5
Microbial Production of Organic Acids
Ram Naraian* and Simpal Kumari
Department of Biotechnology, Mushroom Training and Research Centre, Faculty of Science, Veer Bahadur Singh Purvanchal University, Jaunpur (UP), India
*Corresponding author e‐mail: ramnarain_itrc@rediffmail.com
Introduction
Organic acids are chemical compounds widely distributed in nature as normal constituents of plants or animal tissues. Microbial production of organic acids is a promising approach (Sauer et al. 2008). It is well established that almost all organisms produce organic acids of low molecular weight.
Organic acids represent a rising chemical segment in which several bio‐based compounds such as fumaric, propionic, and itaconic acids are synthesized (Jang et al. 2012). These compounds are formed through the microbial fermentation of carbohydrates and related substrates. Organic acids constitute a key group among the building‐block chemicals that can be produced by microbial processes (Sauer et al. 2008). Several organic acids are produced by natural or genetically engineered micro‐organisms such as bacteria, fungi, and yeast. During fermentation, many anaerobic and facultatively anaerobic micro‐organisms produce organic acids including lactic, succinic, acetic, citric, butyric, and propionic acids (Gottschalk 1985). The gram‐positive facultative anaerobes grow on sugars and produce organic acids (Nishimura et al. 2007 ; Schaffer and Burkovski 2005 ; Takeno et al. 2007). It seems surprising that so many fungi have a high efficiency of organic acid production at high concentrations. Filamentous fungi are commercially used for production of different organic acids. Yeasts are also used for the production of most acids.
Organic acids have been used for many years in the food, chemical, agriculture, and pharmaceutical industries. Production of many organic acids via economical processes will create new markets by providing new opportunities for the chemical industry (Sauer et al. 2008). The chemical industries use organic acids as basic compounds for a wide variety of polymer and solvent production processes. In addition to food safety, supplementation of foods with organic acids imparts flavor and antioxidant activity and maintains organoleptic properties (Gurtler and Mai 2014).
Types of Organic Acid
Organic acids differ on the basis of the involvement of carbon, hydrogen, and oxygen elements. Major types of organic acid produced by microbial activity and discussed below are citric acid, succinic acid, lactic acid, itaconic acid, lactobionic acid, gluconic acid, fumaric acid, propionic acid, and acetic acid (Figure 5.1).
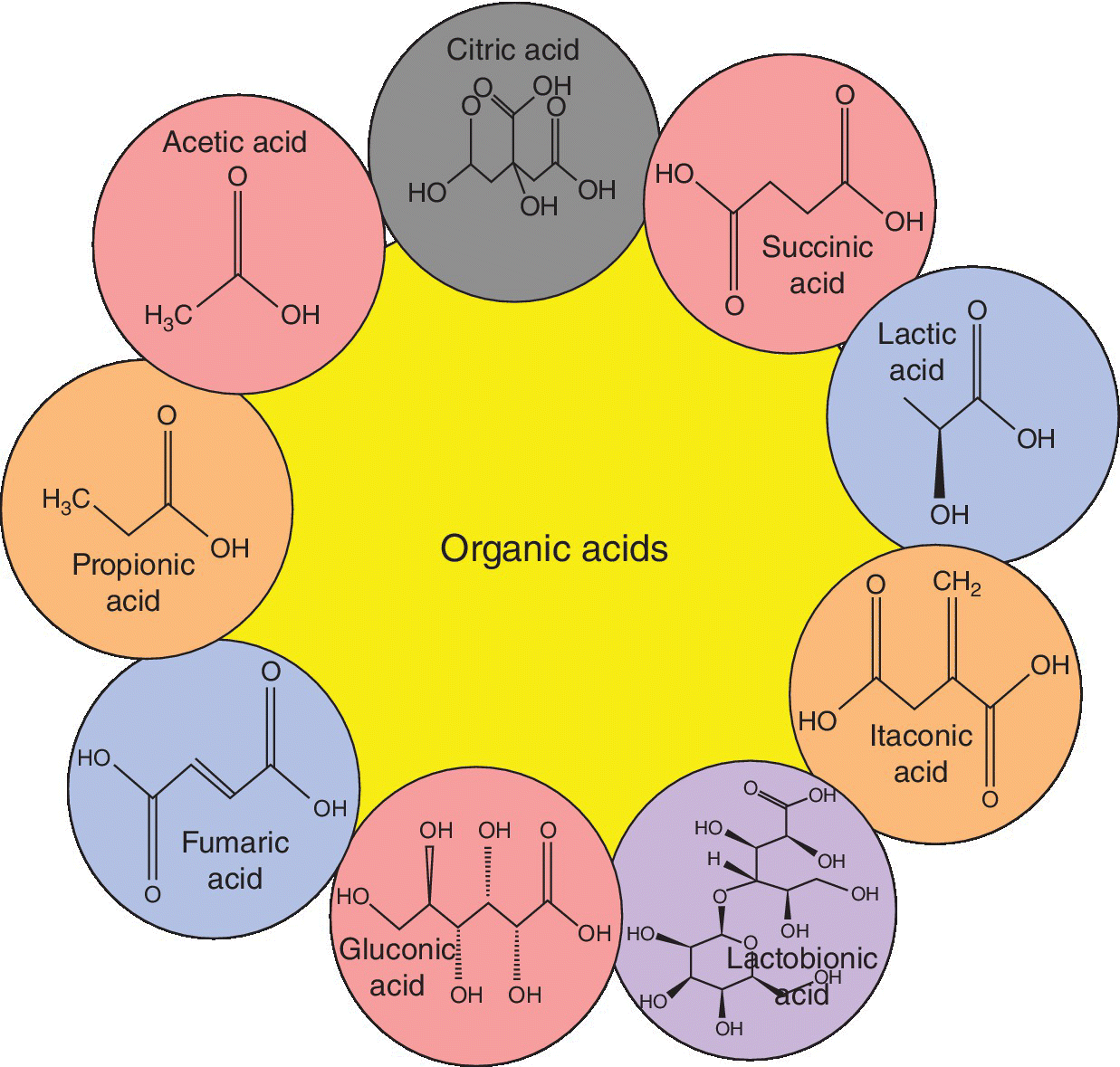
Figure 5.1 Different types of organic acids and summary of their molecular structures.
Citric Acid
Citric acid (C6H8O7, 2‐hydroxy‐1, 2, 3‐propane tricarboxylic acid) (CA) is one of the most versatile and widely used organic acids (Figure 5.2). This is as colorless translucent crystals, odorless, with a strongly acid taste (Franz 1991).
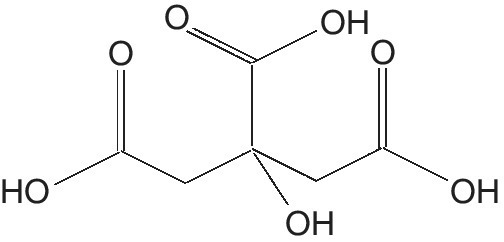
Figure 5.2 Citric acid.
Citric acid was first isolated in 1874, from lemon juice. However, the presence of citric acid was initially reported by Wehmer in 1923 as a byproduct of calcium oxalate produced by Penicillium glaucum (Vandenberghe et al. 1999). Furthermore, Currie (1917) mentioned the process of industrial production using Aspergillus niger in a sugar‐based medium. This technique finally became the method of choice for commercial production, mainly due to its economic advantage and biological production over chemical synthesis. The production of CA by bacteria, yeast, and filamentous fungi is well studied.
Several species of bacteria such as Arthrobacter paraffinens (Kroya Fermentation Industry 1970), Bacillus licheniformis (Sardinas, 1972), and Corynebacterium sp. (Fukuda et al. 1970) have been studied for citric acid production. Kapoor et al. (1983) reported that these bacteria were efficiently grown in medium containing glucose, urea, calcium, and ammonium sulfate. Later, Aerobacter, Pseudomonus, Micrococcus, Bacillus, Brevibacterium, Corynebacterium, and Arthrobacter were evaluated (Kapoor et al. 1983) in media containing isocitric acid which was later converted into citric acid (Table 5.1).
Table 5.1 Summary of studies on citric acid production using three major classes of micro‐organisms (bacteria, yeast, and filamentous fungi).
| Class of micro‐organism | Strains/isolates | Reference |
| Bacteria |
Arthrobacter paraffinens
Bacillus licheniformis Corynebacterium sp. Aerobacter Pseudomonas Micrococcus Bacillus Brevibacterium Arthrobacter |
Kroya Fermentation Industry 1970 Sardinas 1972 Fukuda et al. 1970 Kapoor et al. 1983 |
| Yeast |
Saccharomycopsis lipolytica
Yarrowia lipolytica Candida tropicalis C. oleophila C. guilliermondii C. citroformans C. parapsilosis C. lipolytica Hansenula anomola Brettanomyces Debaromyces Torulopsis Kloeckers Pichia |
Ikeno et al. 1975 ; Wojtatowicz et al. 1993 Ikeno et al. 1975 ; Kubicek et al. 1986 Kapelli et al. 1978 Ishi et al. 1972 Gutierrez et al. 1993 Uchio et al. 1975 Omar and Rehm 1980 Miall and Parker 1975 ; Rane and Sims 1993 Oh et al. 1973 Suzuki et al. 1974 |
| Filamentous fungi |
Aspergillus niger
Aspergillus aculeatus A. awamori A. carbonarius A. foetidus A. phoenicis A. wentii Penicillium janthinellum |
Hang and Woodams 1987 El Dein and Emaish 1979 Grewal and Kalra 1995 El Dein and Emaish 1979 Tran et al. 1998 Yokoya et al. 1992 Karow and Waksman 1947 Grewal and Kalra 1995 |
Barbesgaard et al. (1992) reported various yeast‐based citrate production processes. Yeasts such as Saccharomycopsis lipolytica (Ikeno et al. 1975 ; Wojtatowicz et al. 1993), Candida tropicalis (Kapelli et al. 1978), C. oleophila (Ishi et al. 1972), C. guilliermondii (Gutierrez et al. 1993), C. citroformans (Uchio et al. 1975), C. parapsilosis (Omar and Rehm 1980), C. lipolytica (Miall and Parker 1975 ; Rane and Sims 1993), Hansenula anamola (Oh et al. 1973) and Yarrowia lipolytica have been tested for citric acid production using diverse raw materials (Ikeno et al. 1975 ; Kubicek et al. 1986). Furthermore, Suzuki et al. (1974) also reported some yeasts including Brettanomyces, Debaromyces, Kloeckers, Torulopsis, and Pichia capable of citric acid production (see Table 5.1).
Citric acid production is the oldest microbial process. Several authors (Hang and Woodams 1987 ; Roukas 1991 ; Lu et al. 1997 ; Pintado et al. 1998 ; Vandenberghe et al. 1999) reported sugar‐based citric acid production by the filamentous fungus Aspergillus niger. In several studies many species of fungi were subsequently reported including Aspergillus aculeatus (El Dein and Emaish 1979), A. carbonarius (El Dein and Emaish 1979), A. awamori (Grewal and Kalra 1995), A. foetidus (Tran et al. 1998), A. wentii (Karow and Waksman 1947), A. fonsecaeus, A. phoenicis (Yokoya et al. 1992), and Penicillium janthinellum (Grewal and Kalra 1995) (see Table 5.1).
Citric acid is used in many industrial applications, in variable ways, e.g., as a flavoring agent, acid for vegetable oils and fats, as a stabilizer and preservative in the food and pharmaceutical industries. It eliminates haze due to trace metals, and prevents color and flavor deterioration. It also inhibits turbidity of wine and adjusts pH. It inverts sucrose, prevents oxidation, and produces darker color in candies, jams, jellies, and dairy products as an antioxidant and emulsifier in cheese and cream (Franz 1991). It is also used in cosmetic products as an antioxidant. It is used for the treatment of boiler water, metal plating, detergents, tanning, and textile processes.
Succinic Acid
Succinic acid (SA) is a diprotic, dicarboxylic acid with the molecular formula C₄H₆O₄ and structural formula HOOC‐(CH2)2‐COOH (Zeikus et al. 1999) (Figure 5.3). It is a white, odorless solid and well‐known intermediate metabolite of the tricarboxylic acid cycle (TCA).
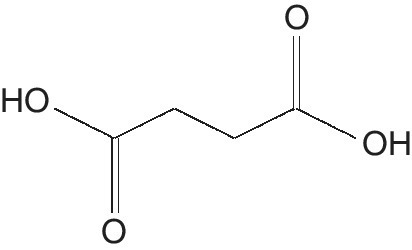
Figure 5.3 Succinic acid.
Georgius Agricola (1546) reported the first purification of succinic acid from amber so it is also called amber acid (Song and Lee 2006). Currently succinic acid is chemically produced from butane through maleic anhydride and petroleum‐based raw materials.
The first microbial production of succinic acid was the engineering of mixed acid fermentation of Escherichia coli using glucose (Chatterjee et al. 2001). The Actinobacillus succinogenes bacterium was investigated for the production of succinic acid using glucose as a carbon source (Li et al. 2011 ; Xi et al. 2012). In several similar studies Anaerobiospirillum succiniciproducens produced succinic acid using glycerol as a carbon source (Jabalquinto et al. 2004 ; Lee et al. 2001). Lee et al. (2003) reported production of succinic acid by Mannheimia succiniciproducens using whey and corn steep liquor. Strains including Bacillus fragilis (Beauprez et al. 2010), Corynebacterium glutamicum (Okino et al. 2005), and genetically engineered E. coli (Khan et al. 2009) were also found to produce succinic acid (Table 5.2).
Table 5.2 Summary of studies on succinic acid production using three major classes of micro‐organisms (bacteria, yeast, and filamentous fungi).
| Class of micro‐organism | Strains/isolates | Reference |
| Bacteria |
Actinobacillus succinogenes
Anaerobiospirillum succiniciproducens Mannheimia succiniciproducens Bacillus fragilis Corynebacterium glutamicum Escherichia coli |
Li et al. 2011, Xi et al. 2012 Lee et al. 2001 ; Jabalquinto et al. 2004 Lee et al. 2003 Beauprez et al. 2010 Okino et al. 2005 Kahn et al. 2009 ; Chatterjee et al. 2001 |
| Yeast |
Saccharomyces cerevisiae
Candida brumptii Candida zeylanoides Candida catenulata Yarrowia lipolytica |
Andreas et al. 2011 Sato et al. 1972 Mandeva et al. 1981 ; Kamzolova et al. 2009 Kamzolova et al. 2009 Yuzbashev et al. 2010 |
| Filamentous fungi |
Penicillium simplicissimum
Fusarium sp. Aspergillus sp. |
Gallmetzer et al. 2002 Foster 1949 Bercovitz et al. 1990 |
The production of succinic acid is industrially exploited by fermentation through host organisms such as Saccharomyces cerevisiae (Raab and Lang 2011). However, succinic acid production by other yeasts, such as Candida brumptii (Sato et al. 1972), C. zeylanoides (Kamzolova et al. 2009), C. catenulata (Kamzolova et al. 2009), and Yarrowia lipolytica (Yuzbashev et al. 2010), is also well established (see Table 5.2).
Gallmetzer et al. (2002) reported that the filamentous fungus Penicillium simplicissimum accumulates succinic acid naturally. A few other species such as Fusarium (Foster 1949) and Aspergillus (Bercovitz et al. 1990) are also common producers (see Table 5.2).
Succinic acid is widely used as a flavoring agent in foods and beverages, in dyes, insecticides, perfumes, lacquers, in vehicle water cooling systems and the manufacture of clothing, paint, inks, and fibers. Chen et al. (2012) reported increased demand for succinic acid in the synthesis of biodegradable polymers such as polybutyrate succinate (PBS), polyamides, and various green solvents. It is also used in therapeutic medicines (Kondrashova 2002 ; Kondrashova et al. 2005).
Lactic Acid
Lactic acid (LA) is an organic compound (2‐hydroxypropanoic acid) with chemical formula C3H6O3 and structural formula CH3CHOHCOOH (Figure 5.4). It is a colorless or white to light yellow hydroxyl acid in either solid or powder form. Lactic acid has an asymmetric carbon atom and is present in two optically active D (‐) and L (+) forms (Senthuran et al. 1997).
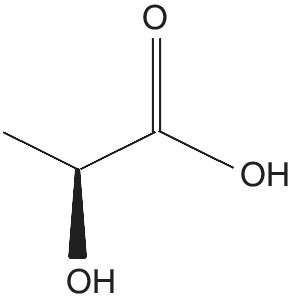
Figure 5.4 Lactic acid.
Originally, lactic acid was recognized in sour milk. However, in 1857, Pasteur discovered that it is not a milk component but a fermentation product generated by certain micro‐organisms (Benninga 1990). Fremy (1881) produced lactic acid by fermentation and it was first used for industrial production (Narayanan et al. 2004).
Microbial production of lactic acid is well established using the bacteria Carnobacterium funditum and Carnobacterium alterfunditum (Franzmann et al. 1991). Lactococcus sp. is widely used for lactic acid production by sucrose inversion (Akerberg et al. 1998 ; Roissart 1994). In general, lactic acid is produced from lactose using Lactobacillus plantarum (Wenge and Matthews 1999) and Enterococcus faecium (Nolasco‐Hipolito et al. 2012). Similarly, Leuconostoc mesenteroides (Fitzpatrick and Keeffe 2001), a lactic acid bacterium, produces optically pure lactic acid using different carbon and nitrogen sources. A few other bacteria including Escherichia, Bacillus, and Kluyveromyces have also been reported to produce lactic acid (Maas et al. 2008). Garde et al. (2002) used a mixed culture of Lactobacillus pentosus and L. brevis to produce lactic acid from wheat straw hemicelluloses (Table 5.3).
Table 5.3 Summary of studies on lactic acid production using three major classes of micro‐organisms (bacteria, yeast, and filamentous fungi).
| Class of micro‐organism | Strains/isolates | Reference |
| Bacteria | Carnobacterium funditum Carnobacterium alterfunditum | Franzmann et al. 1991 |
| Lactobacillus plantarum | Wenge 1999 | |
| Lactococcus sp. | Roissart,1994; Akerberg et al. 1998 | |
| Leuconostoc mesenteroides | Fitzpatrick and Keeffe 2001 | |
| Enterococcus faecium | Nolasco‐Hipolito et al. 2012 | |
| Escherichia | Maas et al. 2008 | |
| Bacillus | ||
| Kluyveromyces | ||
| Lactobacillus pentosus | Garde et al. 2002 | |
| Lactobacillus brevis | ||
| Yeast |
Saccharomyces cerevisiae
Kluyveromyces lactis Pichia stipites Candida boidinii Candida utilis Torulaspora delbruekii Kluyveromyces marxianus |
Dequin and Barre 1994 Bianchi et al. 2001 Llmen et al. 2007 Osawa et al. 2009 Ikushima et al. 2009 Porro et al. 1999 Pecota et al. 2007 |
| Filamentous fungi |
Rhizopus oryzae
R. arrhizus |
Lockwood et al. 1936 ; Bai et al. 2003 Wee et al. 2006 |
Moreover, lactic acid is produced by Saccharomyces cerevisiae in medium containing glucose at low pH (Dequin and Barre 1994). There are several genetically engineered yeasts, such as Kluyveromyces lactis (Bianchi et al. 2001), Pichia stipitis (Llmen et al. 2007), Candida boidinii (Osawa et al. 2009), Candida utilis (Ikushima et al. 2009), Torulaspora delbruekii (Porro et al. 1999) and Kluyveromyces marxianus (Pecota et al. 2007), which have been studied for producing lactic acid (see Table 5.3).
The filamentous fungus Rhizopus under aerobic conditions produced lactic acid using glucose. Rhizopus oryzae produces lactic acid and requires a mineral medium composition (Bai et al. 2003 ; Lockwood et al. 1936). Similarly, R. arrhizus can convert starch directly to L‐lactic acid due to its amylolytic enzyme activity (Wee et al. 2006) (see Table 5.3).
Lactic acid has many potential applications in industry (Datta and Henry 2006), as a descaling agent, pH regulator, neutralizer, chiral intermediate, solvent, cleaning agent, slow acid, metal complexing agent, and antimicrobial agent (Datta et al. 1995). Lactic acid has been utilized by the food industry as an additive for preservation, flavor, mineral fortification, baked goods, pickled vegetables, and beverages. It is frequently used in cosmetics as a moisturizer, pH regulator, skin lightener, and skin hydrater. Use of lactic acid is also reported in a wide variety of mineral preparations including tablets, prostheses, surgical sutures, and controlled drug delivery systems (Narayanan et al. 2004).
Itaconic Acid
Itaconic acid (IA) (methylene butanedioic acid) has the chemical formula C5H6O4 and is commonly known as methylene succinic acid (Figure 5.5). It is a colorless or white crystalline unsaturated dicarbonic acid (Tate 1981). It is stable at acidic, neutral, and middle basic conditions under moderate temperatures (Tate 1981).
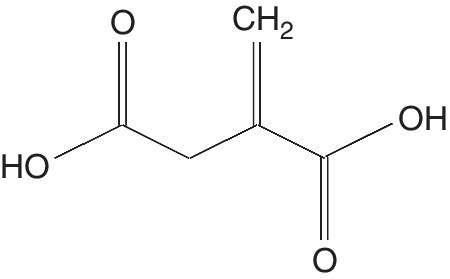
Figure 5.5 Itaconic acid.
Itaconic acid was first discovered by Baup (1837) as a thermal decomposition product of citric acid. Later, Kinoshita (1932) found itaconic acid from Aspergillus itaconicus, which was isolated from dried salted plums. Pfeifer et al. (1952) first reported its large‐scale industrial production.
The fermentation of glucose by yeast belonging to the genus Candida produced itaconic acid (Tabuchi et al. 1981). Willke and Vorlop (2001) also reported itaconic acid production by the Candida mutant strain. Rhodotorula sp. (Kawamura et al. 1981) and Pseudozyma antarctica (William et al. 2006) also produce itaconic acid from glucose and other sugars under nitrogen‐limited growth conditions (Table 5.4).
Table 5.4 Summary of studies on itaconic acid production using two major classes of micro‐organisms (yeast and filamentous fungi).
| Class of micro‐organism | Strains/isolates | Reference |
| Yeast |
Candida sp. Rhodotorula sp. Pseudozyma antarctica |
Tabuchi et al. 1981 Kawamura et al. 1981 William et al. 2006 |
| Filamentous fungi |
Aspergillus itaconicus
Aspergillus terreus Ustilago zeae Helicobasidium mompa Ustilago maydis |
Kinoshita 1932 Calam et al. 1939 Haskins et al. 1955 Araki et al. 1957 Martinez‐Espinoza et al. 2002 |
Itaconic acid was produced from the osmophilic filamentous fungus Aspergillus itaconicus on sugar growth medium (Kinoshita 1932). A few species of the pathogenic fungal genus Ustilago are also known to produce itaconic acid during fermentation (Willke and Vorlop 2001). In later reports, a few other fungal strains, mainly Aspergillus terreus (Calam et al. 1939), Ustilago zeae (Haskins et al. 1955), Helicobasidium mompa (Araki et al. 1957), and mold fungus Ustilago maydis (Martinez‐Espinoza et al. 2002) efficiently produced itaconic acid (see Table 5.4).
This acid supports large industrial applications such as synthesis of lattices, fibers, polyesters, plastics, artificial glass and preparation of bioactive compounds for the agriculture, pharmacy, and medicine sectors (Kin et al. 1998). Itaconic acid is also used in detergents, cleaners, shampoos, pharmaceuticals, and herbicides (Lancashire 1969) and to improve adhesives of paper, cellophane, etc. (Pitzl et al. 1951). It may remove unsaturated polyester resins, paint guns and lines, chopper guns, polyurethane foam, most paints and inks. Applications of IA have been extended to biomedical fields, such as dental and ophthalmic uses, and it has high potential for use in sustained drug release during ocular delivery.
Lactobionic Acid
Lactobionic acid (LBA) is a sugar acid, in the form of a white powder, with the chemical formula C12H22O12 (Figure 5.6). It is also called 4‐O‐β‐galactopyranosyl‐D‐gluconic acid and is soluble in water, and slightly soluble in organic compounds such as glacial acetic acid, ethanol, and methanol.
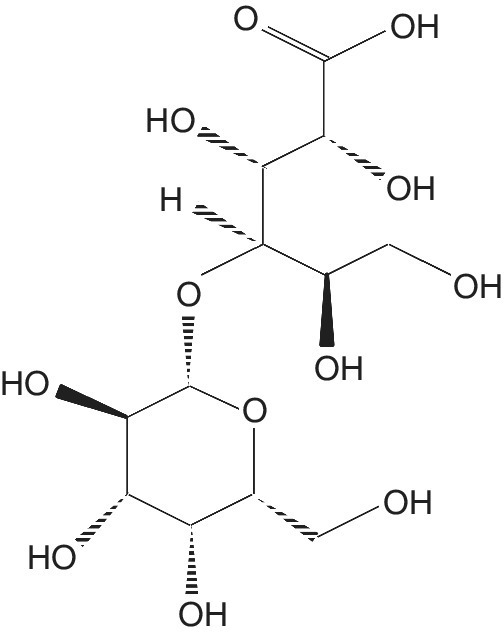
Figure 5.6 Lactobionic acid.
Lactobionic acid was first synthesized by Fischer and Meyer (1889) after oxidation of lactose with bromine. Later, several processes including chemical, electrochemical, biocatalytic and heterogeneous catalytic oxidations were introduced for LBA production. LBA is successfully produced by bacteria and filamentous fungi only.
The production of LBA was first recognized in Pseudomonas species. Several bacterial strains produce LBA from aqueous lactose solutions, such as Pseudomonas graveolens (Stodola and Lockwood 1947), P. fragi, P. mucidolens, P. myxogenes, and P. taetrolen (Stodola and Lockwood 1947). LBA production was also reported through P. quercito‐pyrogallica, P. calco‐acetica, and P. aromatica, which convert lactose into LBA (Masuo et al. 1952). Futhermore, several other bacterial species such as Acetobacter orientalis (Kiryu et al. 2012), Burkholderia cepacia (Murakami et al. 2006), Halobacterium saccharovorum (Tomlinson et al. 1978), Bacterium anitratum (Villecourt and Blachere 1955), Paraconiothyrium sp. (Murakami et al. 2008), Pseudomonas sp. LS13‐1 (Yasumitsu et al. 2000), and Zymomonas mobilis (Satory et al. 1997) have been investigated for LBA production by lactose oxidizing activity (Table 5.5).
Table 5.5 Summary of studies on lactobionic acid production using two major classes of micro‐organisms (bacteria and filamentous fungi).
| Class of micro‐organism | Strains/isolates | Reference |
| Bacteria | Pseudomonas sp. | Yasumitsu et al. 2000 |
| Pseudomonas fragi | Stodola and Lockwood 1947 | |
| P. mucidolens | ||
| P. myxogenes | ||
| P. quercito‐pyrogallica | Masuo et al. 1952 | |
| P. calco‐acetica | ||
| P. aromatica | ||
| Acetobacter orientalis Burkholderia cepacia | Kiryu et al. 2012 Murakami et al. 2006 |
|
| Halobacterium saccharovorum | Tomlinson et al. 1978 | |
| Bacterium anitratum | Villecourt and Blachere 1955 | |
| Paraconiothyrium sp. | Murakami et al. 2008 | |
| Zymomonas mobilis | Satory et al. 1997 | |
| Filamentous fungi | Penicillium chrysogenum | Cort et al. 1956 |
The production of LBA by filamentous fungi has also been studied (Bucek et al. 1956). In one study, production of LBA from lactose by Penicillium chrysogenum (Cort et al. 1956) was reported in shake flask cultures (see Table 5.5).
Lactobionic acid has many applications in the cosmetics (Green et al. 2009 ; West 2004), pharmaceutical (Belzer et al. 1992), food and chemical industries (Gerling 1998). Several brands of cosmetics are currently employing LBA as a key component of novel antiaging products, claiming to reduce photoaging and wrinkles (Grimes et al. 2004), and as a keratinization agent and regenerative skincare product (Green et al. 2009).
Gluconic Acid (Sugar Acid)
Gluconic acid is an organic compound with the molecular formula C6 H12 O7 and structural formula HOCH2(CHOH)4COOH (Figure 5.7). It is charactristically a non‐corrosive, non‐volatile, non‐toxic, and mild organic acid with many applications.
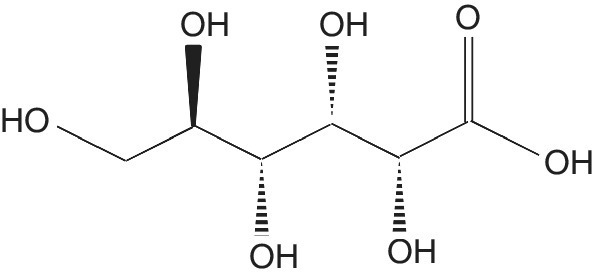
Figure 5.7 Gluconic acid.
Hlasiwetz and Habermann first discovered gluconic acid (Rohr et al. 1983). Bacteria are capable of producing sugar acid, as first reported by Boutroux (1880).
Production of gluconic acid has been predominantly studied with different bacterial species such as Pseudomonas ovalis (Bull et al. 1970), Acetobacter methanolicus (Loffhagen and Babel 1993), and A. suboxydans (Currie and Carter 1930) using diverse media. A few other genera were also found to produce gluconic acid by using glucose, including Zymomonas mobilis (Kim and Kim 1992), Acetobacter diazotrophicus (Attwood et al. 1991), Gluconobacter oxydans (Velizarov and Beschkov 1994), G. suboxydans (Shiraishi et al. 1989), Azospirillum brasiliense (Rodriguez et al. 2004), etc. (Table 5.6). Gluconic acid production is also possible with yeasts such as Aureobasidium pullulans (Anastassiadis et al. 2005 ; Perwozwansky 1939) (see Table 5.6).
Table 5.6 Summary of studies on gluconic acid production using three major classes of microorganisms (bacteria, yeast, and filamentous fungi).
| Class of micro‐organism | Strains/isolates | Reference |
| Bacteria | Pseudomonas ovalis | Bull et al. 1970 |
| Acetobacter methanolicus Acetobacter suboxydans Zymomonas mobilis | Loffhagen et al. 1993 Currie and Carter 1930 Kim et al. 1992 |
|
| Acetobacter diazotrophicus Gluconobacter oxydans | Attwood et al. 1991 Velizarov et al. 1994 |
|
| Gluconobacter suboxydans | Shiraishi et al. 1989 | |
| Azospirillum brasiliense | Rodriguez et al. 2004 | |
| Yeast | Aureobasidium pullulans | Perwozwansky, 1939 ; Anastassiadis et al. 2005 |
| Filamentous fungi |
Aspergillus niger
Penicillium funiculosum P. variabile P. amagasakiense P. purpurescens P. luteum purpurogenum |
Moyer et al. 1940 Kundu et al. 1984 Petruccioli et al. 1994 Kusai et al. 1960 Temash and Olama 1999 May et al. 1927 |
Conversion of glucose to gluconic acid by filamentous fungi is catalyzed by the enzyme glucose oxidase.Various fungal species including Aspergillus niger (Moyer 1940), Penicillium funiculosum (Kundu and Das 1984), P. luteum purpurogenum (May et al. 1927), P. variabile (Petruccioli et al. 1994), and P. amagasakiense (Kusai et al. 1960) produce gluconic acid. Gluconic acid production through Penicillium purpurescens using banana waste has been reported (Temash and Olama 1999).
Gluconic acid has wide applications in the food industries as a flavoring agent in syrups and in reducing fat absorption. It is also used in meat and dairy products, particularly in baked goods as a component of the leavening agent (Znad et al. 2003). Gluconic acid is commonly used in chemical industries for bottle washing, washing painted walls and removal of metal carbonate precipitates without causing corrosion.Textile industries also exploit GA where it prevents the deposition of iron and for desizing polyester and polyamide fabrics. It also finds application as an additive to cement for increasing the strength of water resistance. Cleaning compounds such as mouthwashes, common cold treatments, and wound healing compounds use GA as an ingredient.
Fumaric Acid
Fumaric acid 9FA) is a white solid crystal with no odor and slight acidic taste, with the molecular formula C4H4O4 (Goto et al. 1998) (Figure 5.8). It is an important intermediate in the citrate cycle (Roa Engel et al. 2008). At higher temperatures, it forms traces of maleic anhydride.
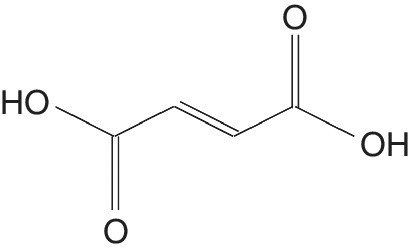
Figure 5.8 Fumaric acid.
Fumaric acid was initially extracted from plants belonging to the genus Fumaria. Ehrlich (1911) first discovered fumaric acid production from fungal fermentation of sugar using R. nigricans.
The bacteria able to produce maleate isomerase are also capable of synthesizing fumaric acid: Pseudomonas sp. (Otsuka 1961), Alcaligenes faecalis (Takamura et al. 1969) and Pseudomonas fluorescens (Scher and Lennarz 1969). Similarly P. alcaligenes has high rates of maleic acid conversion into fumaric acid (Ichikawa et al. 2003 ; Nakajima Kambe et al. 1997). Song et al. (2013) reported on genetically engineered E. coli strains for fumaric acid production (Table 5.7).
Table 5.7 Summary of studies on fumaric acid production using three major classes of micro‐organisms (bacteria, yeast, and filamentous fungi).
| Class of micro‐organism | Strains/isolates | Reference |
| Bacteria |
Pseudomonas sp. Alcaligenes faecalis Pseudomonas alcaligenes Pseudomonas fluorescens E. coli mutant |
Otsuka 1961 Takamura et al. 1969 Nakajima Kambe et al. 1997 ; Ichikawa et al. 2003 Scher and Lennarz 1969 Song et al. 2013 |
| Yeast | Schizosaccharomyces pombe | Saayman et al. 2000 |
| Candida utilis | ||
| Candida sphaerica | Corte‐Real et al. 1989 | |
| Hansenula anomala | Corte‐Real and Leao 1990 | |
| Filamentous fungi |
Rhizopus nigricans
R. arrhizus R. oryzae R. formosa |
Ehrlich 1911 Kautola and Linko 1989 Peleg et al. 1989 Foster and Waksman 1939 |
Fumaric acid production was reported by yeasts such as Schizosaccharomyces pombe (Saayman et al. 2000), Candida utilis (Saayman et al. 2000), Candida sphaerica (Corte‐Real et al. 1989), and Hansenula anomala (Corte‐Real and Leao 1990) which can convert substrates into fumaric acid (see Table 5.7).
Fumaric acid accumulation has been studied in many filamentous fungi of the Rhizopus genus, such as Rhizopus formosa (Foster and Waksman 1939), R. oryzae (Peleg et al. 1989), R. nigricans (Ehrlich 1911), and R. arrhizus (Kautola and Linko 1989).
Fumaric acid has extensive applications in the food, beverage, and pharmaceutical industries. It is currently used in many food products, including corn and wheat tortillas, refrigerated biscuit doughs, sourdough, rye breads, gelatin desserts, gelling aids, pie fillings, fruit juice, nutraceutical drinks, and wines. Its use as a supplement in cattle feed also leads to a large reduction in methane emission (Roa Engel et al. 2008). Ferrous fumarate is used to treat iron deficiencies like anemia.
Propionic Acid
Propionic acid (PA) is colorless liquid with a rancid odor and C3H6O2 molecular formula (Figure 5.9). It is a major intermediate between formic and acetic acid which is miscible in water, but can be removed from water by adding salt. Propionic acid was first discovered by Johann Gottlieb (1844) during the degradation of sugar (Haque et al. 2009).
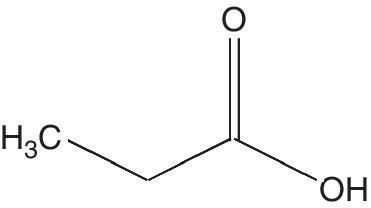
Figure 5.9 Propionic acid.
Available reports suggest that this acid is produced only by bacterial agents such as Propionibacterium freudenreichii (Playne 1985), P. acidipropionici (Steven et al. 1991), and Clostridium propionicum (Cardon and Barker 1947). These species can produce PA as a metabolic end product, through the fermentation of lactate. Recently, large‐scale production of propionic acid was undertaken using genetically engineered Propionibacterium jensenii ATCC 4868 from glycerol (Zhuge et al. 2013) (Table 5.8).
Table 5.8 Summary of studies on propionic acid production using one major class of micro‐organism (bacteria).
| Bacterial strains/isolates | Reference |
|
Clostridium propionicum
Propionibacterium freudenreichii Propionibacterium acidipropionici Propionibacterium jensenii |
Cardon and Barker 1947 Playne 1985 Steven et al. 1991 Zhuge et al. 2013 |
Propionic acid is used in the production of cellulose acetate‐based polymers (Playne 1985). It is widely used in the food industry as an artificial flavor and preservative (yeast and mold inhibitor). Its ammonium salt is employed as a common ingredient in animal feed. It is also used in the manufacturing of herbicides, pesticides, pharmaceuticals, perfumes, and plastics (Boyaval and Corre 1995).
Acetic Acid
Acetic acid (C2H4O2) (AA), the most familiar edible organic acid systematically named ethanoic acid, is an organic compound commonly called vinegar (Figure 5.10). It is a colorless liquid called glacial acetic acid when in undiluted form. In general, acetic acid occurs naturally in trace amounts in fruits, such as pears. Acetic acid was first produced in 1794 in Germany using grapes. The production of acetic acid predominantly depends on the species and efficiency of micro‐organisms. A variety of micro‐organisms including bacteria, yeasts, and a few filamentous fungi are able to produce this organic acid.
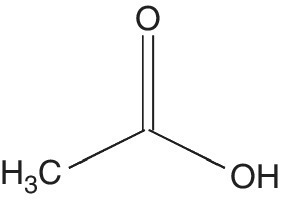
Figure 5.10 Acetic acid.
The literature contains several reports of bacteria producing acetic acid. The most relevant bacteria are gram negative, obligate aerobic, ellipsoidal or cylindrical, found alone, in pairs or in clusters and chains (de Ley et al. 1984). These are able to oxidize ethanol into acetic acid. Acetobacter is the oldest classified genus known for production of vinegar which was reclassified by Yamada et al. (1997) as Gluconacetobacter (Yamada et al. 2012). Based on their ability to oxidize acetate and lactate, bacteria are grouped into two particular genera called Acetobacter and Gluconacetobacter (de Ley et al. 1984 ; Yamada et al. 1976). Vegas et al. (2010) reported that bacterial species like Komagataeibacter europaeus, Acetobacter hansenii, and Acetobacter xylinus are involved in the production of wine vinegar (Table 5.9).
Table 5.9 Summary of studies on acetic acid production using three major classes of micro‐organisms (bacteria, yeast, and filamentous fungi).
| Class of micro‐organism | Strains/isolates | Reference |
| Bacteria | Komagataeibacter europaeus | Vegas et al. 2010 Hidalgo et al. 2012, 2013 |
| Acetobacter hansenii | ||
| A. xylinus | ||
| A. malorum | ||
| A. aceti | ||
| A. polyoxogenes | ||
| A. obodiens | ||
| A. europaeus | ||
| A. lovaniensis | Gullo et al. 2009 | |
| Yeast | Dekkera bruxellensis | Carrascosa et al. 1981 |
| D. anomala | Geros et al. 2000 | |
| Brettanomyces intermedius NRRL Y‐2394 | Castro‐Martinez et al. 2005 | |
| B.custersianus | ||
| B. clausenni | ||
| B. bruxellensis | ||
| Saccharomyces cerevisiae | (Postma et al. 1989) | |
| Filamentous fungi |
Fusarium oxysporum
Polyporus anceps |
Singh et al. 1992 Perlman et al. 1949 |
Spoiling of bottled wine is usually noted as the production of haze, turbidity, and acetic acid by a few genera of yeasts (Sponholz 1993). The production of AA was reported by using different species of yeast such as Dekkera anomala (Geros et al. 2000), Saccharomyces cerevisiae (Postma et al. 1989), and D. bruxellensis (Carrascosa et al. 1981) grown in concentrations of glucose. In a similar study acetic acid production occurred via different yeast Dekkera and Brettanomyces spp. using either glucose or ethanol as a carbon source. Castro‐Martinez et al. (2005) reported that acetic acid can be produced directly from glucose by using different strains of yeasts Brettanomyces bruxellensis YB‐5164, B. intermedius NRRL Y‐2394, B. custersianus, and B. clausenni. A. pasteurianus seems to be the most common in wine vinegars, although other Acetobacter spp. also occur, such as A. malorum, A. aceti, A. polyoxogenes, A. obodiens, and A. europaeus (Hidalgo et al. 2012, 2013) (see Table 5.9).
The production of acetic acid by filamentous fungi has also been reported. Kumar et al. (1991) screened various strains of Fusarium oxysporum and Monilia brunnae for their ability to produce acetic acid. Fusarium oxysporum DSM 841 was selected as a potential strain producing acetic acid and ethanol. Singh et al. (1992) reported involvement of Fusarium oxysporum in acetic acid production. Perlman (1949) mentioned that Polyporus anceps is capable of utilizing substrate to produce acetic acid as a major product.
Because of its pungent odor and taste, acetic acid has an important place in our kitchens, laboratories, and food industries such as in the preparation of pickles, chutney, salad creams, mayonnaise, dressings, and sauces.
Conclusion
Based on the broad applicability of the organic acids (Figure 5.11), it can be concluded that bacteria, yeasts, and filamentous fungi are the responsible bioagents converting substrate into beneficial acids. The exploitation of these micro‐organisms at a large scale should be conducted to achieve a better yield of organic acids for easy and cheap availability. Furthermore, screening of efficient isolates and subsequent efforts regarding strain improvement should be facilitated.

Figure 5.11 Different organic acids used for broad spectrum applications in the field of agriculture, food, pharmaceutical, cosmetics and chemical industries.
References
- Akerberg C., K. Hofvendahl, G. Zacchi and B. Hahn‐Hagerdal (1998) Modeling the influence of pH, temperature, glucose and lactic acid concentration on the kinetics of lactic acid production by Lactococcus lactis sp. lactis ATCC 19435 in whole‐wheat flour. Appl. Microbiol. Biotechnol., 49: 682–690.
- Anastassiadis S., A. Aivasidis, C. Wandrey and H.J. Rehm (2005) Process optimization of continuous gluconic acid fermentation by isolated yeast‐like strains of Aureobasidium pullulans. Biotechnol. Bioeng., 91: 494–501.
- Araki T., Y. Yamazaki and N. Suzuki (1957) Studies on the violet root rot sweet potatoes caused by Helicobasidium mompa. Bull. Natl. Inst. Agric. Sci. Jpn. Ser., 8: 53–59.
- Attwood M., J.P. van Dijken and J.T. Pronk (1991) Glucose metabolism and gluconic acid production by Acetobacter diazotrophicus. J. Ferment. Bioeng., 72: 101–105.
- Bai D.M., M.Z. Jia, X.M. Zhao et al (2003) L(+)‐Lactic acid production by pellet form Rhizopus oryzae R1021 in a stirred tank fermentor. Chem. Eng. Sci., 58: 785–791.
- Barbesgaard P., H.P. Heldt‐Hansen and B. Diderichsen (1992) On the safety of Aspergillus oryzae: a review. Appl. Microbiol. Biotechnol., 36: 569–572.
- Baup S. (1837) Ueber eine neue Pyrogen‐Citronensaure und über Benennung der Pyrogen‐Säuren uberhaupt. Ann. Chim. Phys., 19: 29–38.
- Beauprez J.J., M. de Mey and W.K. Soetaert (2010) Microbial succinic acid production: natural versus metabolic engineered producers. Process Biochem., 45: 1103–1114.
- Belzer F.O., A.M. d’Alessandro, R.M. Hoffmann, S.J. Knechtle, A. Reed and J.D. Pirsch (1992) The use of UW solution in clinical transplantation. A 4‐year experience. Ann. Surg., 215: 579–585.
- Benninga H (1990) A History of Lactic Acid Making: A Chapter in the History of Biotechnology. Dordrecht: Kluwer Academic Publishers.
- Bercovitz A., Y. Peleg, E. Battat, J.S. Rokem and I. Goldberg (1990) Localization of pyruvate carboxylase in organic acid producing Aspergillus strains. Appl. Environ. Microbiol., 56: 1594– 1597.
- Bianchi M.M., L. Brambilla, F. Protani, C.L. Liu, J. Lievense and D. Porro (2001) Efficient homolactic fermentation by Kluyveromyces lactis strains defective in pyruvate utilization and transformed with the heterologous LDH gene. Appl. Environ. Microbiol., 67: 5621–5625.
- Boutroux L. (1880) Physiological chemistry. About a new glucose fermentation. C.R. Hebd. Seances Acad. Sci., 91: 236–238.
- Boyaval P. and C. Corre (1995) Production of propionic acid. Lait., 75: 453–461.
- Bucek W., W.M. Connors, W.M. Cort and H.R. Roberts (1956) Evidence for the formation and utilization of lactobionic acid by Penicillium chrysogenum. Arch. Biochem. Biophys., 63: 477–478.
- Bull D.N. and L.L. Kempe (1970) Kinetics of the conversion of glucose to gluconic acid by Pseudomonas ovalis. Biotechnol. Bioeng., 12: 273–290.
- Calam C.T., A.E. Oxford and H. Raistrick (1939) Studies in the biochemistry of micro organisms. LXIII. Itaconic acid, a metabolic product of a strain of Aspergillus terreus Thom. Biochem. J., 33: 1488– 1495.
- Cardon B.P. and H.A. Barker (1947) Amino acid fermentations by Clostridium propionicum and Diplococcus glycinophilus. Arch. Biochem., 12: 165–180.
- Carrascosa J.M., M.D. Viguera, I. Nunez de Castro, W.A. Schet Ters (1981) Metatabolism of acetaldehyde and Custers effect in the yeast. Antonie van Leeuwenhoek, 47: 209–215.
- Castro‐Martinez C., B.I. Escudero‐Abarca, J. Gomez‐Rodriguez, P.M. Hayward‐Jones and M.G. Aguilar‐Uscanga (2005) Effect of physical factors on acetic acid production in Brettanomyces strain. J. Food Process Eng., 28: 133–143.
- Chatterjee R., C.S. Millard, K. Champion, D.P. Clark and M.I. Donnelly (2001) Mutation of the ptsG gene results in increased production of succinate in fermentation of glucose by Escherichia coli. Appl. Environ. Microbiol., 67: 148–154.
- Chen Z., L. Xiao, W. Liu, D. Liu, Y.Y. Xiao and J. Chen (2012) Novel materials which possess the ability to target liver cells. Expert Opin. Drug Deliv., 9: 649–656.
- Cort W.M., W.M. Connors, H.R. Roberts and W. Bucek (1956) Evidence for the formation and utilization of lactobionic acid by Penicillium chrysogenum. Arch. Biochem. Biophys., 63: 477–478.
- Corte‐Real M. and C. Leao (1990) Transport of malic acid and other carboxylic acids in the yeast Hansenula anomala. Appl. Environ. Microbiol., 56: 1109–1113.
- Corte‐Real M., C. Leao and N. van Uden (1989) Transport of L‐malic acid and other dicarboxylic acids in the yeast Candida sphaerica. Appl. Microbiol. Biotechnol., 31: 551–555.
- Currie J.N. (1917) Citric acid fermentation. J. Biol. Chem., 31: 15–37.
- Currie J.N. and R.H. Carter (1930) Gluconic acid. US Patent 1,896,811.
- Datta R. and M. Henry (2006) Lactic acid: recent advances in products, processes and technologies – a review. J. Chem. Technol. Biotechnol., 81: 1119–1129.
- Datta R., S.P. Tsai, P. Bonsignore, S.H. Moon and J.R. Frank (1995) Technological and economic potential of poly (lactic acid) and lactic acid derivatives. FEMS Microbiol. Rev., 16: 221–231.
- De Ley J., J. Swings and F. Gossele (1984) Genus I Acetobacter Beijerinck. In: Kreig N.R. and J.C. Holt (eds) Bergey’s Manual of Systematic Bacteriology. Baltimore: Williams and Wilkins, pp. 268–274.
- Dequin S. and P. Barre (1994) Mixed lactic acid alcoholic fermentation by Saccharomyces cerevisiae expressing the Lactobacillus casei (L)‐LDH. Biotechnology, 12: 173–177.
- Ehrlich F. (1911) Formation of fumeric acid by means of molds. Ber. Dtsch. Chem. Ges., 44: 3737–3742.
- El Dein S.M.N. and G.M.I. Emaish (1979) Effect of various conditions on production of citric acid from molasses in presence of potassium ferrocyanide by Aspergillus aculeatus and A. carbonarius. Indian J. Exp. Biol., 17: 105–106.
- Fischer E. and J. Meyer (1889) Oxydation des Milchzuckers. Ber. Deut. Chem. Ges., 22: 361–364.
- Fitzpatrick J.J. and U.O. Keeffe (2001) Influence of whey protein hydrolyzate addition to whey permeate batch fermentations for producing lactic acid. Proc. Biochem., 37: 183–186.
- Foster J.W. (1949) Chemical Activities of Fungi. New York: Academic Press.
- Foster J.W. and S.A. Waksman (1939) The production of fumaric acid by molds belonging to the genus Rhizopus. J. Am. Chem. Soc., 61: 127–135.
- Franz A., W. Burgstaller and F. Schinner (1991) Leaching with Penicillium simplicisszmum: influence of metals and buffers on proton extrusion and citric acid production. Appl. Environ. Microbiol., 57: 769–774.
- Franzmann P. D., P. Hopfl, N. Weiss and B. J. Tindall (1991) Psychrotrophic, lactic acid‐producing bacteria from anoxic waters in Ace Lake, Antarctica: Carnobacterium funditum sp. nov. and Carnobacterium alterfunditum sp. nov. Arch. Microbiol., 156: 255–262.
- Fukuda H., T. Susuki, Y. Sumino and S. Akiyama (1970) Microbial preparation of citric acid. German Patent 2: 003–221.
- Gallmetzer M., J. Meraner and W. Burgstaller (2002) Succinate synthesis and excretion by Penicillium simplicissimum under aerobic and anaerobic conditions. FEMS Microbiol. Lett., 210: 221–225.
- Garde A., G. Jonsson, A.S. Schmidt and B.K. Ahring (2002) Lactic acid production from wheat straw hemicellulose hydrolysate by Lactobacillus pentosus and Lactobacillus brevis. Biores. Technol., 81: 217–223.
- Georgius Agricola (1546) Book IV, De natura fossilium. H. frobenius and N.episcopius. Basel, Switzerland.
- Gerling K.G. (1998) Large scale production of lactobionic acid use and new applications. Int. Dairy Fed., 9804: 251–261.
- Geros H., M.M. Azevedo and F. Cassio (2000) Biochemical studies on the production of acetic acid by the yeast Dekkera anomala. Food Technol. Biotechnol., 38: 59–62.
- Goto M., T. Nara, I. Tokumaru et al. (1998) Method of producing fumaric acid. US Patent 5,783,428.
- Gottschalk G. (1985) Bacterial Metabolism, 2nd edn. Berlin: Springer.
- Green B.A., R.J. Yu and E.J. van Scott (2009) Clinical and cosmeceutical uses of hydroxyacids. Clin. Dermatol., 9: 495–501.
- Grewal H.S. and K.L. Kalra (1995) Fungal production of citric acid. Biotechnol. Adv., 13: 209–234.
- Grimes P.E., B.A. Green, R.H. Wildnauer and B.L. Edison (2004) The use of polyhydroxy acids (PHAs) in photoaged skin. Cutis, 73: 3–13.
- Gullo, M., de Vero, L. and Giudici, P. (2009) Succession of selected strains of Acetobacter pasteurianus and other acetic acid bacteria in traditional balsamic vinegar. Appl. Environ. Microbiol., 75(8): 2585–2589.
- Gurtler J.B. and T.L. Mai (2014) Traditional preservatives: organic acids. Encycl. Food Microbiol., 3: 119–130.
- Gutierrez N.A., I.A. Mckay, C.E. French, J. Brooks and I.S. Maddox (1993) Repression of galactose utilization by glucose in the citrate producing yeast Candida guilliermondii. J. Ind. Microbiol., 11: 143–146.
- Hang Y.D. and E. E. Woodams (1987) Microbial production of citric acid by solid state fermentation of kiwifruit peel. J. Food Sci., 52: 226–227.
- Haque M.N., R. Chowdhury, K M.S. Islam and M.A. Akbar (2009) Propionic acid is an alternative to antibiotics in poultry diet. Bang. J. Anim. Sci., 38: 115–122.
- Haskins R.H., J.A. Thorn and B. Boothroyd (1955) Biochemistry of the Ustilaginales. XI. Metabolic products of Ustilago zeae in submerged culture. Can. J. Microbiol., 1: 749–756.
- Hidalgo C., E. Mateo, A. Mas and M.J. Torija (2012) Identification of yeast and acetic acid bacteria isolated from the fermentation and acetification of persimmon. Food Microbiol., 30: 98–104.
- Hidalgo C., E. Mateo, A. Mas and M.J. Torija (2013) Effect of inoculation on strawberry fermentation and acetification processes using native strains of yeast and acetic acid bacteria. Food Microbiol., 34: 88–94.
- Ichikawa S., T. Iino, S. Sato, T. Nakahara and S. Mukataka (2003) Improvement of production rate and yield of fumaric acid from maleic acid by heat treatment of Pseudomonas alcaligenes strain XD‐1. Biochem. Eng. J., 13: 7–13.
- Ikeno Y., Y.M., Masuda, K. Tanno, I. Oomori and N. Takahashi (1975) Citric acid production from various raw materials by yeasts. J. Ferment.Technol., 53: 752–756.
- Ikushima S., T. Fujii, O. Kobayashi, S. Yoshida and A. Yoshida (2009) Genetic engineering of Candida utilis yeast for efficient production of L‐lactic acid. Biosci. Biotechnol. Biochem., 73: 1818–1824.
- Ishi K., Y. Nakajima and T. Iwakura (1972) Citric acid by fermenetation of waste glucose. German Patent 2: 157–847.
- Jabalquinto A.M., F.D. Gonzales‐Nilo, M. Laivenieks, M. Cabezas, J. G. Zeikus and E. Cardemil (2004) Anaerobiospirillum succiniciproducens phosphoenolpyruvate carboxykinase, mutagenesis at metal site 1. Biochemie, 86: 47–51.
- Jang Y.S., B. Kim, J.H. Shin et al. (2012) Bio‐based production of C2–C6 platform chemicals. Biotechnol. Bioeng.,109: 2437–2459.
- Kamzolova S.V., A.I. Yusupova, E.G. Dedyukhina, T.I. Chistyakova, T.M. Kozyreva and I.G. Morgunov (2009) Succinic acid synthesis by ethanol grown yeast. Food Technol. Biotechnol., 47: 144–152.
- Kapelli O., M. Muller and A. Fiechter (1978) Chemical and structural alterations at cell surface of Candida tropicalis, induced by hydrocarbon substrate. J. Bacteriol., 133: 952–958.
- Kapoor K.K., K. Chaudhary and P. Tauro (1983) Citric acid. In: Reed G. (ed.) Prescott and Dunn’s Industrial Microbiology. Basingstoke: Macmillan, pp. 709–747.
- Karow E.O. and S.A. Waksman (1947) Production of citric acid in submerged culture. Ind. Eng. Chem., 39: 821–825.
- Kautola H. and Y.Y. Linko (1989) Fumaric acid production from xylose by immobilized Rhizopus arrhizus cells. Appl. Microbiol. Biotechnol., 31: 448–452.
- Kawamura D., M. Furuhashi, O. Saito and H. Matsui (1981) Production of itaconic acid by fermentation. Japanese Patent 56,137,893.
- Khan M.K., P.K. Mishra, U. Kumar, M. Mishra, T. Sharma and V. Permar (2009) Production of succinic acid of related microbial strain. Glob. J. Biotechnol. Biochem., 4: 47–50.
- Kim D.M. and H.S. Kim (1992) Continuous production of gluconic acid and sorbitol from Jerusalem artichoke and glucose using an oxidoreductase of Zymomonas mobilis and inulinase. Biotechnol. Bioeng., 39: 336–342.
- Kin R., T. Sai and S. So (1998) Itaconate copolymer with quadratic nonlinear optical characteristic. Japanese Patent 10,293,331.
- Kinoshita K. (1932) Uber die Production von Itaconsaure and Mannit durch einen neuen Schimmelpiz Aspergillus itacnicus. Acta Phytochim., 5: 271–287.
- Kiryu K., K. Yamauchi, A. Masuyama et al. (2012) Optimization of lactobionic acid production by Acetobacter orientalis isolated from Caucasian fermented milk Caspian sea yogurt. Biosci. Biotechnol. Biochem., 76: 361–363.
- Kondrashova M.N. (1991) Interaction of carbonic acid reamination and oxidation processes at various functional states of tissue. Biokhimiia (Moscow), 56: 388–405.
- Kondrashova M.N. (2002) Hormone‐similar action of succinic acid (in Russian). Vopr. Biol. Med. Pharmac. Chem., 1: 7–15.
- Kondrashova M.N., E.L. Maevsky, E.V. Grigorenko et al. (2005) The interaction of Krebs cycle with sympathetic and parasympathetic nervous system. Appl. Microbiol. Biotechnol., 83: 1027–1034.
- Kroya Fermentation Industry (1970) Citric acid prepared by fermentation. British Patent 1,187,610.
- Kubicek C.P. and M. Rohr (1986) Citric acid fermentation. Crit. Rev. Biotechnol., 3: 331–373.
- Kumar P.K.R., A. Singh and K. Schugerl (1991) Formation of acetic acid from Cellulosic substrates. Appl. Microbiol. Biotechnol., 34: 570–572.
- Kundu, P. and A. Das (1984) Utilization of cheap carbohydrate sources for production of calcium gluconate by Penicillium funiculosum mutant MN 238. Ind. J. Exper. Biol., 23: 279–281.
- Kusai K., I. Sekuzu, B. Hagihara, K. Okunuki, S. Yamauchi and M. Nakai (1960) Crystallisation of glucose oxidase from Penicillium amagasakiense. Biochim. Biophys. Acta, 40: 555–557.
- Lancashire E (1969) Soap compositions having improved curd dispersing properties. US Patent 3: 454–500.
- Lee P.C., W.G. Lee, S.Y. Lee and H.N. Chang (2001) Succinic acid production with reduced byproduct formation in the fermentation of Anaerobiospirillum succiniciproducens using glycerol as a carbon source. Biotechnol. Bioeng., 72: 41–48.
- Lee P.C., S.Y. Lee, S.H. Hong, H.N. Chang and S.C. Park (2003) Biological conversion of wood hydrolysate to succinic acid by Anaerobiospirillum succiniciproducens. Biotechnol. Lett., 25: 111–114.
- Li J., X.Y. Zheng, X.J. Fang et al. (2011) A complete industrial system for economical succinic acid production by Actinobacillus succinogenes. Biores. Technol., 102: 6147–6152.
- Llmen M., K. Koivuranta, L. Ruohonen, P. Suominen and M. Penttila (2007) Efficient production of L‐lactic acid from xylose by Pichia stipitis. Appl. Environ. Microbiol., 73: 117–123.
- Lockwood L.B., G.E. Ward and O.E. May (1936) The physiology of Rhizopus oryzae. J. Agric. Res., 53: 849–857.
- Loffhagen N. and W. Babel (1993) The influence of energy deficiency – imposing conditions on the capacities of Acetobacter methanolicus to oxidize glucose and to produce gluconic acid. Acta Biotechnol., 13: 251–256.
- Lu M., J.D. Brooks and I.S. Maddox (1997) Citric acid production by solid‐state fermentation in a packed bed reactor using Aspergillus niger. Enzyme Microbiol. Technol., 21: 392–397.
- Maas R.H., R.R. Bakker, M.L. Jansen, D. Visser, E. de Jong and G. Eggink (2008) Lactic acid production from limetreated wheat straw by Bacillus coagulans: neutralization of acid by fed‐batch addition of alkaline substrate. Appl. Microbiol. Biotechnol., 78: 751–758.
- Mandeva R.D., I.T. Ermakova and A.B. Lozinov (1981) Metabolite excretion by yeasts of the genus Candida in media lacking sources N, P, S, or Mg and having different carbon sources. Mikrobiologiia, 50: 62–68.
- Martinez‐Espinoza A.D., M.D. Garcia‐Pedrajas and S.E. Gold (2002) The ustilaginales as plant pests and model systems. Fungal Genet. Biol., 35: 1–20.
- Masuo E., F. Shima and K. Yoshida (1952) Oxidation of carbohydrates by Pseudomonas. II. Formation of lactobionic acid from lactose. Ann. Rep. Shionogi. Res. Lab., 1: 136–141.
- May O.E., H.T. Herrick, G. Thom and N.B. Church (1927) The production of gluconic acid by Penicillium luteum purpurogenum Group I. J. Biol. Chem., 75: 417–422.
- Miall M. and G.F. Parker (1975) Continuous preparation of citric acid by Candida lipolytica. German Patent 2,429,224.
- Moyer A.J. (1940) Process for gluconic acid production. US Patent 2,351,500.
- Murakami H., A. Seko, M. Azumi et al. (2006) Microbial conversion of lactose to lactobionic acid by resting cells of Burkholderia cepacia No. 24. J. Appl. Glycosci., 53: 7–11.
- Murakami H., T. Kiryu, T. Kiso and H. Nakano (2008) Production of calcium lactobionate by a lactose‐oxidizing enzyme from Paraconiothyrium sp. KD‐3. J. Appl. Glycosci., 55: 127–132.
- Nakajima Kambe T., T. Nozue, M. Mukouyama and T. Nakahara (1997) Bioconversion of maleic acid to fumaric acid by Pseudomonas alcaligenes strain XD‐1. J. Ferment. Bioeng., 84: 165–168.
- Narayanan N., P.K. Roychoudhury and A. Srivastava (2004) L (þ) lactic acid fermentation and its product polymerization. Electron. J. Biotechnol., 7: 167–178.
- Nishimura T., A.A. Vertes, Y. Shinoda, M. Inui and H. Yukawa (2007) Anaerobic growth of Corynebacterium glutamicum using nitrate as a terminal electron acceptor. Appl. Microbiol. Biotechnol., 75: 889–897.
- Nolasco‐Hipolito C., O.C. Zarrabal, R.M. Kamaldin, L. Teck‐Yee, S. Lihan and K.B. Bujang (2012) Lactic acid production by Enteroccocus faecium in liquefied sago starch. AMB Express, 2: 1–9.
- Oh M.J., Y.J. Park and S.K. Lee (1973) Citric acid production by Hansenula anamola. Hanguk Sikpum Kawahakhoe Chi, 5: 215–223.
- Okino S., M. Inui and H. Yukawa (2005) Production of organic acids by Corynebacterium glutamicum under oxygen deprivation. Appl. Microbiol. Biotechnol., 68: 475–480.
- Omar S.H. and H.J. Rehm (1980) Physiology and metabolism of two alkane oxidizing and oxidizing and citric acid producing strains of Candida parapsilosis. Eur. J. Appl. Microbiol. Biotechnol., 11: 42–49.
- Osawa F., T. Fujii, T. Nishida et al. (2009) Efficient production of L‐lactic acid by Crabtree‐negative yeast Candida boidinii. Yeast, 26: 485–496.
- Otsuka K. (1961) Cis‐trans isomerase – isomerisation from maleic acid to fumaric acid. Agr. Biol. Chem., 25: 726–730.
- Pecota D.C., V. Rajgarhia and N.A. da Silva (2007) Sequential gene integration for the engineering of Kluyveromyces marxianus. J. Biotechnol., 127: 408–416.
- Peleg Y., E. Battat, M.C. Scrutton and I. Goldberg (1989) Isoenzyme pattern and subcellular localization of enzymes involved in fumaric acid accumulation by Rhizopus oryzae. Appl. Microbiol. Biotechnol., 32: 334–339.
- Perlman D. (1949) Studies on the growth and metabolism of Polyporus anceps in submerged culture. Am. J. Bot., 36: 180–184.
- Perwozwansky W.W. (1939) Formation of gluconic acid during the oxidation of glucose by bacteria. Microbiology (USSR), 8: 149–159.
- Petruccioli M., P. Piccioni, M. Fenice and F. Federici (1994) Glucose oxidase, catalase and gluconic acid production by immobilized mycelium of Penicillium variabile P16. Biotechnol. Lett., 16: 939–942.
- Pfeifer V.F., C. Vojnovich and E.N. Heger (1952) Itaconic acid by fermentation with Aspergillus terreus. Ind. Eng. Chem., 44: 2975–2980.
- Pintado J., A. Torrado, M.P. Gonzalez and M.A. Murado (1998) Optimization of nutrient concentration for citric acid production by solid state culture of Aspergillus niger on polyurethane foams. Enzyme Microbiol. Technol., 23: 149–156.
- Pitzl G. (1951) Vinylidene chloride interpolymer as a coating for regenerated cellulose film. US Patent 2,570‐478.
- Playne M.J. (1985) Propionic and butyric acids. In: Moo‐Young, M. (ed.) Comprehensive Biotechnology: The Principles, Applications and Regulations of Biotechnology in Industry, Agriculture and Medicine. Oxford: Pergamon, pp. 731–759.
- Porro D., M.M. Bianchi, L. Brambilla et al. (1999) Replacement of a metabolic pathway for large scale production of lactic acid from engineered yeasts. Appl. Environ. Microbiol., 65: 4211–4215.
- Postma E., C. Verduyn, W.A. Scheffers and J.P. van Dijken (1989) Enzymatic analysis of the Crabtree effect in glucose limited chemostat cultures of Saccharomyces cerevisiae. Appl. Environ. Microbiol., 55: 468–477.
- Raab A.M. and C. Lang (2011) Oxidative versus reductive succinic acid production in the yeast Saccharomyces cerevisiae. Bioeng. Bugs., 2: 120–123.
- Rane K.D. and K.A. Sims (1993) Production of citric acid by Candida lipolytica Y‐1095: effect of glucose concentration on yield and productivity. Enzyme Microbiol. Technol., 15: 646–651.
- Roa Engel C.A., A.J.J. Straathof and T.W. Zijlmans (2008) Fumaric acid production by fermentation. Appl. Microbiol. Biotechnol., 78: 379–389.
- Rodriguez H., T. Gonzalez, I. Goire and Y. Bashan (2004) Gluconic acid production and phosphate solubilisation by the plant growth promoting bacterium Azospirillum spp. Naturwissenschaften, 91: 552–555.
- Rohr M., C.P. Kubicek and J. Kominek (1983) Gluconic acid. Biotechnology, 3: 455–465.
- Roissart H. and F.M. Luquet (1994) Bacteries Lactiques: Aspects Fondamentaux et Technologiques. Paris: Lorica.
- Roukas T. (1991) Production of citric acid from beet molasses by immobilized cells of Aspergillus niger. J. Food Sci., 56: 878–880.
- Saayman M., H.J.J. van Vuuren, W.H. van Zyl and M. Viljoen‐Bloom (2000) Differential uptake of fumarate by Candida utilis and Schizosaccharomyces pombe. Appl. Microbiol. Biotechnol., 54: 792–798.
- Sardinas J.L. (1972) Fermentative production of citric acid. French Patent 2,113,668.
- Sato M., T. Nakahara and K. Yamada (1972) Fermentative production of succinic acid from n‐paraffin. Agric. Biol. Chem., 36: 1969–1974.
- Satory M., M. Furlinger, D. Haltrich, K.D. Kulbe, F. Pittner and B. Nidetzky (1997) Continuous enzymatic production of lactobionic acid using glucose‐fructose oxidoreductase in an ultrafiltration membrane reactor. Biotechnol. Lett., 19: 1205–1208.
- Sauer M., D. Porro, D. Mattanovich and P. Branduardi (2008) Microbial production of organic acids: expanding the markets. Trends Biotechnol., 26: 100–108.
- Schaffer S. and A. Burkovski (2005) Proteomics. In: Eggeling L and M. Bott (eds) Handbook of Corynebacterium glutamicum. Boca Raton: CRC Press, pp. 99–118.
- Scher M. and W.J. Lennarz (1969) Maleate isomerase. J. Biol. Chem., 244: 1878–1872.
- Senthuran A., V. Senthuran, B. Mattiasson and R. Kaul (1997) Lactic acid fermentation in a recycle batch reactor using immoblized Lactobacillus casei. Biotechnol. Bioeng., 55: 841–853.
- Shiraishi F., K. Kawakami, S. Kono, A. Tamura, S. Tsuruta and K. Kusunoki (1989) Characterisation of production of free gluconic acid by Gluconobacter suboxydans adsorbed on ceramic honeycomb monolith. Biotechnol. Bioeng., 33: 1413–1418.
- Singh A., P.K.R. Kumar and K. Schugerl (1992) Bioconversion of cellulosic materials to ethanol by filamentous fungi. Adv. Biochem. Eng. Biotechnol., 45: 29–55.
- Song C.W., D.I. Kim, S. Choi, J.W. Jang and S.Y. Lee (2013) Metabolic engineering of Escherichia coli for the production of fumaric acid. Biotechnol. Bioeng., 110: 2025–2034.
- Song H. and S.Y. Lee (2006) Production of succinic acid by bacterial fermentation. Enzyme Microbiol. Technol., 39: 352–361.
- Sponholz W.R. (1993) Wine spoilage by microorganisms. In: Fleet G.H. (ed.) Wine Microbiology and Biotechnology. Amsterdam: Harwood Academic, pp. 395–420.
- Steven A.W. and A.G. Bonita (1991) Propionic acid production by a propionic acid tolerant strain of Propionibacterium acidipropionici in batch and semicontinuous fermentation. Appl. Environ. Microbiol., 57: 2821–2828.
- Stodola F.H. and B.L. Lockwood (1947) The oxidation of lactose and maltose to bionic acids by Pseudomonas. J. Biol. Chem., 171: 213–221.
- Suzuki T., Y. Sumino, S. Akiyam and H. Fukuda (1974) Method for producing citric acid. US Patemt 3,801,455.
- Tabuchi T. (1981) Itaconic acid fermentation by a yeast belonging to the genus Candida. Agric. Biol. Chem., 45: 475–479.
- Takamura Y., T. Takamura, M. Soejima and T. Uemura (1969) Studies on induced synthesis of maleate cis‐trans isomerase by malonate. Purification and properties of maleate cis‐trans isomerase indiced by malonate. Agric. Biol. Chem., 33: 718–728.
- Takeno S., J. Ohnishi, T. Komatsu, T. Masaki, K. Sen and M. Ikeda (2007) Growth and potential for amino acid production by nitrate respiration in Corynebacterium glutamicum. Appl. Microbiol. Biotechnol., 75: 1173–1182.
- Tate B.E. (1981) Itaconic acid and derivatives. In: Mark H.F., D.F. Othmer, C.G. Overberger and G.T. Seaborg (eds) Encyclopedia of Chemical Technology. New York: Wiley, pp. 865–873.
- Temash S. and Z.A. Olama (1999) Optimization of glucose oxides and gluconic acid production by Penicillium purpurescensusing banana waste under solid state fermentation. J. Med. Res., 20: 54–62.
- Tomlinson G.A., M.P. Strohm and L.I. Hochstein (1978) The metabolism of carbohydrates by extremely halophilic bacteria: the identification of lactobionic acid as a product of lactose metabolism by Halobacterium saccharovorum. Can. J. Microbiol., 24: 898–903.
- Tran C.T., L.I. Sly and D.A. Mitchell (1998) Selection of a strain of Aspergillus for the production of citric acid from pineapple waste in solid state fermentation. World J. Microbiol. Biotechnol., 14: 399–404.
- Uchio R., I. Maeyashiki, K. Kikuchi and Y. Hirose (1975) Citric acid production by yeast. Japanese Patent 76,63,059.
- Vandenberghe L.P.S., C.R. Soccol A. Pandey, and J.M. Lebeault (1999) Review: Microbial production of citric acid. Braz. Arch. Biol. Technol., 42: 263–276.
- Vegas C., E. Mateo and A. Gonzalez (2010) Population dynamics of acetic acid bacteria during traditional wine vinegar production. Int. J. Food Microbiol., 138: 130–136.
- Velizarov S. and V. Beschkov (1994) Production of free gluconic acid by cells of Gluconobacter oxydans. Biotechnol. Lett., 16: 715–720.
- Villecourt P. and H. Blachere (1955) Oxidation of lactose in lactobionic acid by pathogenic or saprophytic bacteria (Bacterium anitratum). Ann. Inst. Pasteur (Paris), 88: 523–526.
- Wee Y.J., J.N. Kim and H.W. Ryu (2006) Biotechnological production of lactic acid and its recent applications. Food Technol. Biotechnol., 442: 163–172.
- Wenge F. and A.P. Mathews (1999) Lactic acid production from lactose by Lactobacillus plantarum: kinetic model and effects of pH, substrate, and oxygen. Biochem. Engin. J., 3: 163–170.
- West D. (2004) AHA wrinkle creams come of age. Chem. Week, 166: 20.
- William E.L., P.K. Cletus and M.K. Tsung (2006) Production of itaconic acid by Pseudozyma Antarctica NRRL Y‐7808 under nitrogen‐limited growth conditions. Enz. Microbiol. Technol., 39: 824–827.
- Willke T. and K.D. Vorlop (2001) Biotechnological production of itaconic acid. Appl. Microbiol. Biotechnol., 56: 289–295.
- Wojtatowicz M., G.L. Marchin and L.E. Erickson (1993) Attempts to improve strain A‐10 of Yarrow lipolytica for citric acid production from n‐paraffins. Proc. Biochem., 28: 453–460.
- Xi Y.L., K.Q. Chen, R. Xu et al. (2012) Effect of biotin and a similar compound on succinic acid fermentation by Actinobacillus succinogenes in a chemically defined medium. Biochem. Eng. J., 69: 87–92.
- Yamada Y., Y. Okada and K. Kondo (1976) Isolation and characterization of polarly flagellated intermediate strains in acetic acid bacteria. J. Gen. Appl. Microbiol., 22: 237–245.
- Yamada Y., K.I. Hoshino and T. Ishikawa (1997) The phylogeny of acetic acid bacteria based on the partial sequences of 16S ribosomal RNA: the elevation of the subgenus Gluconoacetobacter to the generic level. Biosci. Biotechnol. Biochem., 61: 1244–1251.
- Yamada Y., P. Yukpan and H.T.L. Vu (2012) Description of Komagataeibacter gen. nov.with proposals of new combinations (Acetobacteraceae). J. Gen. Appl. Microbiol., 58: 397–404.
- Yasumitsu M., T. Ooi and S. Kinoshita (2000) Production of lactobionic acid from whey by Pseudomonas sp. LS13‐1. Biotechnol. Lett., 22: 427–430.
- Yokoya F. (1992) Citric acid production. In: Industrial Fermentation Series. Brazil: Campinas.
- Yuzbashev T.V., E.Y. Yuzbasheva, T.I. Sobolevskaya et al. (2010) Production of succinic acid at low pH by a recombinant strain of the aerobic yeast Yarrowia lipolytica. Biotechnol. Bioeng., 7: 673–682.
- Zeikus J.G., M.K. Jain and P. Elankovan (1999) Biotechnology of succinic acid production and markets for derived industrial products. Appl. Microbiol. Biotechnol., 51: 545–552.
- Zhuge X., L. Liu, H.D. Shin et al. (2013) Development of a Propionibacterium, Escherichia coli shuttle vector for metabolic engineering of Propionibacterium jensenii, an efficient producer of propionic acid. Appl. Environ. Microbiol., 79: 4595–4602.
- Znad A., J. Markos and V. Bales (2003) Production of gluconic acid by Aspergillus niger growth and non‐growth conditions. Food Technol., 34: 1078–1080.