content of common mushroom species is given in Table 4.2.
Table 4.2 Crude protein contents of some popular edible mushrooms.
| Mushroom | Protein content (%) |
| Agaricus bisporus | 23.9‐34.8 |
| Agaricus campestris | 33.2 |
| Boletus edulis | 29.7 |
| Pleurotus ostreatus | 30.4 |
| Pleurotus sajor‐caju | 26.6 |
| Volvariella dysplasia | 28.5 |
| Volvariella volvacea | 25.9 |
Amino Acids
The nutritional value of mushrooms can be determined based on their content of essential amino acids. The essential amino acid (EAA) index is measured according to dietary protein in terms of an essential amino acid pattern, and the amount of high‐quality protein and the nutritional quality of mushroom are calculated using the formula (Crisan and Sands 1978):
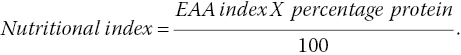
The EAA index, amino acid scores, and nutritional index values for various mushrooms compared with other foods are shown in Table 4.3.
Table 4.3 Comparison of nutritive value of mushrooms with other foods.
Source: Crisan and Sands (1978).
| Essential amino acid index | Food | Amino acid scores | Food | Nutritional indexes | Food |
| 100 | Pork, chicken, beef | 100 | Pork | 59 | Chicken |
| 99 | Milk | 98 | Chicken, beef | 43 | Beef |
| 98 | Mushrooms (high) | 91 | Milk | 35 | Pork |
| 91 | Potatoes, kidney beans | 89 | Mushrooms (high) | 31 | Soybeans |
| 88 | Corn | 63 | Cabbage | 28 | Mushrooms (high) |
| 86 | Cucumbers | 59 | Potatoes | 26 | Spinach |
| 79 | Peanuts | 53 | Peanuts | 25 | Milk |
| 76 | Spinach, soybeans | 50 | Corn | 21 | Kidney beans |
| 72 | Mushrooms (low), cabbage | 46 | Kidney beans | 20 | Peanuts |
| 69 | Turnips | 42 | Cucumbers | 17 | Cabbage |
| 53 | Carrots | 33 | Turnips | 14 | Cucumbers |
| 44 | Tomatoes | 32 | Mushrooms (low) | 11 | Corn |
| 31 | Carrots | 10 | Turnips | ||
| 28 | Spinach | 09 | Potatoes | ||
| 23 | Soybeans | 08 | Tomatoes | ||
| 18 | Tomatoes | 06 | Carrots | ||
| 05 | Mushrooms (low) |
Table 4.3 shows the rankings based on EAA index, amino acid scores, and nutritional indexes calculated against the FAO reference protein pattern; biological values correlate closely with the EAA indexes. Values for mushrooms represent the mean of the three highest values (high) and lowest values (low) (Huang et al. 1985).
Proteins are made up of over 20 amino acids, out of which only nine (lysine, methionine, tryptophan, threonine, valine, leucine, isoleucine, histidine and phenylamine) are present simultaneously and help in protein synthesis. Animal products provide better and more balanced essential amino acids than plant products. For example, cereal grains lack some important amino acids like lysine, and legumes usually lack methionine and tryptophan, but edible mushrooms contain all nine amino acids which are essential for protein synthesis in humans. According to Bano and Rajarathnam (1988), the most abundant essential amino acid present in mushroom is lysine and very scarce essential amino acids are methionine and tryptophan.
In addition to the nine essential amino acids, some edible mushrooms contain sodium salts of glutamic and aspartic amino acids which give foods a pleasant savory taste known as umami. The taste of the mushroom also depends on the presence of amino acids as shown in Table 4.4. According to chemical analysis, the umami flavor of mushroom was attributed to MSG‐like amino acids such as aspartic acid (Asp) and glutamic acid (Glu) and flavor 5′‐nucleotides such as 5′‐guanosine monophosphate (5′‐GMP), 5′‐inosine monophosphate (5′‐IMP) and 5′‐xanthosine monophosphate (5‐XMP) (Sommer 2008). The presence of Asp and Glu by themselves gives a sour taste whereas sodium salts of Glu and Asp elicit the umami taste (Fuke and Shimadzu 1993). Umami is also found in various vegetables (eggplant, tomato, potato, cabbage, edible mushroom, carrot, and soybean), sea foods (fish, seaweed, oyster, prawn, crab, sea urchin, clam, and scallop) and meat (beaf, pork, and chicken) (Fuke and Ueda 1996). Glu and Asp amino, acids are found most abundantly in mushrooms compared to all these foods.
Table 4.4 Classification of amino acids of edible mushrooms.
| Sl. No. | Characteristics of groups | Amino acids |
| 1 | MSG‐like or palatable amino acids | Aspartic acid and glutamic acid |
| 2 | Sweet amino acids | Alanine, glycine, serine, and threonine |
| 3 | Bitter amino acids | Arginine, histidine, isoleucine, and leucine |
| 4 | Tasteless amino acids | Lysine and tyrosine |
Many researchers have found that the umami taste has been elicited by di‐or tripeptides of glutamic acids such as Glu‐Glu, Glu‐Asp, Glu‐Asp‐Glu, Glu‐Gly‐Ser, Gly‐Asp, Ala‐Glu, Gly‐Asp‐Gly, Val‐Asp‐Val, Asp‐Leu, and Val‐Glu‐Leu (Ohyama et al. 1988). The presence of umami was influenced by many factors including the species type, maturity stage, part of mushroom, and quality. Umami amino acid contents in various mushroom species are listed in Table 4.5.
Table 4.5 Umami taste amino acid content of various mushroom species.
| Mushroom | L‐Asp | L‐Glu | MSG‐like | Reference |
| Agaricus blazei | 1.11 | 3.29 | 4.4 | Tsai et al. 2008 |
| Agaricus bisporus (J.E.Lange) Imbach | 1.33 | 4.71 | 6.04 | Dijkstra and Wiken 1976 |
| Agaricus campestris L. | 0.21 | 34.78 | 34.99 | Beluhan and Ranogajec 2011 |
| Agrocybe cylindracea (DC) Maire | 0.94 | 2.18 | 3.12 | Tsai et al. 2008 |
| Amanita rubescens | 2.77 | 17.53 | 20.30 | Guzman et al. 1997 |
| Auricularia fuscosiccinea (brown) | 0.12 | 0.14 | 0.26 | Mau et al. 1998b |
| Auricularia fuscosuccinea (white) | 0.06 | 0.16 | 0.22 | Mau et al. 1998b |
| Auricularia mesenteria | 0.07 | 0.27 | 0.34 | Mau et al. 1998b |
| Boletus edulis Bull. | 0.33 | 39.09 | 39.42 | Beluhan and Ranogajec 2011 |
| Calocybe gambosa | 0.19 | 25.67 | 25.86 | Beluhan and Ranogajec 2011 |
| Cantharellus cibaricus | 0.06 | 29.99 | 30.05 | Beluhan and Ranogajec 2011 |
| Coriolus versicolor | 0.41 | 0.09 | 0.50 | Mau et al. 2001a |
| Craterellus cornucopioides | nd | 45.85 | 45.85 | Beluhan and Ranogajec 2011 |
| Dictyophoraindusiata | 0.31 | 0.54 | 0.85 | Mau et al. 2001a |
| Entolomaclypeatum | nd | 23.89 | 23.89 | Beluhan and Ranogajec 2011 |
| Flammulina velutipes (Curtis) Singer | 0.03 | 1.54 | 1.57 | Yang et al. 2001 |
| Ganoderma lucidum (Curtis) P. Karst | 0.06 | 0.11 | 0.17 | Mau et al. 2001a |
| Ganoderma tsugae | 0.22 | 0.06 | 0.28 | Mau et al. 2001a |
| Hericium erinaceus (Bull.) Pers. | 0.50 | 0.50 | 1.00 | Mau et al. 2001a |
| Lentinula edodes (Berk.) Pegler | 0.41 | 1.30 | 1.71 | Yang et al. 2001 |
| Macrolepiota procera | 0.12 | 33.65 | 33.77 | Beluhan and Ranogajec 2011 |
| Morchellaelata | nd | 38.29 | 38.29 | Beluhan and Ranogajec 2011 |
| Pleurotus cystidiosus | 0.05 | 1.16 | 1.21 | Yang et al. 2001 |
| Pleurotus ferulae | 0.36 | 1.40 | 1.76 | Tsai et al. 2009 |
| Pleurotus ostreatus (Jacq. ex Fr.) P. Kumm. | 0.17 | 41.09 | 1.21 | Yang et al. 2001 |
| Ramria flava | 1.63 | 9.42 | 11.05 | Leon‐Guzman et al. 1997 |
| Tremella fuciformis Berk. | 0.03 | 0.06 | 0.09 | Mau et al. 2001a |
| Tricholoma giganteum | 0.34 | 0.34 | 0.68 | Mau et al. 2001a |
The umami taste of edible mushrooms is the reason for their frequent use as a flavor enhancer in new food product development. Several functional foods have been patented using the umami taste. The glutamic acid content in most edible mushrooms is higher than the aspartic acid content, establishing the fact that Glu is the main umami amino acid in most edible mushrooms. Wild mushroom species have a stronger umami taste than commercial or cultivated ones (Zhang et al. 2013). It is also reported that the presence of glutamate plays an important role in the regulation of gastrointestinal disorders such as gastric problems (functional dyspepsia, chronic atrophic gastritis, and indigestion) (Nakamura et al. 2008). All these health‐improving functions of glutamine and glutamate imply that mushrooms can be used not only as nutritious and palatable food, but also as a functional food or as a raw material for functional foods.
Flavored condiments are used as a functional food by adding edible mushroom powder (A. bisporus) including sodium glutamate, salt, sugar, maltodextrin, yeast extract, and edible oil (Sun et al. 2007). Soybean sauce was produced by fermenting pine mushroom with soybeans to produce a strong pine mushroom taste without any alteration of the sauce (An 2007). Mushroom powder is used as a functional food in Asian countries, usually fresh Agaricus bisporus with sodium glutamate, salt, sugar, yeast extract, flavor nucleotides, and egg yolk powder (Shao and Huang 2009). The dried mushroom slices are mixed with salt, granulated, dried, and sterilized to produce edible mushroom essence spices (Tu 2012).
Solid‐state fermentation by Phellinus linteus and Inonotus obliquus added to cooked rice resulted in a fermented rice product with umami taste having improved nutritional value, and also enhanced the production of enzymes and total antioxidant phenolic products. Pleurotus eryngii, Cordyceps, Termitomyces, and Antrodia camphorata were used to ferment buckwheat, polished rice, wheat, and rice germ, respectively (Chen et al. 2012). Mushrooms such as A. salmonea and Grifola frondosa were used to ferment oat and wheat (Huang et al. 2006).
Fat
Mushrooms are considered low‐calorie foods as they provide low amounts of fat (Leon‐Guzman et al. 1997) ranging from 1.1% to 8.3% on a dry weight basis, with an average fat content of 4.0%. Lentinula edodes has the highest fat content of 4.9–8.0% and Pleurotus has the lowest crude fat. Generally, mushrooms contain all classes of lipid compounds including monoglycerides, diglycerides, triglycerides, free fatty acids, sterols, esters, and phospholipids.
According to Huang et al. (2006), edible mushroom contains a higher percentage of saponifiable lipids than non‐saponifiable lipids. These authors reported that saponifiable lipid ranges from 78.1% in Auricularia auricula‐judae to 58.8% in Volvariella volvacea expressed in terms of percentage of dry weight of lipids. Miles and Chang (2004) reported that high content of saponifiable lipid was due to the presence of provitamin D2 and ergosterol.
The unsaturated fatty acid content of mushroom ranges from 70% to 72% of total fatty acids which is mainly due to the linoleic acid content. Chang (1987) reported that 85.4% of total free fatty acids in Volvariella volvacea, 80.5% in A. bisporus, and at least 74.1% in A. auricula‐judae are unsaturated fatty acids. The higher proportion of linoleic acid in edible mushrooms plays a significant role as a functional food. Total fatty acid composition in edible mushroom varies from species to species. Mushroom contains short chain fatty acids with 14 to 18 carbon atoms, including myristic acid (C14), palmitic acid (C16), palmitoleic acid (16:1), stearic acid (C18:0), oleic acid (18:1), and linoleic acid (C18:2).
Vitamins
Edible mushroom are a good source of several vitamins, including thiamine (B1), riboflavin (B2), niacin, biotin, and ascorbic acid (C) (Crisan and Sands 1978). Lau et al. (1985) reported that the thiamin content of V. volvacea is 0.35 mg, Agaricus species 1.14 mg, Pleurotus species 1.16–4.6 mg and L. edodes 7.8 mg per 100 g dry weight of mushroom. The niacin content varies from 54.9 mg in V. volvacea and 64.9 mg in A. bisporus to 46.0–108.7 mg per 100 g dry weight in Pleurotus species. The riboflavin content was higher in A. bisporus (5.0 mg) and L.edodes (4.9 mg) than in V. volvacea (1.63‐2.98 mg). Lau et al. (1985) reported that vitamin C content in edible mushroom ranges from 7.4 mg to 9.4 mg/100 g dry weight. γ‐Ergosterol, a provitamin form of D2, is the common sterol found in edible mushrooms. V. volvacea had the highest provitamin D2 content on a dry weight basis (0.47%) followed by L. edodes (0.27%) and A. bisporus (0.23%) while Tremella fuciformis had the least (0.01%). The mature stage of V. volvacea had a higher content of provitamin D2 than the egg stage and the higher content of provitamin D2 was found in the cap rather than the stalk. V. volvacea contains 0.2% provitamin D2, 0.07% provitamin D4 and 0.07% γ‐ergosterol followed by Agaricus spp., which contains 0.23% of provitamin D1.
Carbohydrate
Carbohydrate constitutes the major component of mushroom dry matter, usually about 50–60%. This group comprises various compounds like monosaccharides, their derivatives, and oligosaccharides. Mannitol and α‐trehalose are the main representatives of the polyols and oligosaccharides which constitute the prevailing component of mushroom dry matter. Crisan and Sands (2003) reported that pentoses, methyl pentoses, and hexoses as well as disaccharides, amino acids, sugars, alcohols, and sugar acids are the constituents of mushroom. Mushroom contains glycogen and chitin as the major polysaccharides which are usually present in animals and not as starch and cellulose as in plants. Chitin is a water‐soluble structural N‐containing polysaccharide present in 80–90% of dry matter of mushroom cell walls. Chitin content increases with the maturation of several cultivated species and decreases during cooking. Lectin‐carbohydrate has been shown to have antitumor and immunomodulatory activities (Wang et al. 2000). The lectin isolated from Agaricus campestris has proved to be more effective in hemagglutination (Cheung 2010). Numerous mushroom polysaccharides are considered as a potential source of prebiotics and β‐glucans and are used as probiotics (Aida et al. 2009).
β‐Glucans
Mesomo et al. (2010) produced β‐glucans from the aqueous extraction of A. blazie. They analyzed their role in the shelf‐life of cheese bread made from A. blazie and found that β‐glucans were an excellent source of nutrients and had no side effects on the cheese bread formulation. The product was stored for 30 days without any major changes in taste, texture or appearance. Lemos ( 2009 ) developed and characterized a product similar to burger from A. brasiliensis mushroom with 12% of mushroom. The product was rich in protein (20.31%), carbohydrates (27.84%), dietary fiber (24.47%), ash (6.12%), and lipid (1.60%) when compared with commercial products like ground beef and vegetable burger and had high consumer acceptance. Bassan et al. (2011) developed a gluten‐free sponge‐like functional food from A. brasiliensis mushroom which was proved to have a high level of nutritional value.
Medicinal Properties
Mushrooms are not only sources of nutrients but also have been reported as therapeutic foods, useful in preventing diseases (Silva et al. 2010). They have been proved to enhance the immune system and natural defenses against several diseases. Mushrooms will also help patients receiving radiotherapy and chemotherapy by increasing the number of leukocytes in the blood and enhancing immune function, increasing and improving appetite, reducing pain, stopping hair loss, with potential antioxidant, antidiabetic, hypoglycemic, antitumor, immunomodulator, antimicrobial, antiviral, antiallergic, anti‐inflammatory, hepatoprotective, anticancer and genoprotective properties, and general health‐improving effect (Figure 4.1) (Lindequist et al. 2005).
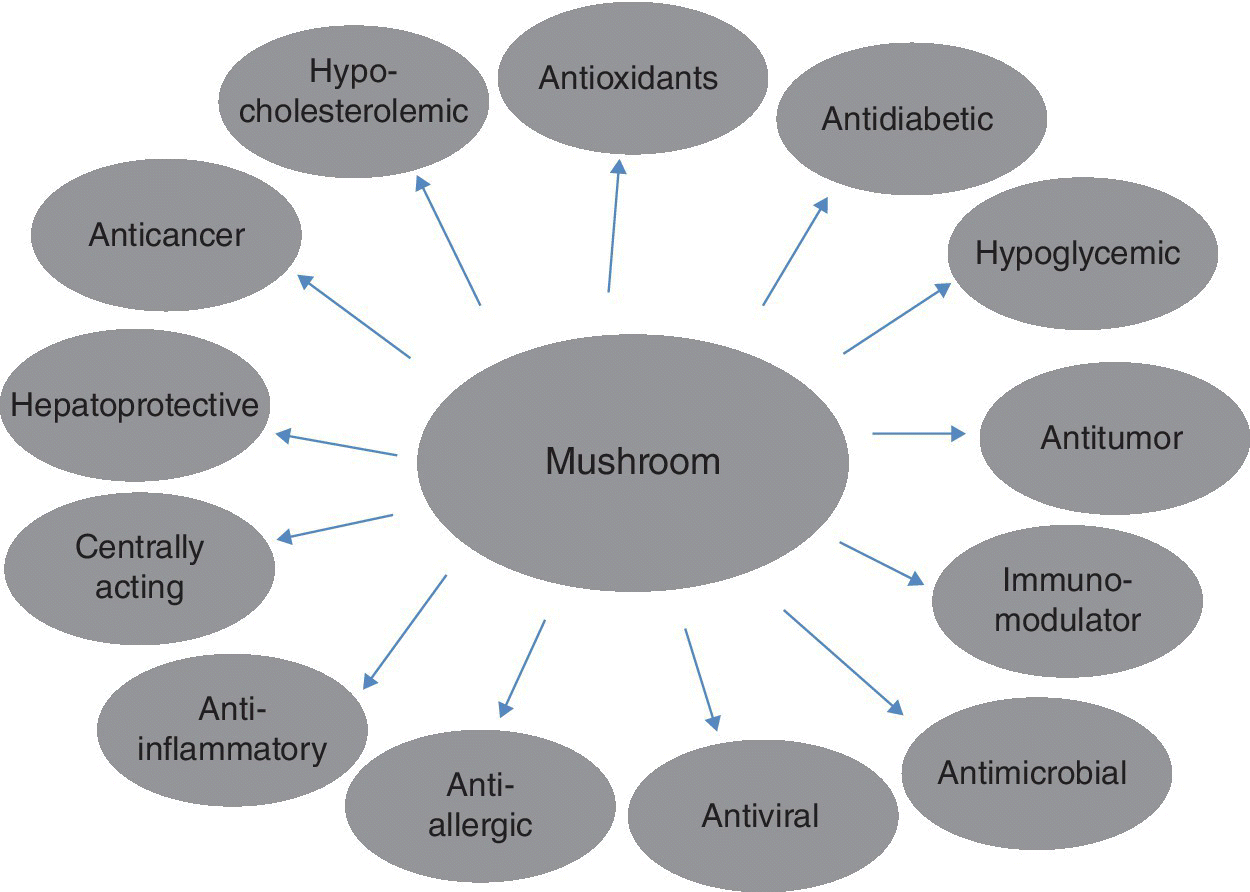
Figure 4.1 Pharmacological properties of edible mushroom.
Source: Adapted from Lindequist et al. (2005).
Research into medicinal, pharmaceutical, and functional foods during the last few decades suggests that mushrooms have much to offer for human healthcare. In treatments where chemical medicines might have side effects and might not provide a complete remedy, use of mushrooms and their products may augment success. Mushrooms have either good nutritional value or medicinal value. Some of them possess both nutritional and medicinal properties and provide health benefits beyond basic nutrition and are therefore considered as functional foods (Rathee et al. 2012).
A database of scientific evidence exists about the specific health effects of mushrooms and their bioactive molecules.
Antitumor Effects
Polysaccharides are the best known and most potent mushroom‐derived substances with antitumor and immunomodulating properties (Liu 2002 ; Mizuno 1995). Antitumor polysaccharides from mushroom have been extensively studied in recent years. These polysaccharides vary in chemical composition, structure, and antitumor activity. Many mushroom polysaccharides are present mainly as glucans with different types of glycosidic linkages. Polysaccharides such as (1‐3), (1‐6)‐β‐glucans, and (1‐3)‐α‐glucans are helpful in antitumor action. However, antitumor polysaccharide may have different chemical structures, such as hetero‐β‐glycan, heteroglycan, β‐glucan‐protein, and heteroglycan‐protein complexes including glycoproteins and triterpenoids. Glucans with higher molecular weight are more effective than glucans of lower molecular weight (Wasser 2002). Mushrooms such as L. edodes, Grifola frondosa, Agaricus blazei, and Pleurotus spp., taken as part of the diet, are likely to provide some protection against some diseases, particularly tumors. Polysaccharides from mushrooms do not attack cancer cells directly, but produce antitumor effects by activating T‐cells through a thymus‐dependent immune mechanism (Konno et al. 2002).
Several researchers have focused their studies on L. edodes with the objective of identification and purification of the responsible ingredients against tumor effects. Chihara et al. (1969) reported that water‐soluble polysaccharide isolated from L. edodes inhibited the growth of mouse sarcoma 180 in albino mice and lead to complete regression at the dose of 1–10 mg/kg body weight. Fujimoto et al. (1991) isolated and characterized antitumor polysaccharide KS‐2 composed of xylose‐containing polysaccharide and protein from L. edodes, which completely suppressed the growth of Ehrlich ascites carcinoma in mice treated with 1–5 mg/kg body weight. Similar results were also obtained by Sugiyama et al. (1995), who identified anticarcinogenic actions of a water‐soluble compound from L. edodes which suppressed the growth of Ehrlich ascites carcinoma in mice.
Fiber is considered to be an important ingredient in a balanced and healthy diet. According to Kalac (2009), soluble protein of 4–9% and insoluble fiber of 22–30% are present in mushroom. These fibers play an important role for diabetic patients by reducing their daily insulin requirements and stabilizing their blood glucose profile. Further research on mushroom fiber is required to confirm its health benefits. Various biological activities of mushrooms reported by several researchers are shown in Table 4.6.
Table 4.6 Biological activity of mushrooms and active constituents.
| Mushroom | Compounds | Uses | References |
| Auricularia auricula‐judae (Bull.) J.S. | Dietary fiber | May be used as antioxidant | Tsuchida et al. 1999b |
| Agaricus blazei | Riboglucan, glucomannan | Cancer, infections, inflammation, allergy, asthama, and diabetes | Cho et al. 1999 |
| Agaricus bisporus (J.E. Lange) Imbach | Fibers, lectins | Hypocholesterolemic and hypoglycemic | Tsai et al. 2007 |
| Agaricus campestris L. | Lectins | Hypoglycemic, ulcers | Ahmad et al. 1984 |
| Agrocybe aegerita | Glucan | Antioxidant | Zhang et al. 2002 |
| Agrocybe cylindracea (DC) Maire | Agrocybin | Anti‐inflammatory, antioxidant | Elsayed et al. 2014 |
| Amanita muscaria | Glucan | Antitumor and immunostimulator | Kiho et al. 1992 |
| Auricularia fuscosuccinea (brown) | Glucan | Antiaging, antioxidant, antitumor and anticoagulant | Lo et al. 2012 |
| Auricularia fuscosuccinea (white) | Glucan | Antioxidant | Lin, et al. 2013 |
| Auricularia mesenterica | Glucan | Antimutagenic, antioxidant | Yoon et al. 2003 |
| Auricularia polytricha | Polysaccharides | Antinociceptive, antioxidant | Mau et al. 2001b |
| Boletus edulis Bull. | Polysaccharides | Antitumor | Mau et al. 2001b |
| Calocybe gambosa | Polysaccharides | Antimutagenic, anti‐inflammatory, antioxidant | Alves et al. 2013 |
| Calvatia gigantea (Batsch ex Pers.) Lloyd | Polysaccharides | Antioxidant | Badshah et al. 2012 |
| Cantharellus cibaricus | Phenols | Antioxidant, antimicrobial | Ramesh and Pattar 2010 |
| Clitocybe maxima | Phenols, polysaccharides | Antihyperglycemic and antioxidant | Liu et al. 2012 |
| Collybia maculata (Alb. &Schwein.) P. Kumm | Purine derivatives | Antiviral and antioxidant | Leonhardt et al. 1987 |
| Coprinus comatus | Polysaccharides, tocopherols | Antioxidant | Han et al. 2006 |
| Coriolus versicolor | Polysaccharide | Anticancer | Patel and Goyal 2012 |
| Craterellus cornucopioides | Glucan | Antioxidant | Barros et al. 2008 |
| Dictyophora indusiata | Fucamannogalacton | Antitumor, antioxidant | Ramesh and Pattar 2010 |
| Flammulina velutipes (Curtis) Singer | Fibers, polysaccharides | Antioxidant, hypocholesterolemic, antiallergic | Fukushima et al. 2000 |
| Ganoderma lucidum (Curtis) P. Karst | Glucans, triterpenes polysaccharide | Hypoglycemic antioxidant and antitumor Antiviral (HIV‐1) Antiallergic Anti‐inflammatory Antihepatotoxic |
Hikino et al. 1985 Lin et al. 1995 |
| Ganoderma pfeifferi Bres. | Polysaccharide, sesquiterpenoid hydroquinones | Antimicrobial, antiviral | Mothana et al. 2000 |
| Ganoderma tsugae | Arabinoglucan | Antitumor and anti‐immunomodulatory | Zhang et al. 2007 |
| Grifola frondosa (Dicks.) Gray | Ergosterol (1) | Antioxidant, hypotensive, hypoglycemic, immunotherapy, anti‐inflammatory | Lee et al. 2003 |
| Hericium erinaceus (Bull.) Pers. | Phenol‐analogous compounds | Antioxidant, ameliorative effect in Alzheimer’s dementia | Kim et al. 2011 |
| Hypsizygus marmoreus | Polysaccharide | Antioxidant and antiallergic | Kim et al. 2014 |
| Inonotus hispidus (Bull.) P. Karst. | Phenolic compounds | Antiallergic and antiviral | Ali et al. 1996 |
| Inonotus obliquus Linn. | Polysaccharides | Used as a folk medicine for cancer and stomach diseases, anticancer | Chen et al. 2010 |
| Laricifomes officinalis (Vill.) Kotl. & Pouzar | Phenols, triterpenoids, coumarins, antithrombin | Hypoglycemic, antioxidant, antimicrobial, and antitumor | Molitoris 1994 |
| Lentinula edodes (Berk.) Pegler | Lentinan, oxalic acid | Antioxidant Hypocholesterolemic Immunotherapy Antimicrobial and antiprotozoal |
Wang et al. 1996 Lee et al. 2007 Cheung 2009 |
| Macrolepiota procera | Phenols | Antioxidant | Fernandes et al. 2013 |
| Morchella esculenta | Tocopherol | Antioxidant | Kim et al. 2011 |
| Phellinus linteus | Proteoglycan | Anti‐inflammatory | Song et al. 2003 |
| Pholiota nameko (T. Ito) S. Ito & S. Imai | Polysaccharide | Antiallergic | Ji et al. 2012 |
| Pleurotus citrinopileatus | Polysaccharide | Antitumor | Zhang et al. 1994 |
| Pleurotus cystidiosus | Tocopherol, arabinogalacton | Antioxidant, antitumor and immunostimulating polysaccharide | Menikpurage et al. 2009 |
| Pleurotus eryngii (DC) Quel. | Glucans | Antiallergic, antiproliferative, antiangiogenic | Sano et al. 2002 Ngai and Ng 2006 |
| Pleurotus ferulae | Polysaccharide | Antihyperlipidemic | Alam et al. 2011 |
| Pleurotus ostreatus (Jacq. ex Fr.) P. Kumm. | Phenolic compound, tocopherol | Antioxidant and hypocholesterolemic | Wang et al. 1996 |
| Polyporus confluens | Glucan | Hypocholesterolemic, antitumor, and immunostimulatory | Sugiyama et al. 1992 |
| Schizophyllum commune Fries | Schizophyllan | Immunotherapy | Hazama et al. 1995 |
| Trametes versicolor (L. Fr.) Quel. | Coriolan, a β‐glucan‐protein complex | Immunotherapy and hypoglycemic | Hazama et al. 1995 |
| Termitomyces albuminosus | Termitomycesphins G and H | Antioxidant and neuritogenetic | Qu et al. 2012 |
| Tricholoma giganteum | Polysaccharide | Antioxidant, antitumor, and antihypertensive | Mizuno et al. 1995 |
| Volvariella volvacea (Bulliard ex. Fries) Singer | β‐glucosidase | Antioxidant, antitumo,r and anti‐inflammatory | Cai et al. 1998 |
Silva et al. (2011) developed a natural potential antioxidant ingredient to preserve soybean oil. They studied the antioxidant activity of extracts of mushroom Agaricus blazei and proved that mushroom extract is effective in preserving soybean oil. Mushrooms possess many antioxidant properties. Kasuga et al. (1993) studied the antioxidant activity of crude ethanol extract of 150 Japanese mushrooms using peroxide value in the methyl linoleate system and found that the peroxide value of Suillus genus mushrooms was 80% lower than the control. They also studied antioxidant activity of polar and non‐polar extracts of oogitake, kugitake, and Maitake mushrooms and found that both polar and non‐polar extracts had the highest antioxidant activity (Kasuga et al. 1993). Methanolic extracts of D. indusiata, H. erinaceus, T. giganteum, F. velutipes, L. edodes, and P. cystidiosus mushrooms showed inhibitory effect on lipid peroxidase, 1,1‐diphenyl‐2‐picrylhydrazyl (DPPH) radical scavenging, and hydroxyl scavenging and could chelate ferrous ion (Mau et al. 2002).
Cheung (2010) reported that crude methanol and water extracts of the common Chinese edible mushrooms L. edodes and V. volvacea showed highest antioxidant activity during β‐carotene bleaching, DPPH radical scavenging activity, and erythrocyte hemolysis assay. Similar antioxidant properties have also been reported for A. cylindracea and A. aegerita (Tsai et al. 2007). Inhibition of lipid peroxidation by L. edodes and V. volvacea showed antioxidant behavior by scavenging free radicals (Cheung and Cheung 2003). These studies confirmed that edible mushrooms have potential as natural antioxidants due to the ability of their phenolics to inhibit lipid oxidation.
Wang et al. (1996) successfully isolated and characterized two novel structural isomers, asiaticusin A and asiaticusin B, from the fruiting body of A. asiaticus using spectrometry, mass spectrometry (MS), and nuclear magnetic resonance. Cheung (2010) identified a polyphenolic compound, epigallocatechin 3‐gallate, through liquid chromatography mass spectrometry (LCMS) analysis from P. tuber‐regium mushroom that showed potential antioxidant activity. In addition, the gas chromatography mass spectrometry (GCMS) method revealed the presence of several phenolics, including cinnamic acid, hydroxybenzoic acid, protocatechuic acid, and caffeic acid, from A. bisporus and L. edodes (Mattila et al. 2001).
The ability of mushroom‐derived preparations (MDPs) to prevent oxidative damage to cellular DNA has been evaluated using the single‐cell gel electrophoresis assay (Wang et al. 2011). MDPs obtained from nine common mushrooms including A. bisporus and Ganoderma lucidum varied in their ability to protect against oxidative DNA (Fukushima et al. 2000). These findings indicated that some edible mushrooms consist of biologically active compounds which have possible commercial value as dietary supplements for offsetting adverse biological effects associated with coronary heart disease, cancer, and age‐related neurodegenerative diseases. They might also facilitate the development of treatments for the repair of indiscriminate cellular DNA damage that occurs during certain forms of chemotherapy and radiotherapy (Hazama et al. 1995). The edible mushroom G. frondosa contains ergosterol(1), ergosta‐4‐6‐8(14), 22‐tetraen‐3‐one and 1‐oleoyl‐2‐linoleoyl‐3‐palmitoylglycerol, which inhibit cyclo‐oxygenase I and II activity (Zhang et al. 2002).
There is no doubt that mushroom‐based products can serve as superior dietary supplements. In China several mushrooms, particularly Ganoderma, have been used as dietary supplements for the past 2000 years. Ganoderma contains several compounds including tetero‐glucans, lectins, terpenoids, steroids, nucleic acids, and immunomodulatory proteins such as Ling Zhi‐8 and it could be used as an anticancer, antitumor, antiviral, and antibacterial agent. Synergistic effects of several components in an extract are responsible for its therapeutic value and it can be tentatively concluded that mushroom products are of multifunctional value.
Conclusion
Mushrooms are defined as “a macro fungus with distinctive fruiting bodies that could be hypogeous or epigeous, large enough to be seen by naked eyes and to be picked by hands.” The Basidiomycetes and some species of Ascomycetes are categorized as mushrooms. Mushrooms constitute 22 000 known species which are widely available and about 10% of them have been explored for their nutritional value and health benefits. Only a few species of mushrooms are edible. Edible mushrooms are appreciated not only for their texture and flavor but also for their chemical and nutritional characteristics. Many Asian countries traditionally use wild edible mushrooms as nutritional foods and medicine. Mushrooms have higher protein contents and minerals and contain less fat but are rich in B vitamins, vitamin D, vitamin K, and sometimes vitamins A and C. Mushrooms are not only sources of nutrients but also have been reported as therapeutic foods, useful in preventing diseases such as hypertension, diabetes, hypercholesterolemia, and cancer. Certain mushroom species have antitumor, antiviral, antithrombotic, and immunomodulating properties. A limited number of mushroom species have the potential to lower elevated blood sugar levels. The health beneficial characteristics of mushrooms are mainly due to the presence of dietary fiber, in particular chitin and β‐glucans. Therefore, it would be useful to carry out more research on mushrooms with a view to identifying active ingredients which contribute health‐promoting functional food characteristics and also nutraceutical and disease‐preventing functions.
References
- Ahmad, N., Bansal, A.K. and Kidwai, J.R. (1984) Effect of PHA‐B fraction of Agaricus bisporus lectin on insulin release and 45Ca2C uptake by islet of Langerhans in vitro. Acta Diabetol., 21: 63–70.
- Aida, F.M., Shuhaimi, M., Yazid, M. and Maaruf, A.G. (2009) Mushroom as a potential source of prebiotics: a review. Trends Food Sci. Technol., 20(11): 567–575.
- Alam, N., Yoon, K.N. and Lee, T.S. (2011) Antihyperlipidemic activities of Pleurotus ferulae on biochemical and histological function in hypercholesterolemic rats. J. Res. Med. Sci., 16(6): 776.
- Ali, N.A., Jansen, R., Pilgrim, H., Liberra, K. and Lindequist, U. (1996) Hispolon, a yellow pigment from Inonotus hispidus. Phytochemistry, 41(3): 927–929.
- Alves, M.J., Ferreira, I.C., Froufe, H.J., Abreu, R.M.V., Martins, A. and Pintado, M. (2013) Antimicrobial activity of phenolic compounds identified in wild mushrooms, SAR analysis and docking studies. J. Appl. Microbiol., 115(2): 346–357.
- An, Y.H. (2007) Method for producing soybean paste containing pine mushroom. Google Patent US20090053363.
- Badshah, H., Qureshi, R.A., Khan, J. et al. (2012) Pharmacological screening of Morchella esculenta (L.) Pers., Calvatia gigantea (Batsch ex Pers.) Lloyd and Astraeus hygrometricus Pers., mushroom collected from South Waziristan (FATA). Chemistry. 45–65.
- Bano, Z. and Rajarathnam, S. (1988) Pleurotus mushrooms. Part II. Chemical composition, nutritional value, post‐harvest physiology, preservation, and role as human food. Crit. Rev. Food Sci. Nutr., 27: 87–158.
- Barros, L., Cruz, T., Baptista, P., Estevinho, L.M. and Ferreira, I.C. (2008) Wild and commercial mushrooms as source of nutrients and nutraceuticals. Food Chem. Toxicol., 46(8): 2742–2747.
- Bassan, J.C., Ferreira, G.A.O., Bueno, M. and Escouto, L.F.S. (2011)_ Physical characteristics and sensory type in gluten‐free cake sponge cake with mushroom Agaricus brasiliensis. Rev. Alimentus, 1(1): 273–287.
- Beluhan, S. and Ranogajec, A. (2011) Chemical composition and nonvolatile components of Croatian wild edible mushrooms. Food Chem., 124(3): 1076–1082.
- Cai, Y.J., Buswell, J.A. and Chang, S.T. (1998) β‐Glucosidase components of the cellulolytic system of the edible straw mushroom, Volvariella volvacea. Enzyme Microb. Technol., 22(2): 122–129.
- Chang, S.T. (1980) Mushroom production in Southeast Asia. Mushroom Newsl. Trop., 1(2): 18–22.
- Chang, S.T. (1999) World production of cultivated edible and medicinal mushrooms in 1997 with emphasis on Lentinus edodes (Berk.) Sing, in China. Int. J. Med. Mushrooms, 1(4): 43–48.
- Chang, S.T. and Buswell, J.A. (1996) Mushroom nutriceuticals. World J. Microb. Biot., 12: 473–476.
- Chang, S.T. and Miles, P.G. (1987) Historical record of the early cultivation of Lentinus in China. Mushroom J. Trop., 7: 47–52.
- Chang, S.T. and Miles, P.G. (1997) Mushroom biology: concise basics and current developments. World Scientific, 144.
- Chang, S.T. and Miles, P.G. (2004) Mushrooms: Cultivation, Nutritional Value, Medicinal Effect, and Environmental Impact. Boca Raton: CRC Press, pp. 27–38.
- Chang, S.T., Buswell, J.A. and Chiu, S.W. (1993) Mushroom Biology and Mushroom Products. Hong Kong: Chinese University Press.
- Chen, S.Y., Ho, K.J., Liang, C.H., Tsai, C.H., Huang, L.Y. and Mau, J.L. (2012) Preparation of culinary‐medicinal king oyster mushroom Pleurotus eryngii fermented products with high ergothioneine content and their taste quality. Int. J. Med. Mushrooms, 14: 85–93.
- Chen, Y., Gu, X., Huang, S.Q., Li, J., Wang, X. and Tang, J. (2010) Optimization of ultrasonic/microwave assisted extraction (UMAE) of polysaccharides from Inonotus obliquus and evaluation of its anti‐tumor activities. Int. J. Biol. Macromol., 46(4): 429–435.
- Cheung, L.M. and Cheung, C.K. (2003) Antioxidant activity and total phenolics of edible mushroom extracts. Food Chem., 81: 249–265.
- Cheung, P.C.K. (2009) Nutritional value and health benefits of mushrooms. In: Cheung, P.C.K. (ed.) Mushrooms as Functional Foods. Chichester: Wiley, pp. 71–109.
- Cheung, P.C.K. (2010) Mushroom and health. Nutr. Bull., 35: 292–299.
- Chihara, G., Maeda, Y., Sasaki, T. and Fukuoka, F. (1969) Inhibition of mouse sarcoma 180 by polysaccharides from Lentinus edodes (Berk.). Nature, 222: 687–688.
- Cho, S.M., Park, J.S., Kim, K.P., Cha, D.Y., Kim, H.M. and Yoo, I.D. (1999) Chemical features and purification of immunostimulating polysaccharides from the fruit bodies of Agaricus blazei. Korean J. Mycol., 27: 170–174.
- Chung, M.J., Chung, C.K., Jeong, Y. and Ham, S.S. (2010) Anticancer activity of subfractions containing pure compounds of Chaga mushroom (Inonotus obliquus) extract in human cancer cells and in Balbc/c mice bearing Sarcoma‐180 cells. Nutr. Res. Pract., 4(3): 177–182.
- Crisan, E.V. and Sands, A. (1978) Nutritional Value. New York: Academic Press, pp. 137–168.
- Dijkstra, F. and Wiken, T. (1976) Studies on mushroom flavours. 1. Organoleptic significance of constituents of the cultivated mushroom, Agaricus bisporus. Zeitschrift fur Lebensmittel‐ Untersuchung und‐Forschung, 160: 255–262.
- El‐Mekkawy, S., Meselhy, M.R., Nakamura, N., Tezuka, Y., Hattori, M. and Kakiuchi, N. (1998) Anti‐HIV‐1 and anti‐ HIV‐1‐protease substances from Ganoderma lucidum. Phytochemistry, 49: 1651– 1657.
- Elsayed, E.A., El Enshasy, H., Wadaan, M.A. and Aziz, R. (2014) Mushrooms: a potential natural source of anti‐inflammatory compounds for medical applications. Mediat. Inflamm., 3(2): 24–36.
- FAO, IFAD, WFP (2014) The State of Food Insecurity in the World. 1(1): 24–32.
- Fernandes, A., Barros, L., Barreira, J.C. et al. (2013) Effects of different processing technologies on chemical and antioxidant parameters of Macrolepiota procera wild mushroom. LWT‐Food Sci. Technol., 54(2): 493–499.
- Fortes, R.C. and Novaes, M.R.C. (2006) Effects of dietary supplementation with Agaricales mushrooms and other fungi in medicinal therapy against cancer. Rev. Bras. Cancerol., 52(4): 363–371.
- Fujimoto, S., Furue, H., Kimura, T., Kondo, T., Orita, K. and Taguchi, T. (1991) Clinical outcome of postoperative adjuvant immunochemotherapy with sizofiran for patients with resectable gastric cancer‐a randomized controlled study. Eur. J. Cancer, 27: 1114–1118.
- Fuke, S. and Shimizu, T. (1993) Sensory and preference aspects of umami. Trends Food Sci. Technol., 4(8): 246–251.
- Fuke, S. and Ueda, Y. (1996) Interactions between umami and other flavor characteristics. Trends Food Sci. Technol., 7(12): 407–411.
- Fukushima, M., Nakano, M., Morii, Y., Ohashi, T., Fujiwara, Y. and Sonoyama, K. (2000) Hepatic LDL receptor mRNA is increased by dietary mushroom (Agaricus bisporus) fiber and sugar beet fiber. J. Nutr., 130: 2151–2156.
- Han, C., Yuan, J., Wang, Y. and Li, L. (2006) Hypoglycemic activity of fermented mushroom of Coprinus comatus rich in vanadium. J. Trace Elements Med. Biol., 20(3):191–196.
- Hasler, C.M. (1998) Functional foods: their role in disease prevention and health promotion. Food Technol., 52: 63–70.
- Hazama, S., Oka, M., Yoshino, S., Iizuka, N. and Kyamamoto, W. (1995) Clinical effects and immunological analysis of intraabdominal and intrapleural injection of lentinan for malignant ascites and pleural effusion of gastric carcinoma. Cancer Chemother. Pharmacol., 22: 1595–1597.
- Hikino, H., Konno, C., Mirin, Y. and Hayashi, T. (1985) Isolation and hypoglycemic activity of ganoderans A and B, glycans of Ganoderma lucidum fruit bodies. Planta Med., 51(4): 339–340.
- Huang, B.H., Yung, K.H. and Chang, S.T. (1985) The sterol composition of Volvariella volvacea and other edible mushrooms. Mycologia, 77: 959–963.
- Huang, S.J., Tsai, S.Y., Lee, Y.L. and Mau, J.L. (2006) Nonvolatile taste components of fruit bodies and mycelia of Cordyceps militaris. LWT–Food Sci. Technol., 39(6): 577–583.
- Ji, H.F., Zhang, L.W., Zhang, H.Y., Li, G.L. and Yang, M.D. (2012) Antioxidant activities of extracts from Pholiota Nameko. Adv.Materials Res., 343: 457–462.
- Jones, P.J. (2002) Clinical nutrition: 7. Functional foods – more than just nutrition. Can. Med. Assoc. J., 166(12): 1555–1563.
- Kalac, P. (2009) Chemical composition and nutritional values of European species of wild growing mushrooms: a review. Food Chem., 113: 9–16.
- Kasuga, A., Aoyagi, Y. and Sugahara, T. (1993) Antioxidative activities of several mushroom extracts. J. Japan. Soc. Food Sci. Technol., 40: 56–63.
- Kiho, T., Nagai, K., Ukai, S. and Haga, M. (1992) Structure and antitumor activity of a branched (1 → 3)‐β‐d‐glucan from the alkaline extract of Amanita muscaria. Carbohydrate Res., 224: 237–243.
- Kim, J.A., Lau, E., Tay, D. and de Blanco, E.J.C. (2011) Antioxidant and NF‐κB inhibitory constituents isolated from Morchella esculenta. Nat. Prod. Res., 25(15): 1412–1417.
- Kim, S.P., Kang, M.Y., Kim, J.H., Nam, S.H. and Friedman, M. (2011) Composition and mechanism of antitumor effects of Hericium erinaceus mushroom extracts in tumor‐bearing mice. J. Agric. Food Chem., 59(18): 9861–9869.
- Kim, T., Park, K., Jung, H. S., Kong, W.S., Jeon, D. and Lee, S.H. (2014) Evaluation of anti‐atopic dermatitis activity of Hypsizigus marmoreus extract. Phytother. Res., 28(10): 1539–1546.
- Konno, S., Aynehchi, S., Dolin, D.J., Schwartz, A.M., Choudhury, M.S. and Tazakin, H.N. (2002) Anticancer and hypoglycaemic effects of polysaccharides in edible and medicinal Maitake mushroom [Grifola frondosa (Dicks.:Fr.) S.F.Gray]. Int. J. Med. Mushrooms, 4: 185–195.
- Lau, O.W., Shiu, K.K. and Chang, S.T. (1985) Determination of ascorbic acid in vegetables and fruits by differential pulse polarography. J. Sci. Food Agric., 36: 733–739.
- Lee, B.C., Bae, J.T., Pyo, H.B. et al. (2003) Biological activities of the polysaccharides produced from submerged culture of the edible Basidiomycete Grifola frondosa. Enzyme Microb. Technol., 32(5): 574–581.
- Lemos, F.M.R. (2009) Preparation and characterization of the product similar to burgers mushroom Agaricus brasiliensis. Thesis, Federal University of Parana.
- Leon‐Guzman, M.F., Silva, I. and Lopez, M.G. (1997) Proximate chemical composition, free amino acid contents, and free fatty acid contents of some wild edible mushrooms from Queretaro, Mexico. J. Agric. Food Chem., 45(11): 4329–4332.
- Leonhardt, K., Anke, T. and Hillen‐Maske, E. (1987) 6‐Methylpurine, 6‐methyl‐9‐β‐D‐ribofuranosylpurine, and 6‐hydroxymethyl‐9‐β‐D‐ribofuranosylpurine as antiviral metabolites of Collybia maculata (Basidiomycetes). Zeitschrift für Naturforschung C, 42(4): 420–424.
- Lin, J.M., Lin, C.C., Chen, M.F., Ujiie, T. and Takada, A. (1995) Radical scavenger and antihepatotoxic activity of Ganoderma formosanum, Ganoderma lucidum and Ganoderma neo‐japonicum. J. Ethnopharmacol., 47(1): 33–41.
- Lin, W.Y., Yang, M.J., Hung, L.T. and Lin, L.C. (2013) Antioxidant properties of methanol extract of a new commercial gelatinous mushrooms (white variety of Auricularia fuscosuccinea) of Taiwan. Afr. J. Biotechnol., 12: 6210–6221.
- Lindequist, U., Niedermeyer, T.H.J. and Julich, W.D. (2005) The pharmacological potential of mushrooms. Evidence‐Based Comp. Altern. Med., 2: 285–299.
- Liu, J. (2002) Biologically active substances from mushrooms in Yunnan, China. Heterocycles, 57: 157–167.
- Liu, Y.T., Sun, J., Luo, Z.Y. et al. (2012) Chemical composition of five wild edible mushrooms collected from Southwest China and their antihyperglycemic and antioxidant activity. Food Chem. Toxicol., 50(5): 1238–1244.
- Lo, Y.C., Lin, S.Y., Ulziijargal, E. et al. (2012) Comparative study of contents of several bioactive components in fruiting bodies and mycelia of culinary‐medicinal mushrooms. Int. J. Med. Mushrooms, 14(4): 85–97.
- Manzi, P., Aguzzi, A. and Pizzoferrato, L. (2001) Nutritional value of mushrooms widely consumed in Italy. Food Chem., 73: 321–325.
- Mattila, P., Konko, K., Euvola, M., Pihlava, J., Astola, J. and Vahteristo, L. (2001) Contents of vitamins, mineral elements and some phenolic compound in cultivated mushrooms. J. Agric. Food Chem., 42: 2449–2453.
- Mau, J.L., Wu, K.T., Wu, Y.H. and Lin, Y.P. (1998a) Nonvolatile taste components of ear mushrooms. J. Agric. Food Chem., 46(11): 4583–4586.
- Mau, J.L., Lin, Y.P., Chen, P.T., Wu, Y.H. and Peng, J.T. (1998b) Flavor compounds in king oyster mushrooms Pleurotus eryngii. J. Agric. Food Chem., 46: 4587–4591.
- Mau, J.L., Lin, H.C. and Chen, C.C. (2001a) Non‐volatile components of several medicinal mushrooms. Food Res. Int., 34: 521–526.
- Mau, J.L., Lin, H.C. and Chen, C.C. (2001b) Non‐volatile taste components of several medicinal mushrooms. Food Res. Int., 34: 521–526.
- Mau, J.L., Lin, H.C. and Chen, C.C. (2002) Antioxidant properties of several medicinal mushrooms. J. Agric. Food Chem., 50(21): 6072–6077.
- Menikpurage, I.P., Abeytunga, D.T.U., Jacobsen, N.E. and Wijesundara, R.L.C. (2009) An oxidized ergosterol from Pleurotus cystidiosus active against anthracnose causing colletotrichumgloeosporioides. Mycopathologia, 167(3): 155.
- Menikpurage, I.P., Soysa, S.S. and Abeytunga, D.T.U. (2012) Antioxidant activity and cytotoxicity of the edible mushroom, Pleurotus cystidiosus against Hep‐2 carcinoma cells. J. Natl Sci Found Sri Lanka, 40: 2–18.
- Mesomo, M.C., Helms, K.M., Zuim, D.R., Visentainer, J.V., Costa, S.M.G. and Pintro, P.T.M. (2010) Evaluation of shelf life of cheese added to the residue of the extract Agaricus blazei Murrill. Amb. Rev. Sector Agric.Environ. Sci., 6(3).
- Miles, P.G. and Chang, S.T. (1997) Mushroom Biology: Concise Basics and current Developments. Singapore: World Scientific.
- Miles, P. G. and Chang, S.T. (2004) Mushrooms: Cultivation, Nutritional Value, Medicinal Effect, and Environmental Impact. Boca Raton: CRC Press.
- Mizuno, T. (1995) Bioactive biomolecules of mushrooms: food function and medicinal effect of mushroom fungi. Food Rev. Int., 11: 7–12.
- Mizuno, T. (1999) The extraction and development of antitumoractive polysaccharides from medicinal mushrooms in Japan (review). Int. J.Med. Mushrooms, 1: 9–30.
- Mizuno, T., Kinoshita, T., Zhuang, C., Ito, H. and Mayuzumi, Y. (1995) Antitumor‐active heteroglycans from Niohshimeji mushroom, Tricholoma giganteum. Biosci. Biotechnol. Biochem., 59(4): 568–571.
- Molitoris, H.P. (1994) Mushrooms in medicine. Folia Microbiol., 39(2): 91–98.
- Mothana, R.A., Jansen, R., Jülich, W.D. and Lindequist, U. (2000) Ganomycins A and B, new antimicrobial farnesyl hydroquinones from the basidiomycete Ganoderma pfeifferi. J. Nat. Prod., 63(3): 416–418.
- Nakamura, E., Torii, K. and Uneyama, H. (2008) Physiological roles of dietary free glutamate in gastrointestinal functions. Biol. Pharmaceut. Bull., 31(10): 1841–1843.
- Ngai, P.H. and Ng, T.B. (2006) A hemolysin from the mushroom Pleurotus eryngii. Appl. Microbiol. Biotechnol., 72(6): 1185–1191.
- Ohyama, S., Ishibashi, N., Tamura, M., Nishizaki, H. and Okai, H. (1988) Synthesis of bitter peptides composed of aspartic acid and glutamic acid. Agric. Biol. Chem., 52(3): 871–872.
- Ouwehand, A.C., Salminen, S. and Isolauri, E. (2002) Probiotics: an overview of beneficial effects. Antonie Van Leeuwenhoek, 82(1‐4): 279–289.
- Patel, S. and Goyal, A. (2012) Recent developments in mushrooms as anti‐cancer therapeutics: a review. 3 Biotech, 2(1): 1–15.
- Qu, Y., Sun, K., Gao, L. et al. (2012) Termitomycesphins G and H, additional cerebrosides from the edible Chinese mushroom Termitomyces albuminosus. Biosci. Biotechnol. Biochem., 76(4): 791–793.
- Ramesh, C. and Pattar, M.G. (2010) Antimicrobial properties, antioxidant activity and bioactive compounds from six wild edible mushrooms of western ghats of Karnataka, India. Pharmacog. Res., 2(2): 107.
- Rathee, S., Rathee, D., Rathee D., Kumar, V. and Rathee, P. (2012) Mushroom as therapeutic agents. Brazil. J. Pharmacog., 22(2): 459–474.
- Roberfroid, M.B. (2000) Concepts and strategy of functional food science: the European perspective. Am. J. Clin. Nutr., 71: 1660–1664.
- Sano, M., Yoshino, K., Matsuzawa, T. and Ikekawa, T. (2002). Inhibitory effects of edible higher basidiomycetes mushroom extracts on mouse type IV allergy. Int. J. Med. Mushrooms, 4(1).
- Shao,W. and Huang, Y. (2009) A composite mushroom flavor seasoning and its preparation method. Chinese Patent CN200910063604.X.
- Silva, M.C.S., Nozuka, J., Oliveira, P.V. et al. (2010) In vivo bioavailablity of selenium in enriched Pleurotus ostreatus mushrooms. Metallomic, 2: 162–166.
- Siro, I., Kapolna, E., Kapolna, B. and Lugasi, A. (2008) Functional food. Product development, marketing and consumer acceptance – a review. Appetite, 51: 456–467.
- Sommer, I. (2008) Effect of gamma irradiation on selected compounds of fresh mushrooms. MSc thesis, Wien: Uniwien, 0105517.
- Song, Y.S., Kim, S.H., Sa, J.H., Jin, C., Lim, C.J. and Park, E.H. (2003) Anti‐angiogenic, antioxidant and xanthine oxidase inhibition activities of the mushroom Phellinus linteus. J. Ethnopharmacol., 88(1): 113–116.
- Stojkovic, D., Reis, F.S., Barros, L. et al. (2013) Nutrients and non‐nutrients composition and bioactivity of wild and cultivated Coprinus comatus (OF Müll.) Pers. Food Chem. Toxicol., 59: 289–296.
- Sugiyama, K., Saeki, S., Tanaka, A., Yoshida, S., Sakamoto, H. and Ishiguro, Y. (1992) Hypocholesterolemic activity of Ningyotake (Polyporus confluens) mushroom in rats. J. Jpn Soc. Nutri. Food Sci., 45(3): 265–270.
- Sugiyama, K., Akachi, T. and Yamakawa, A. (1995) Hypocholesterolemic action of eritadenine is mediated by a modification of hepatic phospholipid metabolism in rats. J. Nutr., 125: 2134–2144.
- Sun, J.Y., Sun, M.Q., He, C.S, and Zhao, J. (2007) A mushroom refined condiments and its manufacturing method. Chinese Patent CN101011135.
- Tsai, S., Tsai, H. and Mau, J. (2007) Nonvolatile taste components of fruit bodies and mycelia of shaggy ink cap Mushroom Coprinus comatus (OF Mull.: Fr.) Pers. (Agaricomycetideae). Int. J. Med. Mushrooms, 9: 47–55.
- Tsai, S.Y., Tsai, H.L. and Mau, J.L. (2008) Non‐volatile taste components of Agaricus blazei, Agrocybe cylindracea and Boletus edulis. Food Chem., 107: 977–983.
- Tsai, S.Y., Huang, S.J., Lo, S.H., Wu, T.P., Lian, P.Y. and Mau, J.L. (2009) Flavour components and antioxidant properties of several cultivated mushrooms. Food Chem., 113(2): 578–584.
- Tsuchida, H., Mizuno, M., Taniguchi, Y., Ito, H., Kawade, M. and Akasaka, K. (1999) Glucomannan separated from Agaricus blazei mushroom culture and antitumor agent containing as active ingredient. Japanese Patent 11‐080206.
- Tu, H.M. (2012) A method for preparing mushrooms essence and its application in seasoning production. Chinese Patent CN201210116730.9.
- Wang, H., Gao, J. and Ng, T.B. (2000) A new lectin with highly potent antiheptoma and antisarcoma activities from the oyster mushroom Pleurotus ostreatus. Biochem. Biophys. Res. Commun., 275: 810–816.
- Wang, H.X., Liu, W.K., Ng. T.B. and Chang S.T. (1996) The immunomodulatory and antitumor activities of lectins from the mushroom. Immunopharmacognosy, 31: 205–211.
- Wang, Z.M., Peng, X., Lee, K.L.D., Tang, J.C.O., Cheung, P.C.K. and Wu, J.Y. (2011) Structural characterisation and immunomodulatory property of an acidic polysaccharide from mycelial culture of Cordyceps sinensis fungus Cs‐HK1. Food Chem., 125(2): 637–643.
- Wasser, S.P. (2002) Medicinal mushrooms as a source of antitumor and immunomodulating polysaccharides. Appl. Microbiol. Biot., 60: 258–274.
- Yang, J.H., Lin, H.C. and Mau, J.L. (2001) Non‐volatile taste components of several commercial mushrooms. Food Chem., 72: 465–471.
- Yang, J.H., Lin, H.C. and Mau, J.L. (2002) Antioxidant properties of several commercial mushrooms. Food Chem., 77: 229–237.
- Yoon, S.J., Yu, M.A., Pyun, Y.R. et al. (2003) The nontoxic mushroom Auricularia auricula contains a polysaccharide with anticoagulant activity mediated by antithrombin. Thromb. Res., 112(3): 151–158.
- Zhang, J., Wang, G., Li, H. et al. (1994) Antitumor polysaccharides from a Chinese mushroom,“yuhuangmo” the fruiting body of Pleurotus citrinopileatus. Biosci Biotechnol. Biochem., 58(7): 1195–1201.
- Zhang, M., Cui, S.W., Cheung, P.C.K. and Wang, Q. (2007) Antitumor polysaccharides from mushrooms: a review on their isolation process, structural characteristics and antitumor activity. Trends Food Sci. Technol., 18(1): 4–19.
- Zhang, Y., Mills, G. and Nair, M.G. (2002) Cyclooxygenase inhibitory and antioxidant compounds from the mycelia of the edible mushroom Grifola frondosa. J. Agric. Food Chem., 50: 7581–7585.
- Zhang, Y., Venkitasamy, C., Pan, Z. and Wang, W. (2013) Recent developments on umami ingredients of edible mushrooms–a review. Trends Food Sci. Technol., 33(2): 78–92.