10
Production of High‐Quality Probiotics by Fermentation
Marimuthu Anandharaj1, Rizwana Parveen Rani2, and Manas R. Swain3*
1 Agricultural Biotechnology Research Center, Academia Sinica, Taipei, Taiwan
2 Gandhigram Rural Institute, Department of Biology, Tamil Nadu, India
3 Department of Biotechnology, Indian Institute of Technology Madras, Chennai, India
*Corresponding author e‐mail: manas.swain@gmail.com
Introduction
Over recent years, our knowledge of intestinal microbiota and modulating factors, and interest in supplementing various types of food products with probiotic bacteria has grown significantly. Probiotics are defined as live micro‐organisms or a product containing viable micro‐organisms in sufficient numbers to alter the microflora (mainly lactobacilli and bifidobacteria) which when administered in adequate amounts confer a health benefit on the host (Lacroix and Yildirim 2007). The most extensively used probiotics are lactobacilli, bifidobacteria, bacilli, and yeasts (Hasler 2002 ; Kołozyn‐Krajewskaa and Dolatowski 2012). These probiotic strains, which are the major microbiota of the human gut, may provide protection against gastrointestinal disorders including gastrointestinal infections and inflammatory bowel diseases (Mitsuoka 1982 ; Salminen et al. 1998). Probiotic lactic acid bacteria are thought to be beneficial to human health and therefore, a wide variety of lactic acid bacteria strains are available in both traditional fermented foods and in supplement form (Kourkoutas et al. 2005). A major development has been foods fortified with probiotics and prebiotics, which enhance health‐promoting microbiota in the intestine when consumed in a regular diet (Hasler 2002).
In recent years, functional food and beverage consumption has increased tremendously and the global market has grown from $33 billion in 2000 to $176.7 billion in 2013, which accounts for 5% of the overall food market (Granato et al. 2010). In the global functional food market, 60–70% are probiotic foods (Holzapfel 2006 ; Kołozyn‐Krajewskaa and Dolatowski 2012 ; Stanton et al. 2001).
The viability of probiotics is a key parameter which affects therapeutic benefits and varies as a function of the strain and health effect desired. In general, a minimum level of more than 106 viable probiotic bacteria per milliliter or gram of food product is accepted. Another issue to be addressed is the viability of probiotic micro‐organisms in the product during its manufacture and shelf‐life and its resistance in upper gastrointestinal tract conditions (Roy 2011). Strains which are being isolated from human or animal intestine are able to proliferate outside their natural environment and therefore exhibit reduced technological properties (Ross et al. 2005). Furthermore, probiotic micro‐organisms must exhibit their resistance against the acidic environment of the stomach and bile secreted in the duodenum in order to achieve high survival rates in the gastrointestinal tract (Chassard et al. 2011).
Currently, commercial strains are largely selected for their technological properties, ruling out some strains with promising health properties. Many strains of intestinal origin are difficult to propagate and high survival is important for both economic reasons and health effects. In addition, more efficient technologies could lead to greater product efficacy and strain diversification with the development of technologically unsuitable strains. Some fermentation technologies, which include fed‐batch, continuous fermentation, membrane bioreactors, and immobilized cell technology, are considered as suitable for proliferation of probiotic bacteria. They are designed to produce increased cell yield and productivity and to decrease the downstream processing capacity required to harvest the biomass. The limitations of these technologies include operational difficulties in industrial conditions due to the susceptibility of continuous cultures to contamination; subjecting sensitive cells to low nutrient concentration, oxygen, and osmotic and mechanical stresses in membrane bioreactors; and changes in the growth, morphology, and physiology of bacterial cells in immobilized reactors (Lacroix and Yildirim 2007).
This chapter discusses the selection criteria for an assortment of probiotic strains and the latest developments in fermentation technologies for producing probiotic bacteria as well as potential new approaches for enhancing the performance of these fastidious organisms during fermentation, downstream processing, and utilization in commercial products, and for improving functionality in the gut. Processes that include sublethal stress applications during cell production and new fermentation technologies, such as immobilized cell biofilm‐type fermentations, are promising in this respect and could be used to profile cell physiology to optimize survival and functionality in the gut.
Assessment of Probiotic Functionality
Although there is evidence that probiotics can play a role in disease prevention and health promotion, safety considerations should be paramount. In addition, technological performance of probiotics has to be tested and must be suitable for large‐scale applications. Safety of a probiotic strain can be assessed by three approaches: studies of the strain’s intrinsic properties, studies of the pharmacokinetics of the strain (survival, activity in the intestine, dose–response relationships, fecal and mucosal recovery) and studies looking for interactions between the strain and the host. The selection criteria for potential probiotic strains are illustrated in Figure 10.1.
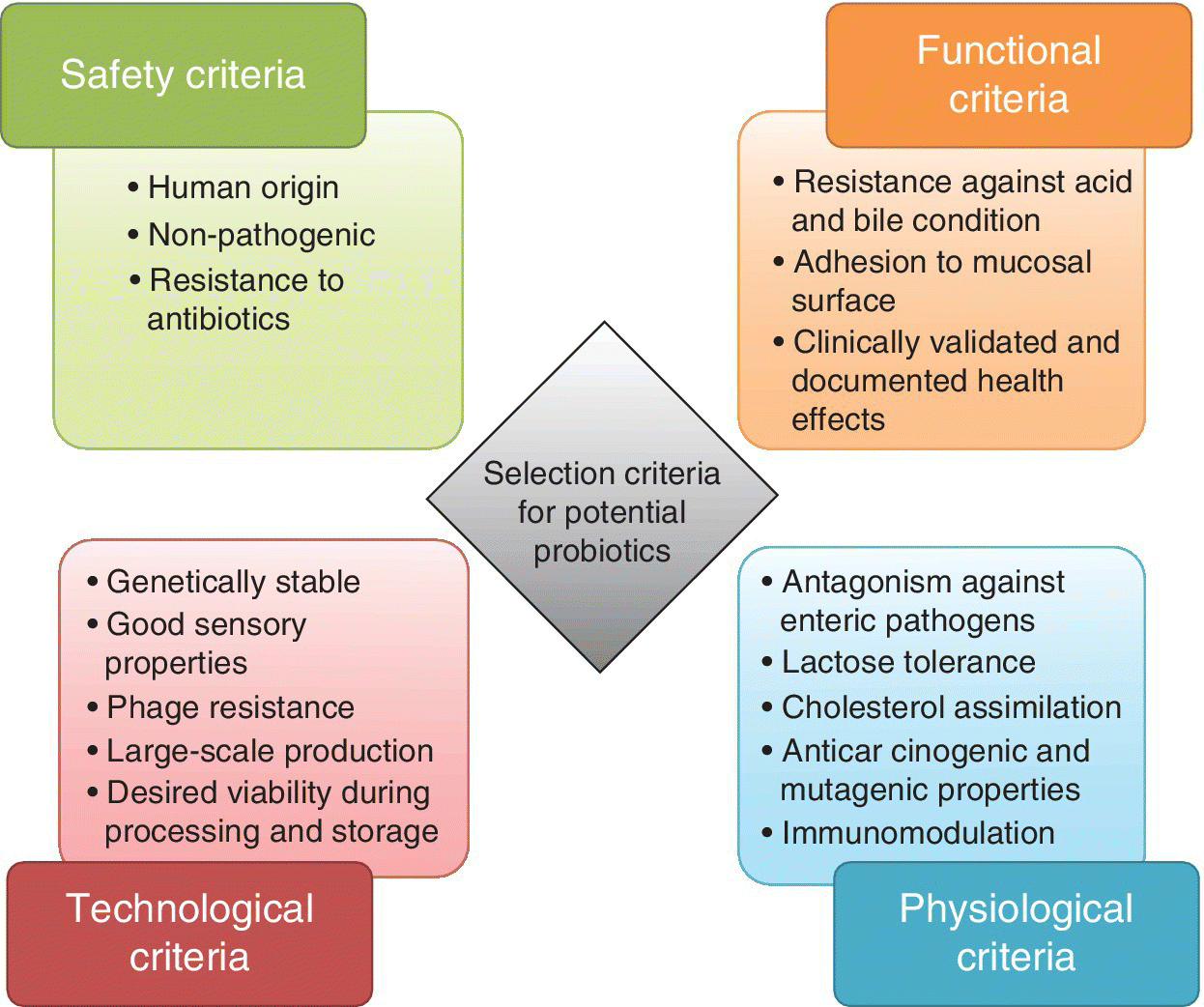
Figure 10.1 Desirable selection criteria for potential probiotic micro‐organisms.
A probiotic micro‐organism should be able to withstand a range of harsh physicochemical factors during transit through the stomach and small intestine to exhibit its beneficial effects and it should be able to persist in the gut, as outlined in a joint report by the FAO and WHO in 2002 (FAO/WHO 2002).
Great viability and activity of probiotics are considered mandatory for optimal functionality. However, quite a few studies have shown that non‐viable probiotics can have positive effects such as immune modulation and carcinogen binding. Therefore, for certain probiotic strains it might be enough that they grow well during initial production processes (to obtain sufficient cell numbers in the product) but they do not necessarily need to maintain good viability during storage (Mattila‐Sandholm et al. 2002).
The food industry has to fulfill consumer demand to succeed in increasing the consumption of functional probiotic products. All probiotic foods should be safe and have good sensory properties. They should also include specific probiotic strains at a suitable level during storage. They must first be able to be manufactured under industrial conditions, then survive and retain their functionality during storage as frozen or freeze‐dried cultures, and also in the food products into which they are finally formulated. Some commercially available probiotic products are listed in Table 10.1. They must be able to be incorporated into foods without producing off‐flavors or textures. The packaging materials used and the storage conditions are vital for the quality of products containing probiotic bacteria (Saarela et al. 2000).
Table 10.1 Some important commercially used probiotic strains, manufacturers, and products.
Source: Adapted from Muller et al. (2009), Krishnakumar and Gordon (2001), Playne et al. (2003), Holm (2003).
| Species | Strain | Manufacturer | Product |
| L. acidophilus | LA‐1/LA‐5 NCFM La1 DDS‐1 SBT‐2062 LA5 |
Chr. Hansen Rhodia Nestle Nebraska Cultures Snow Brand Milk Products Muller |
TopOntbijt, Nu Trish, Biospega, Snow yoghurt + 2, Vitality, YoMi |
| L. delbrueckii subsp. bulgaricus | 2038 | Meiji | Yoghurt |
| L. casei immunitas | Danone | ||
| L. casei | DN‐114 001 Shirota |
Danone Yakult |
Yakult, DanActive, Actimel, Joie |
| L. plantarum | 299v (DSM 9843) Lp01 |
Probi AB | |
| L. rhamnosus | GG GR‐1 LB21 LC‐705 |
Campina Urex Biotech Essum AB Valio |
Vifit, Femdophilus, Bio Profit, Culturelle, Gefilac, RepHresh Pro‐B |
| L. reuteri | SD2112/MM2 RC‐14 ATCC 55730 |
Biogaia Chr. Hansen Biogai |
Femdophilus, Stonyfield Farm yoghurts |
| L. paracasei | CRL 431 | Chr. Hansen Nestlé, Nutricia |
Chamyto, Fyos |
| L. fermentum | RC‐14 | Urex Biotech | Femdophilus, RepHresh Pro‐B |
| L. helveticus | B02 | ||
| B. lactis | DR10™ Bb‐12, Bb‐02, LaftiTM, B94 DR10/HOWARU |
Parmalat Chr. Hansen DSM Danisco |
Active‐piu, Nu Trish, YoMi, Yo‐plus yoghurt, TopOntbijt, Teddy |
| B. animalis | DN173 010 | Danone | Activia yoghurt |
| B. bifidum | BF2 | Cell Biotech Europe | |
| B. longum | BB536 SBT‐2928 UCC 35624 |
Morinaga Snow Brand Milk Products UCCork |
Bifidus yoghurt |
| B. infantis | 35264 744 Immunitas |
Procter and Gamble | Align |
| B. adolescentis | ATCC 15703, 94‐BIM |
Assessment of probiotic strains must focus on microbial activity following passage through the stomach as probiotics need to be functionally active in the gut. This could include physiological profiling of probiotics, i.e., bar‐coding, by “omics” technologies. Bar‐coding of cells involves characterization of the viable and active physiological state of a putative probiotic bacterium. During the production process, this physiological state is compared to the physiological state of the same bacterium at the final stage of fermentation. Transcriptomics, proteomics, or metabolomics can be used to compare physiological states. It is suggested that by bar‐coding, new fermentation conditions can be developed that better mimic the active physiological state of a probiotic bacterium at its place of action (e.g., in various parts of the gastrointestinal tract) (Ledeboer et al. 1999).
Stress Factors Encountered by Probiotics
In order to achieve health benefits in the living system, some specific requirements regarding food products need be fulfilled. First, probiotics must have the ability to resist various stresses during the manufacturing process; next, they should retain their viability under very low storage temperatures. Several features influence the viability of probiotic micro‐organisms in food products during production and storage periods, including pH, acidity, dissolved oxygen, redox potential, H2O2, bacteriocins, short chain fatty acids, flavoring agents, competitive microbes, packaging materials and packaging conditions, rate and concentration of inoculation, fermentation conditions, microencapsulation, milk solid non‐fat content, supplementation of milk with nutrients, incubation temperature and timing, storage temperature, carbonation, salt addition, sugar and sweeteners, cooling rate of the product and scale of production (Mortazavian et al. 2012).
There are two possible outcomes when probiotic micro‐organisms respond to stress (Yousef and Juneja 2003):
- initiation of a sequence of proteins that repair damage, maintain the cell, or exclude the stress agent
- transient elevation in resistance or tolerance to harmful factors.
Probiotic bacteria may encounter various stress condition during transition to the gastrointestinal tract following ingestion. However, probiotics, like other bacteria, have the ability to develop defense mechanisms to withstand these stressful environmental conditions. The following section deals with the various stress factors encountered by probiotics and the cellular mechanisms used to overcome them.
Oxidative Stress (OxS)
Probiotic micro‐organisms can be exposed to different levels of oxygen. Strict anaerobic conditions cannot be easily maintained during production, downstream processing, and storage of probiotic bacteria. Probiotic cultures, generally isolated from the human gut, can be classified according to their oxygen tolerance as microaerophilic (e.g., Lactobacillus acidophilus) and strictly anaerobic (most of the Bifidobacterium spp.). However, oxygen sensitivity is species and strain dependent (Talwalkar and Kailasapathy 2004).
Oxidative stress may be defined as the disproportion between cellular production of oxidants (reactive oxygen species (ROS)) and antioxidative capacity and is known to cause acinar injury in the early course of acute pancreatitis (Rau et al. 2000). In living micro‐organisms the major ROS are superoxide radicals, lipid peroxyl radicals, hydroxyl radicals and non‐radical H2O2, and reactive nitrogen species (RNS) are nitric oxide and non‐radical peroxynitrite. These hydroxyl radicals are rapidly generated through the Fenton cycle, in which free iron reacts with H2O2. Most of the mentioned reactive species (RS) come from endogenous sources as by‐products of normal essential metabolic processes, while exogenous sources involve exposure to cigarette smoke, environmental pollutants, radiation, drugs, bacterial infections, excess of food iron, unbalanced intestinal microflora, etc. Several diseases are associated with the toxic effects of the transition metals (iron, copper, cadmium) (Halliwell and Gutteridge 1999).
A special probiotic, LfME‐3, has good potential in cardiovascular health management. LfME‐3, an antioxidative, antiatherogenic and antimicrobial probiotic, decreases OxS levels in the human body. Foodstuffs enriched with this probiotic decrease oxidized LDL, increase HDL, modulate postprandial lipid profile and OxS, and decrease the level of 8‐isoprostanes in urine (markers of systemic OxS) and the overall OxS load, indicating an atherogenic potential (Kullisaar et al. 2003, 2011 ; Mikelsaar 2009; Songisepp et al. 2005).
Several studies have shown that LfME‐3 alleviates inflammation and OxS‐associated shifts in gut, skin, and blood (Kaur et al. 2008 ; Kullisaar et al. 2003). This occurs via complicated cross‐talk between probiotic and host body cells via the integrated influence of several factors of LfME‐3, including having complete glutathione system, the expression of antioxidative enzymes, the production of CLA and NO, etc. (Kullisaar et al. 2010, 2011 ; Mikelsaar and Zilmer 2009).
Some mechanisms have been proposed to explain the tolerance of lactic acid bacteria to oxygen toxicity, including reduction of the intracellular redox potential, repair of oxidative damage by overexpression of chaperones, and protection of potential targets (e.g., proteins) from oxidation (van de Guchte et al. 2002). The development of molecular tools and the increasing number of available genome sequences have permitted identification of proteins or genes involved in these mechanisms but the role of some of them remains unclear (Dubbs and Mongkolsuk 2007 ; Klijn et al. 2005 ; O’Connell‐Motherway et al. 2000).
Acid Stress
Acid stress is the primary challenge faced by probiotics due to the acidic pH (>2) in the stomach. When cells are exposed to the low pH, proton accumulation inside the cell may alter the proton motive force (PMF) across the cell membrane. The changes in PMF cause cell membrane and structural damage, and acid stress also causes damage to nucleic acids and proteins (Collado et al. 2005). Bifidobacterium strains are less acid tolerant than Lactobacillus strains, and this is reflected by their poor survival in human gastric juice. Bifidobacteria are more resistant to acetate (acetic acid) rather than Hcl in milk‐based media, since acetate is the major by‐product after Bifidobacterium fermentation (Borgstrom et al. 1957). Bacillus clausii spore supplementation of antibiotic therapies has demonstrated efficacy, owing to the fact that the spores are resistant to acid stress and drugs (Nista et al. 2004).
The lack of standard procedures for evaluating tolerance to gastrointestinal conditions makes comparison difficult. In general, tolerance to gastrointestinal conditions is low; as a consequence, several methodologies, including those based on stress adaptation mechanisms of probiotic bacteria, are being considered as possible mechanisms to improve their acid and bile tolerance (Collado 2005; Noriega et al. 2004).
Bile Stress
Bile plays a basic role in the defense mechanisms of the intestinal tract and its inhibitory effect is determined primarily by bile salt concentration. Therefore, bile tolerance is considered as an important characteristic of Lactobacillus strains, which enables them to survive, grow, and exert their action in gastrointestinal transit (Argyri et al. 2013). According to Sanders et al. (1996), Lactobacillus strains which can to grow and metabolize in normal physical bile concentration also survive in the human gastrointestinal tract. In addition, Gänzle et al. (1999) and du Toit et al. (1998) proposed that some components of food could protect and promote the resistance of strains to bile salt. Gilliland et al. (1984) reported that a culture of L. acidophilus with high bile tolerance was significantly better than a strain with low bile tolerance for increasing the number of facultative lactobacilli in the upper part of the small intestines of calves.
Tanaka et al. (1999) suggested that the presence of bile salt hydrolase (BSH) had a selective advantage for the probiotic micro‐organism in bile salt‐rich environments. BSH activity benefits them by increasing resistance to conjugated bile salts and survival in the gastrointestinal tract for colonization (Jones et al. 2008 ; Ridlon et al. 2006).
Heat Stress
During the production process, the probiotic micro‐organism may be exposed to heat. Several researchers have studied the effect of heat on physiological functions and the induction of a stress response by probiotics.The heat shock response is a complex process, involving various heat shock proteins (HSPs) with different roles in physiology, including chaperone activity, ribosomal stability, temperature sensing, and regulation of ribosome functions (de Angelis and Gobbetti 2004). The heat shock response by probiotics differs from strain to strain. When exposed to higher temperatures, the proteins inside the cell are denatured and start aggregating. Several studies have revealed that the heat stress response includes the destabilization of macromolecules as ribosomes and RNA as well as alterations of membrane fluidity (Earnshaw et al. 1995 ; Teixeira et al. 1997).
The transient induction of several heat shock proteins (such as DnaK, GroEL and GroES) in various probiotics in response to heat stress has been demonstrated (Earnshaw et al. 1995 ; Serrazanetti et al. 2013 ; Teixeira et al. 1997). The HSPs were dramatically upregulated when the cell was exposed to heat stress, induced by heat shock factor (HSF). HSPs are present in almost all living organisms, from bacteria to humans. Their major role is to bind proteins in a transient non‐covalent manner during heat shock, prevent premature folding, and stabilize the proteins. The increased heat resistance was achieved by pre‐exposure of cells to elevated temperatures, allowing them to adapt to these temperatures. Heat acclimation triggers the expression of HSP genes mediated by HSF and enhances tolerance to noxious high temperatures, a phenomenon known as acquired thermotolerance (Gardiner et al. 2000 ; Prasad et al. 2003).
Cold Stress
Probiotics bacteria can be exposed to extremely cold conditions during storage. Understanding the behavioral changes in extreme cold environments is important to increase cell viability. Probiotic micro‐organisms have been subjected to various temperatures, sometimes below the optimal growth temperature. When bacteria are exposed to extreme cold conditions, several physiological changes may occur, including decreases in membrane fluidity and stabilization of secondary structures of RNA and DNA (leading to decreased transcription and translation), and decreased or no DNA replication. Similar to the heat shock response, several proteins, called cold‐induced proteins (CIPs), were expressed. These play a critical role in adjusting membrane fluidity, DNA supercoiling and transcription and translation. Several researchers have demonstrated the functional contribution of CIPs in Lactococcus lactis and L. plantarum (Derzelle et al. 2000 ; Kim et al. 1998). Kim et al. (1998) studied various lactic acid bacteria (LAB) strains to determine the cold shock response and found that frozen or freeze‐dried bacterial cells have improved cryotolerance, due to the overexpression of several CIPs. Furthermore, the response of bacterial strains to freezing may depend on exposure time and the induction of cryotolerance appears to be dependent on the growth phase in which the cold shock took place (Serrazanetti et al. 2013).
Osmotic Stress
From the production to storage process, probiotic strains encounter osmotic stresses. During storage processes, like freezing, freeze‐drying, and spray drying, the osmolality of the environment increases as a result of cryoconcentration of solutes or dehydration, leading to excessive passage of water from the cell to the extracellular environment (Poolman et al. 2002). Addition of various amounts of sugars and salts also alters the osmolality of probiotic cells. Researchers found that hyperosmotic conditions occurring due to high sugar levels do very little harm to the cell and are only transient; the major reason for this is the cell’s ability to balance the extra‐ and intracellular lactose and sucrose concentration. Probiotic strains have to adapt to these environmental changes to survive and they tackle this by taking up or synthesizing compatible solutions (organic compounds) when they sense osmotic stress (van de Guchte et al. 2002). These compatible solutes are defined as osmoprotectants, which act as a stabilizing agent for various enzymes, thereby offering protection against other stress conditions (high temperature, freezing, and drying). Water loss from the cell due to the high external osmolality was prevented by the intracellular accumulation of compatible solutes, which maintains turgor (Piuri et al. 2003). The compatible solutes synthesized by various LAB strains include carnitine, betaine, proline, and 3‐methylbutanoic acid (Serrazanetti et al. 2013). LAB can synthesize these compatible solutes, at very low levels, which are then taken up from the medium by specific transporters (Poolman et al. 2002).
Competitive Stress
Almost all food fermentation is carried out by various groups of micro‐organisms including LAB, yeasts, and several non‐pathogenic filamentous fungi. This mixed culture fermentation has several advantages when compared with single strain fermentation, including reduced production cost and time, exchange of metabolites and growth factors or inhibiting compounds (Sieuwerts et al. 2008). The co‐culture of several closely related bacterial species prevents the growth of other unwanted micro‐organisms. However, this may create several types of stress for the micro‐organisms including:
- nutrient competition
- generation of unfavorable environments
- competition for attachment/adhesion sites (Serrazanetti et al. 2013).
Due to the heterogeneous physicochemical composition in food fermentation, micro‐organisms start to compete for available nutrients, each strain utilizing the nutrients via multiple mechanisms (Sieuwerts et al. 2008). In most cases, the concentration of available carbon sources is very high at the initial stage of fermentation, which leads to competition and rapid utilization of the carbon source which produces a higher biomass. Similarly, in dairy fermentation the available nitrogen source is very limited, so the fermentative micro‐organisms compete for the utilization of free amino acids and small peptides. During fermentation, the microbes produce diffusible chemical compounds to communicate with other bacteria. Apart from the competition for available nutrients, micro‐organisms also compete for adhesion sites.
Overall, in recent years many researchers have tried to determine the mechanisms behind the stress response by micro‐organisms. This basic understanding about the bacterial stress response is crucial for the identification of the most efficient probiotic strains to improve food fermentation. The overall stress conditions faced by probiotics from production to consumption are illustrated in Figure 10.2.
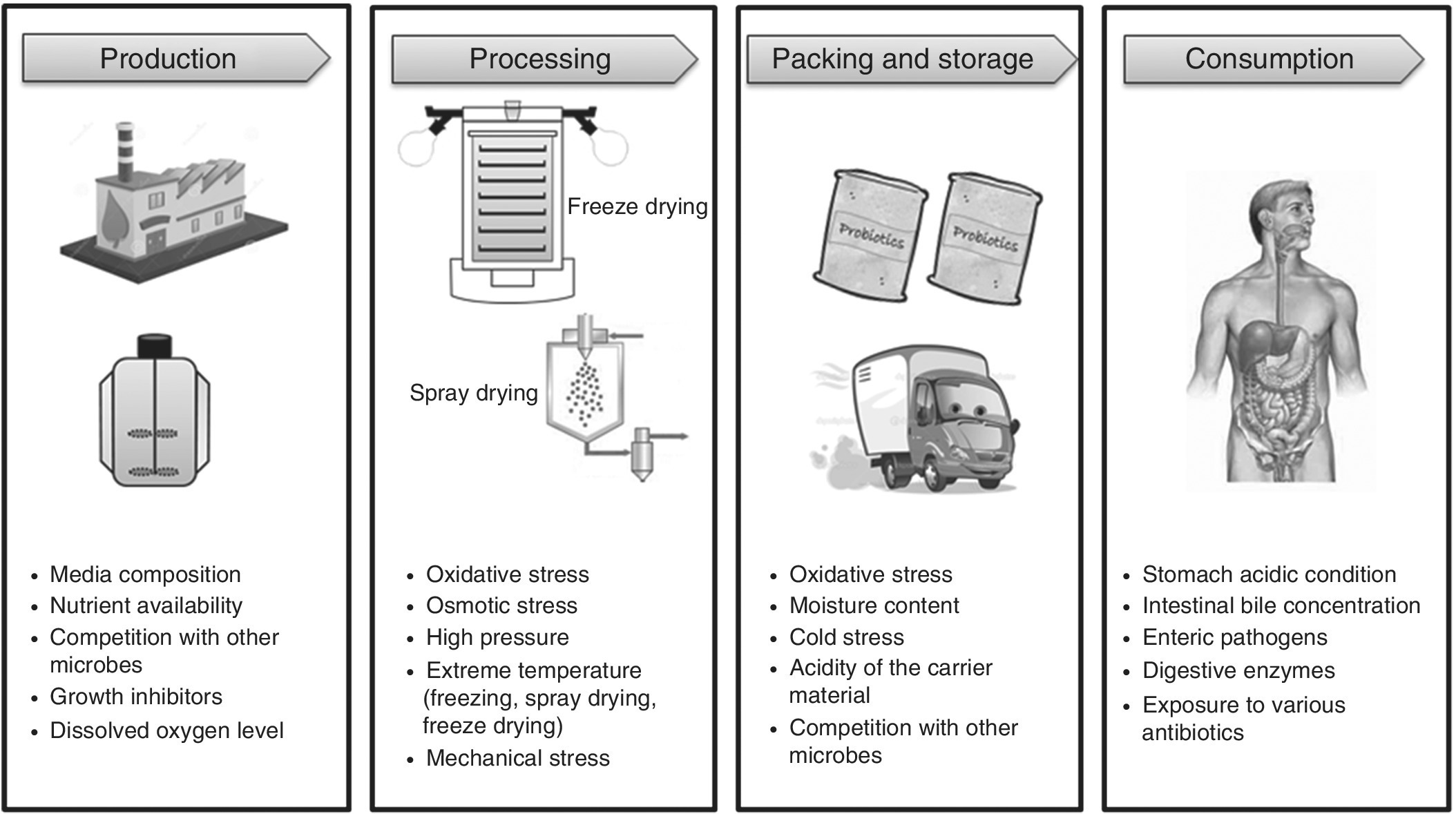
Figure 10.2 Various stressful environments encountered by probiotics from production to consumption.
Current Technologies Used to Increase Cell Viability
Industrial demand for new technologies that enable high cell yields at large scale and ensure probiotic stability in food remains strong, because many strains of intestinal origin are difficult to propagate and high survival is important for both economic reasons and health effects. In addition, more efficient technologies could lead to greater product efficacy and strain diversification with the development of technologically unsuitable strains into products.
Industrial processes for food culture production, including probiotics, almost exclusively use conventional batch fermentation with suspended cells. Several approaches have been investigated to enhance cell viability during downstream processing, storage, and eventually digestion. These include the application of sublethal stresses during fermentation, the addition of protectants, including compatible solutes (e.g., betaine which has been extensively studied), and cell protection by microencapsulation. The ability of micro‐organisms to grow and survive depends largely on their capacity to adapt to changing environments. Adaptation to adverse environments is usually associated with the induction of a large number of genes, the synthesis of stress response proteins, and the development of cross‐resistance to various stresses.
Genetic Modification of the Strain
Advancements in genomics and proteomics have made both lactobacilli and bifidobacteria more amenable to genetic manipulation by increasing their viability when exposed to stressful environments. Genetically modified (GM) probiotics are mainly concerned with the improved survival and persistence of probiotics within the human and animal gut, tolerance of packing and storage conditions of food, and the delivery of therapeutics by live probiotics. With the identification of genetic elements that confer tolerance to increased osmolarity, bile salt and reduced water activity, the improvement of probiotics for survival has become a reality. With the expression of various molecules such as antigens, enzymes, and molecules of immunological importance within probiotic microbes, the use of GM probiotics in the field of therapeutics looks promising.
Basically, there are two different approaches that can be followed:
- modify the expression/production of genes already present in the micro‐organism (homologous expression)
- introduce genes from other microbial species (heterologous expression) (Desmond et al. 2004).
Trials have also been undertaken to produce mutants which overexpress protective stress metabolites, i.e., heat shock proteins GroEL and GroES, thereby improving tolerance to abusive heat treatments. Increasing the available GroES and GroEL concentration prior to the stresses associated with freezing, lyophilization, or spray‐drying may offer additional protection against protein denaturation and produce a more viable and physiologically active product (Walker et al. 1999). For example, overexpression of the HSP chaperones GroES and GroEL in Lactobacillus paracasei NFBC 338 improved its ability to withstand thermal stress, but it did not perform as well as the heat‐adapted parent culture. The heat shock response involves a consort of proteins including DnaK, DnaJ, and GrpE, tagatose‐6‐phosphate‐aldolase; glyceraldehyde‐3‐phosphate dehydrogenase; and triose phosphate isomerase (Desmond et al. 2004).
The effect of homologous excess production of each of the three HSPs of Lactobacillus plantarum WCFS1 has been studied to determine the stress tolerance of transformed cells. A change from the optimum temperature (i.e., 28 °C) to 12 °C, 37 °C, and 40 °C lead to a decrease in cell viability ranging between 2.0 and 4.0 log CFU/mL for the untransformed cells while the three HSP‐overproducing strains were not affected (Fiocco et al. 2007). The auxotrophic mutant strains of Saccharomyces boulardii showed functional recombinant protein even after being recovered from gastrointestinal immune tissues in mice. A UV mutagenesis approach was applied to generate three uracil auxotrophic S. boulardii mutants that show a low rate of reversion to wild‐type growth. These mutants can express recombinant protein and are resistant in vitro to low pH, bile acid salts, and anaerobic conditions (Hudson et al. 2014).
However, within the latest legislative situation, i.e., Novel Food Regulations, which sets a high standard of safety, as well as the general consumer rejection of the presence of genetically modified micro‐organisms in food, this approach seems unlikely to be applicable in future (Rastall and Maitin 2002).
Enhancing Stress Tolerance of Probiotic Strains
Intrinsic stress tolerance seems to be a critical factor in the overall resistance to manufacture and storage of probiotic products. Different approaches can be used to this end, which can be divided into three main categories: selection of naturally occurring strains, stress adaptation of naturally occurring strains, and genetic modification of the strains. The first two approaches use already existing diversity and genetic potential, whilst the third would imply genetic manipulation leading to a genetically modified organism (GMO) (Sanchez et al. 2012).
Physiological Adaptation and General Stress Response
A brief pretreatment of bacteria with stress can lead to physiological adaptation to forthcoming more severe stress caused by the same stressor. The storage stability of Lactobacillus rhamnosus HN001 was substantially increased after a sublethal stress such as heat or osmotic stress. The largest increase in the storage stability of L. rhamnosus HN001 was observed after sublethal heat stress during the stationary phase of growth (Prasad et al. 2003). Saarela et al. (2004) reported that sublethal treatments of acid and heat treatments of stationary phase probiotic cells (Lactobacilli strains) increased their viability and survival during lethal treatments and their ability to adapt at fermentor‐scale production of probiotic cultures. Hence, strain‐specific and sublethal treatments of specific growth phase probiotic cultures can be utilized in the production of probiotic cultures with improved viability. The methods of pretreatment, heat adaptation and other factors that affect such investigation are very important for further studies on the viability of probiotic bacteria during heat processing.
Cell responses to sublethal stresses are directly proportional to the strain selection and applied stresses. Therefore, in the absence of general mechanisms for sublethal stress adaptation, fermentation conditions must be determined for each strain. This process is labor intensive and costly. In addition, sublethal stresses can lead to decreases in cell activity and yield and eventually to changes in the functionality of probiotic cells in the intestinal tract. Clearly, there is a need to develop a more in‐depth understanding of stress response mechanisms of probiotics (Lacroix and Yildirim 2007).
Methods to Increase Survival and Viability of Probiotics
Researchers have long been encouraged to find novel, proficient methods of improving the viability of probiotics in food products (especially fermented types). The most recent developments focus on fermentation technologies for producing probiotic bacteria; new approaches for enhancing the performance of these fastidious organisms during fermentation, downstream processing, and utilization in commercial products; and improving functionality in the gut (Mortazavian et al. 2007).
Fermentation Technology
The large‐scale preparation of bacterial strains is difficult, time‐consuming, and expensive. In addition, most of the probiotic strains show poor growth rates in milk‐based media. Therefore, it can be difficult to achieve high numbers of viable cells after fermentation (Ross et al. 2005). The media composition and type of substrate used for fermentation can have a great impact on probiotic strain viability during production and downstream processing (Lacroix and Yildirim 2007). The overall fermentation process of probiotic starter culture is depicted in Figure 10.3.
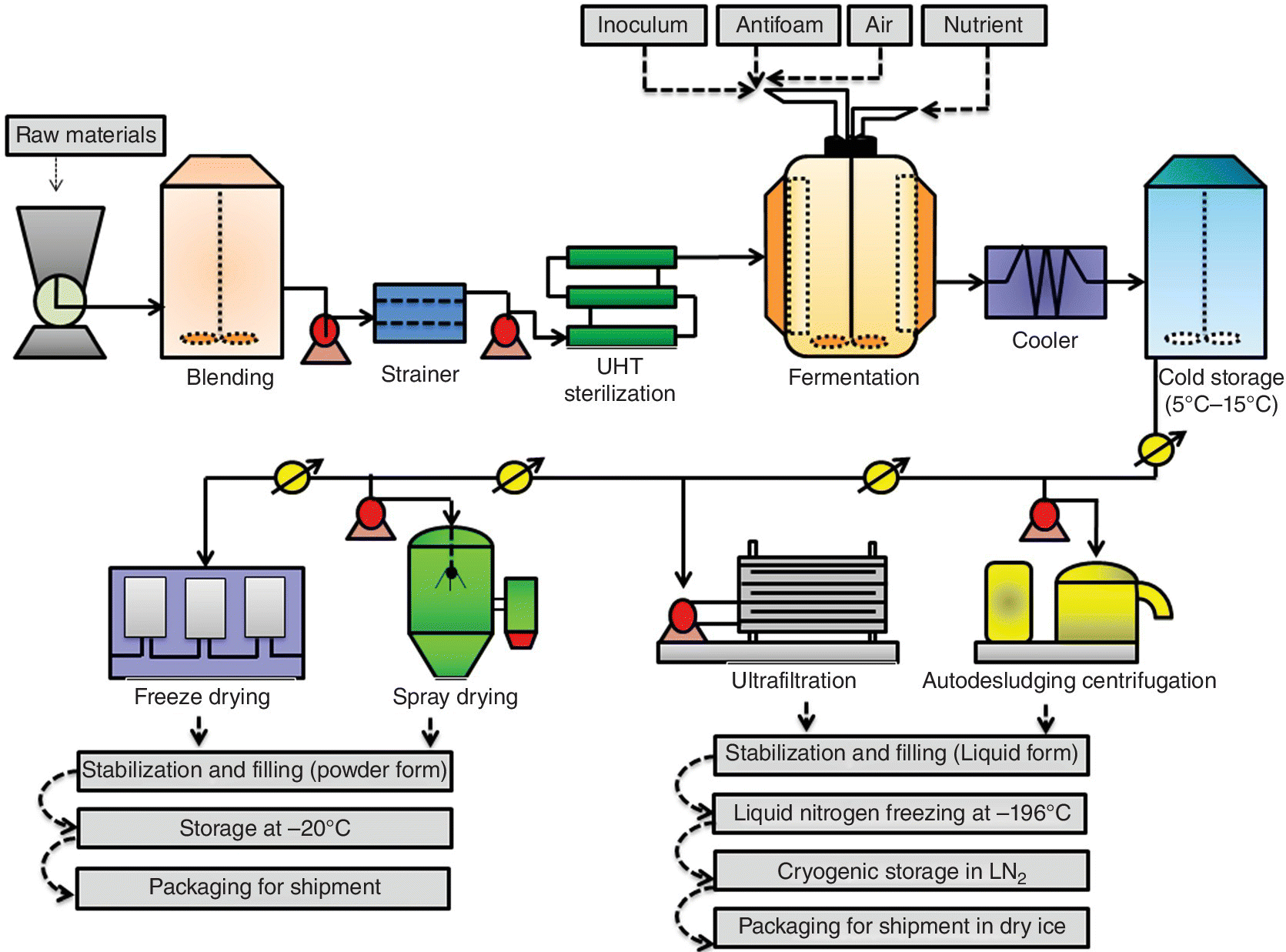
Figure 10.3 Flowchart for the production process of probiotic starter cultures.
Optimization of Growth Medium
The foremost demerit of probiotic strains is the rapid growth and acidification rate of typical starter cultures. Therefore, they are added to dairy products after fermentation (Champagne et al. 2005). The cultivation conditions are directly proportional to growth, culture stability, and activity as well as drying and subsequent storage. The stability and function of probiotics can be enhanced by changing the culture conditions. Usually probiotic cells are produced by large‐scale fermentation. It is important to design growth media and change the fermentation technology to improve biomass yield and enhance cell stability. MRS broth (de Man et al. 1960) is the most widely used medium for cultivation of LAB or bifidobacteria. Higher cell counts of bifidobacterial strains were produced in ultra‐filtered skim milk with various protein concentrations or inclusion of milk with nitrogenous substrates such as whey and casein fractions from human or cow milk when compared to growth in skim milk only (Ventling and Mistry 1993). Soy milk or animal product‐free vegetable medium (based on soy peptone, glucose, and yeast extract) resulted in lower yields or lower viability of bifidobacteria during storage because of the low buffer capacity of the vegetable medium (Heenan et al. 2002). Redox‐reducing compounds like cysteine were used in growth media of bifidobacteria to enhance growth (Doleyres and Lacroix 2005). In some cases, the disulfide bonds were reduced and hence these supplements may lose their growth‐enhancing properties (Ibrahim et al. 1994).
Types of Fermentation Involved in Probiotic Production
Membrane Bioreactor
Some researchers have reported on probiotic cell production in membrane bioreactors. They have supported high cell yields and volumetric productivity. Taniguchi et al. (1987) reported seven‐fold higher Bifidobacterium longum biomass levels in a membrane bioreactor than that attained in free cell batch fermentors. Similarly, Corre et al. (1992) reported that there was a high cell yield and a 15‐fold enhancement in volumetric productivity compared to free cell batch cultures of Bifidobacterium bifidum.
Immobilized Cell (IC) Technology in Continuous Cultures
Although the fermentation technologies, such as continuous culture and immobilized cell systems, are not common in food industries, they have the potential to improve the role of fastidious probiotic cells. Hence, the physiology and functionality of ingested probiotics can be improved with the implementation of these technologies. It helps to extend the range of commercially available probiotics (Lacroix and Yildirim 2007).
In continuous cultures, bacterial cells grow in a fermentor with continuous supplementation of fresh medium and removal of fermented broth at a given dilution rate. Metabolism, growth rate, and gene expression of bacteria of continuous culture can be monitored under constant conditions during long time periods (Hoskisson and Hobbs 2005). In continuous culture of B. longum SH2, there was increased volumetric biomass productivity when compared to traditional batch cultures (Kim et al. 2003). In one study, a B. longum NCC2705 strain grown in prolonged chemostat culture for 200 hours resulted in higher metabolic and transcriptomic stability throughout the whole culture time which suggests applying these cells as a novel method to screen for sublethal treatments (Doleyres et al. 2002). Beyond this application, contamination is considered to be the main drawback. The constant feeding of new medium must be supplemented at dilution rates lower than the maximum specific growth rate to avoid wash‐out of active biomass. There is a chance of competitive contaminant strain due to low dilution rate.
Continuous Fermentation
In addition, cells produced with continuous IC cultures are in the exponential or early stationary growth phase, and exhibit both high viability and metabolic activity compared with starving cells produced with conventional batch cultures. Immobilized cell technology combined with long‐term continuous culture can be used to efficiently produce, in a one‐step process, cells with improved tolerance to environmental stresses, without the need for preconditioning treatments which are sometimes used for better survival of probiotics during production and use in functional foods, but eventually result in reduced cell activity and yield (Desmond et al. 2004). A change in the metabolic pathway from homofermentative to heterofermentative has also been observed during continuous cultures with immobilized cells of Lactobacillus. Probiotic strains produced with continuous IC fermentations could better as they pass through the gastrointestinal tract and could be used to manufacture products with new or enhanced health benefits, as well as new food or pharmaceutical bio‐ingredients (Benech et al. 2002 ; Bouksaim et al. 2000).
Stabilization of Probiotics
Microencapsulation
Microencapsulation is a fascinating field of biopharmacy that has originated and developed rapidly in the past decade. Several micro‐organisms have been immobilized within semi‐permeable and biocompatible materials that modulate the delivery of cells using this technique (Vidhyalakshmi et al. 2009). Microencapsulation is a technology for packaging solids, liquids or gaseous materials in tiny, sealed capsules that can release their contents at controlled rates under specific conditions (Shahidi and Han 1993). Microencapsulation of probiotics in hydrocolloid beads has been found to enhance their viability and activity in food products and the intestinal tract by entrapping the cells inside a bead matrix, thus separating them from harsh environmental conditions, as well as protecting them against bacteriophages (Krasaekoopt et al. 2003).
A microcapsule consists of a semi‐permeable, spherical, thin, strong membrane surrounding a solid or liquid core, with a diameter varying from a few microns to 1 mm (Anal and Singh 2007). Hence, the bacterial cells are retained within the microcapsule (Jankowski et al. 1997). The most widely used materials in microencapsulation of probiotic bacteria include polysaccharides originated from seaweed (alginate, κ‐carrageenan), other plants (starch and its derivatives, gum arabic), bacteria (gellan, xanthan), and animal proteins (milk, gelatin). The chief techniques for microencapsulation of probiotics are extrusion techniques, spray drying, and spray cooling (Chavarri et al. 2012).
The microencapsulation techniques applied to probiotic cells for use in fermented milk products or biomass production can be classified into two groups, based on the method used to form the beads: extrusion (droplet method) and emulsion or two‐phase system. Both extrusion and emulsion techniques show high survival of probiotic bacteria by up to 80–95% (Kebary et al. 1998). Lack of oxygen in the interior of the capsule may result in cell death, but conversely it may help anaerobic bacteria such as bifidobacteria (Kailasapathy 2002).
Using an electric potential, the electrostatic bead generator pulls the droplets from a needle tip. The electrostatic potential is placed between the needle feeding the mixture of alginate and bacterial cell solution and the gelling bath. Although viability of LAB could be enhanced by microencapsulation, the process may change the heat susceptibility and health benefits of the cell since the cells undergo heat treatment and dehydration during microencapsulation (Ananta et al. 2005).
Merits of Microencapsulation
Entrapment of probiotic micro‐organisms in a biodegradable polymer matrix has various advantages. Once entrapped in matrix beads, the cells are easier to handle than a suspension or slurry. The number of cells in each bead can be quantified allowing dosages to be readily controlled. Cryo‐ and osmoprotective components can be incorporated in the polymer matrix, increasing survival of cells during processing and storage. Finally, once the matrix beads have been dried, a surface coating can be applied. This outer layer can be used to alter the esthetic and sensory properties of the product and may also be functional, providing an extra level of protection for the cells. In addition, the coating layer can have preferable dissolution properties which permit overdue release of the cells or release upon, for example, change in pH. Cells produced during continuous immobilized cell cultures show changes in cell membranes which may induce formation of cell aggregates and tolerance to bile salts and aminoglycosidic antibiotics. Cell immobilization in combination with continuous culture can be used to produce probiotic micro‐organisms with improved stress tolerance.
Voluminous formation of cell aggregates has already been reported during continuous culture with immobilized lactobacilli (Bergmaier et al. 2005), whereas no cell aggregates were reported for the B. longum NCC2705 grown for more than 8 days in continuous mode with free cells in MRS.
A parallel decrease of the ratio of unsaturated to saturated fatty acids of cell membrane from IC cultures was reported for a bile‐adapted mutant strain of B. animalis compared to its mother strain (Ruiz et al. 2007). These changes might be involved in restricted diffusion of bile salts into the cytoplasm (Begley et al. 2005). Doleyres et al. (2004) reported a progressive increase of survival of B. longum ATCC 15707 in simulated intestinal conditions and tolerance to nisin Z with fermentation time induced by cell immobilization in continuous co‐culture with Lactobacillus lactis MD. The elevation of bile tolerance with time correlated with increased tolerance to nisin Z, which targets bacterial cell membranes.
Spray Drying
In general, dairy starter and probiotic cultures are preserved and distributed in frozen or dried form. High costs of storage, shipping, and energy are the primary reason to preserve probiotic cultures in dried forms (Johnson and Etzel 1995). Bacterial cells require water activity (aw) of about 0.98 in the resultant matrix for their growth and survival. Hence, the physical removal of water to convert the bacterial cells into a dried form is a risky process. It is necessary to maintain either high aw for metabolic activity or low aw to preserve the bacteria in live state, so that they can survive in a dormant state in powders (Paul et al. 1993).
Freeze or spray drying is the predominant method used to dry the bacterial suspension. The common method used to formulate starter and probiotic cultures is freeze drying. Its major demerit is that it is an expensive process with low yields. But in the case of inexpensive spray drying, higher production rates result (Zamora et al. 2006). Spray drying is a well‐organized tool in food industries for the production of milk powders and instant coffee. Although there are several restrictive process conditions for micro‐organisms (inlet reaching ≥180 °C), the rapidity of drying combined with the ability to dry large amounts of bacterial cultures has caught the attention of research and industry (Meng et al. 2008 ; Zamora et al. 2006).
Freeze Drying
Freeze drying is a commonly used method, which provides higher survival rates compared with spray drying. Generally, freeze drying is achieved by three important steps: freezing, primary and secondary drying. To increase the survival ratio of bacterial cultures, they are typically frozen at –196 °C in liquid nitrogen. After freezing, the frozen samples are sublimated with ice under high vacuum conditions to complete the primary freezing. In this step, high temperature under pressure causes the phase transition from solid to gas. After the primary drying step, almost 95% of the water content in the sample is removed. However, secondary drying is also important to remove the remaining hydrogen‐bound water molecules to achieve a final water content below 4%, thus improving survival rates and long‐term storage efficiency and preventing spoilage (Santivarangkna et al. 2007).
Freeze drying is an expensive method with a low yield. When bacterial cells are exposed to extremely low temperatures (i.e., freezing), osmotic pressure across the membrane is increased due to formation of extracellular ice and the cells dehydrate until an eutectic point is reached (Fowler and Toner 2005). During the freeze‐drying process, the bacteria face various stresses which damage the cell; generally, longer rod‐shaped lactobacilli are more susceptible to damage than small round enterococci, because of their larger surface area (Fonseca et al. 2000). The cell membrane lipids are more sensitive and easily damaged during freezing, and destabilization of nucleic acids also limits several important growth functions, including replication of DNA, transcription, and translation (van de Guchte et al. 2002).
To reduce these problems during freeze drying, several approaches have been developed, including the addition of protectants, such as trehalose, betaine, adonitol, sucrose, skim milk powder, lactose, commercial cryoprotectants (e.g., Unipectine, Satialgine), and several commercially available polymers also increase the stability of bacteria after freezing (Burns et al. 2008).
Fluidized Bed and Vacuum Drying
Fluidized bed dryers use an upward‐moving flow of heated air and mechanical shaking to create a fluidized effect in a solid product. Particles are freely suspended in air and are dried by rapid heat exchange (Santivarangkna et al. 2007). This method is more economical than others, including spray drying. In this method, the length of bacterial exposure to heat is easily controlled, which reduces the risk of heat inactivation. Using the fluidized bed drying method, several yeast strains have been successfully dried and this method is also employed for LAB (Bayrock and Ingledew 1997). It is suitable for granular particles so bacterial solutions are encapsulated with alginate, potato starch, skim milk or casein before being introduced into the drier. The desired moisture content is achieved by drying the granulated particles under gentle temperatures.
The vacuum drying method is used to dry heat‐sensitive compounds; here the water molecules in the samples are removed at low temperature under vacuum conditions. This method is similar to freeze drying but the temperature is maintained as low as –2 °C. Due to the high vacuum conditions, oxidation reactions are reduced in the vacuum drying method, which is more suitable for oxygen‐sensitive bacteria, but this method is not extensively used for drying LAB strains. It has several limitations including long drying time (10–100 hours) compared with the spray drying or fluidized bed method (Santivarangkna et al. 2007). However, these problems can be overcome by modifications such as using continuous vacuum drying. Continuous vacuum drying is used in large‐scale industries for drying enzymes, food additives, and other pharmaceutical products (Hayashi et al. 1983).
Enumeration of Viable Probiotic Cells
Minimum levels of viable cells in probiotic products are essential so quantification methods are required to detect viable probiotic strains in functional food and dairy products. The quality of probiotic products can be evaluated using fast and reliable methods to enumerate probiotic cells in mono‐ and mixed cultures. Plate counting on selective media is the most commonly used method but cell clumping, inhibition by adjacent cells, and time consumption are limitations (Bergmaier et al. 2005). The lack of a standard medium to discriminate bifidobacteria from other LAB has prompted the search for alternative, non‐culture‐based technologies for cell enumeration (Masco et al. 2005). Fluorescent stains alone or combined with enzymatic assays help to distinguish between live, metabolically active, injured, dormant, viable but not cultivable, and dead bacterial cells and also enable quantification of viable bacteria (Alakomi et al. 2005). Molecular tools such as fluorescence in situ hybridization (FISH) and flow cytometry are applied to estimate viable cells in probiotic products (Maukonen et al. 2006). Flow cytometry can distinguish between subpopulations of stressed and unstressed bifidobacteria (Ben Amor et al. 2002). However, single cell suspension is required for culture‐based and fluorescent methods because cells in clusters or chains may cause distortion (Bibiloni et al. 2001).
Several other methods exist to detect or quantify bifidobacteria in fecal samples and pharmaceutical or probiotic products. DNA fingerprinting approaches such as denaturing gradient gel electrophoresis (DGGE) and pulsed‐field gel electrophoresis (PFGE) are used to detect bifidobacteria. Quantitative approaches include the use of quantitative real‐time PCR (qRT‐PCR) (Masco et al. 2007 ; Matsuda et al. 2007). Since DNA and rRNA are very stable and can be used as viability markers, these methods cannot provide information regarding metabolic activity or viability of microbial cells. mRNA with short half‐life times is a useful viable cell marker (Hellyer et al. 1999). qRT‐PCR has been used in medical applications to enumerate viable pathogenic micro‐organisms by quantifying mRNA (Birmingham et al. 2008) but no such method with mRNA has been applied to probiotic bacteria. Increasing counts of accessible genome sequences of food‐related micro‐organisms and upcoming microarray technology will extend our knowledge regarding gene expression profiles of micro‐organisms in various environments which will increase the number of target mRNAs to accurately quantify viable micro‐organisms.
Conclusion
The ability of probiotic micro‐organisms to survive the severe environments existing during processing and gastrointestinal transit has been a primary factor in their use. Undoubtedly, initiation of the probiotic stress response through preadaptation approaches may not always ensure the improved performance of a culture in compromising environments. Further research will be needed to discriminate the influence of specific stressors and their combined effect on cell growth and survival, particularly distinguishing between changes due to growth stage and the impact of specific stressors, given that most studies to date have not controlled variables. Therefore, a complete approach is required for incorporating the emerging food processing technologies, which may improve and maintain survival of probiotics during processing and storage, with recent knowledge on genotypes and expressed characteristics of probiotics. The benefits of the technology should benefit the consumer rather than supporting corporate profit. Genetically modified probiotics should directly benefit the consumer but studies which investigate the safety of engineered probiotics are vital. Hence, it is very important to understand the bacterial stress response which helps scientists to modify probiotic strains to obtain their maximum potential. To enhance the viability and health functionality of probiotics, novel cultivation technologies need to be devised with the ability to produce functional probiotics, which is not achieved by traditional fermentation methods. Microencapsulation of probiotics is a promising technology which can protect bacterial cells from various environmental stresses and is also useful for the targeted delivery of probiotics to desired regions.
References
- Alakomi, H.L., Mättö, J., Virkajärvi, I. and Saarela, M. (2005) Application of a micro plate scale fluorochrome staining assay for the assessment of viability of probiotic preparations. J. Microbiol. Methods, 62: 25–35.
- Anal, A.K. and Singh, H. (2007) Recent advances in microencapsulation of probiotics for industrial applications and targeted delivery. Trends Food Sci. Technol., 18: 240–251.
- Ananta, E., Volkert, M. and Knorr, D. (2005) Cellular injuries and storage stability of spray‐dried Lactobacillus rhamnosus GG. Int. Dairy J., 15(4): 399–409.
- Argyri, A., Zoumpopoulou, G., Karatzas, K.A., Tsakalidou, E., Nychas, G.J.E. and Panagou, E.Z. (2013) Selection of potential probiotic lactic acid bacteria from fermented olives by in vitro tests. Food Microbiol., 33: 282–291.
- Bayrock, D. and Ingledew, W.M. (1997) Mechanism of viability loss during fluidized bed drying of baker’s yeast. Food Res. Int., 30: 417–425.
- Begley, M., Gahan, C.G.M. and Hill, C. (2005) The interaction between bacteria and bile. FEMS Microbiol. Rev., 29: 625–651.
- Ben Amor, K., Breeuwer, P., Verbaarschot, P. et al. (2002) Multi parametric flow cytometry and cell sorting for the assessment of viable, injured, and dead Bifidobacterium cells during bile salt stress. Appl. Environ. Microbiol., 68: 5209–5216.
- Benech, R.O., Kheadr, E.E., Lacroix, C. and Fliss, I. (2002) Antibacterial activities of nisin Z encapsulated in liposomes or produced in situ by mixed culture during Cheddar cheese ripening. Appl. Environ. Microbiol., 68: 5607–5619.
- Bergmaier, D., Champagne, C.P. and Lacroix, C. (2005) Growth and exopolysaccharide production during free and immobilized cell chemostat culture of Lactobacillus rhamnosus RW‐9595M. J. Appl. Microbiol., 98: 272–284.
- Bibiloni, R., Zavaglia, A.G. and de Antoni, G. (2001) Enzyme‐based most probable number method for the enumeration of Bifidobacteriumin dairy products. J. Food Protect., 64: 2001–2006.
- Birmingham, P., Helm, J.M., Manner, P.A. and Tuan, R.S. (2008) Simulated joint infection assessment by rapid detection of live bacteria with real‐time reverse transcription polymerase chain reaction. J. Bone Joint Surg., 90A: 602–608.
- Borgstrom, B., Dahlqvist, A., Lundh, G. and Sjovall, J. (1957) Studies of intestinal digestion and absorption in the human. J. Clin. Invest., 36: 1521–1536.
- Bouksaim, M., Lacroix, C., Audet, P. and Simard, R.E. (2000) Effects of mixed starter composition on nisin Z production by Lactococcus lactis subsp. lactisbiovar. diacetylactis UL 719 during production and ripening of Gouda cheese. Int. J. Food Microbiol., 59: 141–156.
- Burns, P., Vinderola, G., Molinari, F. and Reinheimer, J. (2008) Suitability of whey and buttermilk for the growth and frozen storage of probiotic lactobacilli. Int. J. Dairy Technol., 61: 156–164.
- Champagne, C.P., Gardner, N.J. and Roy, D. (2005) Challenges in the addition of probiotic cultures to foods. Crit. Rev. Food Sci. Nutr., 45: 61–84.
- Chassard, C., Grattepanche, F. and Lacroix, C. (2011) Probiotics and health claims: challenges for tailoring their efficacy. In: Kneifel, W. and Salminen, S. (eds) Probiotics and Health Claims. Oxford: Wiley‐Blackwell.
- Chavarri, M., Maranon, I. and Villara, M.C. (2012) Encapsulation technology to protect probiotic bacteria. In: Rigobelo, E.C. (ed.) Probiotics. InTech, 501–540.
- Collado, M.C., Hernandez, M. and Sanz, Y. (2005) Production of bacteriocin‐like inhibitory compounds by human fecal Bifidobacterium strains. J. Food Protect., 68: 1034−1040.
- Corre, C., Modec, M.N. and Boyaval, P. (1992) Production of concentrated Bifidobacterium bifidum. J. Chem. Technol. Biotechnol., 53: 189–194.
- De Angelis, M. and Gobbetti, M. (2004) Environmental stress responses in Lactobacillus: a review. Proteomics, 4: 106–122.
- De Man, J.D., Rogosa, M. and Sharpe, M.E. (1960) A medium for the cultivation of Lactobacilli. J. Appl. Bacteriol., 23: 130–135.
- Derzelle, S., Hallet, B., Francis, K.P., Ferain, T., Delcour, J. and Hols, P. (2000) Changes in cspL, cspP, and cspC mRNA abundance as a function of cold shock and growth phase in Lactobacillus plantarum. J. Bacteriol., 182: 5105–5113.
- Desmond, C., Fitzgerald, G.F., Stanton, C. and Ross, R.P. (2004) Improved stress tolerance of GroESL‐overproducing Lactococcus lactis and probiotic Lactobacillus paracasei NFBC 338. Appl. Environ. Microbiol., 70: 5929–5936.
- Doleyres, Y. and Lacroix, C. (2005) Technologies with free and immobilised cells for probiotic bifidobacteria production and protection. Int. Dairy J., 15: 973–988.
- Doleyres, Y., Paquin, C., LeRoy, M. and Lacroix, C. (2002) Bifidobacterium longum ATCC 15707 cell production during free‐ and immobilized‐cell cultures in MRS‐whey permeate medium. Appl. Microbiol. Biotechnol., 60: 168–173.
- Doleyres, Y., Fliss, I. and Lacroix, C. (2004) Increased stress tolerance of Bifidobacterium longum and Lactococcus lactis produced during continuous mixed‐strain immobilized‐cell fermentation. J. Appl. Microbiol., 97: 527–539.
- Dubbs, J.M.and Mongkolsuk, S., (2007) Peroxiredoxins in bacterial antioxidant defense. Subcell. Biochem., 44: 143–193.
- Du Toit, M., Franz, C.M., Dicks, L.M. et al. (1998) Characterization and selection of probiotic lactobacilli for a preliminary minipig feeding trial and their effect on serum cholestrerol levels, faeces pH and faeces moisture content. Int. J. Food Microbiol., 40: 93–104.
- Earnshaw, R.G., Appleyard, J. and Hurst, R.M. (1995) Understanding physical inactivation processes: combined preservation opportunities using heat, ultrasound and pressure. Int. J. Food Microbiol., 28: 197–219.
- FAO/WHO (2002) Guidelines for the Evaluation of Probiotics in Food. Available at: www.scribd.com/doc/110123033/Guidelines‐for‐the‐Evaluation‐of‐Probiotics‐in‐Food (accessed 2 May 2017).
- Figueroa‐Gonzalez, I., Quijano, G., Ramirez, G. and Cruz‐Guerrero, A. (2011) Probiotics and prebiotics – perspectives and challenges. J. Sci. Food Agric., 91: 1341–1348.
- Fiocco, D., Capozzi, V., Goffin, P., Hols, P. and Spano, G. (2007) Improved adaptation to heat, cold, and solvent tolerance in Lactobacillus plantarum. Appl. Microbiol. Biotechnol., 77: 909–915.
- Fonseca, F., Beal, C. and Corrieu, G. (2000) Method of quantifying the loss of acidification activity of lactic acid starters during freezing and frozen storage. J. Dairy Res., 67: 83–90.
- Fowler, A. and Toner, M. (2005) Cryo‐injury and bio‐preservation. Ann. NY Acad. Sci., 1066: 119–135.
- Gänzle, M.G., Hertel, C., van der Vossen, J.M. and Hammes, W.P. (1999) Effect of bacteriocin‐producing lactobacilli on the survival of Escherichia coli and Listeria in a dynamic model of the stomach and the small intestine. Int. J. Food Microbiol., 48(1): 21–35.
- Gardiner, G.E., O’Sullivan, E., Kelly, J. et al. (2000) Comparative survival rates of human‐derived probiotic Lactobacillus paracasei and L. salivarius strains during heat treatment and spray drying. Appl. Environ. Microbiol., 66: 2605–2612.
- Gilliland, S.E., Staley, T.E. and Bush, L.J. (1984) Importance of bile tolerance of Lactobacillus acidophilus used as a dietary adjunct. J. Dairy Sci., 67: 3045–3051.
- Granato, D., Branco, G.F., Cruz, A.G., Faria, J.A.F. and Nazzaro, F. (2010) Functional foods and nondairy probiotic food development: trends, concepts and products. Compr. Rev. Food Sci. F., 9: 292–302.
- Halliwell, B. and Gutteridge, J.M.C. (1999) Free Radicals in Biology and Medicine, 3rd edn. New York: Oxford University Press, pp. 617–783.
- Harzallah, D. and Belhadj, H. (2013) Lactic acid bacteria as probiotics: characteristics, selection criteria and role in immunomodulation of human gi muccosal barrier. Available at: http://cdn.intechopen.com/pdfs/42329/intech‐lactic_acid_bacteria_as_probiotics_characteristics_selection_criteria_and_role_in_immunomodulation_of_human_gi_muccosal_barrier.pdf (accessed 2 May 2017).
- Hasler, C.M. (2002) Functional foods: benefits, concerns and challenges – a position paper from the American Council on Science and Health. J. Nutr., 132(12): 3772–3781.
- Hayashi, H., Kumazawa, E., Saeki, Y. and Lahicka, Y. (1983) Continuous vacuum dryer for energy saving. Drying Technol., 1: 275–284.
- Heenan, C.N., Adams, M.C., Hosken, R.W. and Fleet, G.H. (2002) Growth medium for culturing probiotic bacteria for applications in vegetarian food products. Food Sci. Technol., 35: 171–176.
- Hellyer, T.J., DesJardin, L.E., Hehman, G.L., Cave, M.D. and Eisenach, K.D. (1999) Quantitative analysis of mRNA as a marker for viability of Mycobacterium tuberculosis. J. Clin. Microbiol., 37: 290–295.
- Holm, F. (2003) Gut health and diet: the benefits of probiotic and prebiotics on human health. World Ingredients, 2: 52–55.
- Holzapfel, W.H. (2006) Introduction to prebiotics and probiotics. In: Goktepe, I., Juneja, V.K. and Ahmedna, M. (eds) Probiotics in Food Safety and Human Health. New York: CRC Press, pp. 1–35.
- Hoskisson, P.A. and Hobbs, G. (2005) Continuous culture – making a comeback. Microbiology, 151: 3153–3159.
- Hudson, L.E., Fasken, M.B., McDermott, C.D. et al. (2014) Functional heterologous protein expression by genetically engineered probiotic yeast Saccharomyces boulardii. PLoS One, 9(11): e112660.
- Ibrahim, S.A. and Bezkorovainy, A. (1994) Growth promoting factors for Bifidobacterium longum. J. Food Sci., 59: 189–191.
- Jankowski, T., Zielinska, M. and Wysakowska, A. (1997) Encapsulation of lactic acid bacteria with alginate/starch capsules. Biotechnol. Technol., 11: 31–34.
- Johnson, J.A.C. and Etzel, M.R. (1995) Properties of Lactobacillus helveticus CNRZ‐32 attenuated by spray drying, freeze drying, or freezing. J. Dairy Sci., 78: 761–768.
- Jones, B.V., Begley, M., Hill, C., Gahan, C.G.M. and Marchesi, J.R. (2008) Functional and comparative metagenomic analysis of bile salt hydrolase activity in the human gut microbiome. Proc. Natl Acad. Sci. USA, 105: 13580–13585.
- Kailasapathy, K. (2002) Microencapsulation of probiotic bacteria: technology and potential applications. Curr. Iss. Intest. Microbiol., 3: 39–48.
- Kaur, S., Kullisaar, T., Mikelsaar, M. et al. (2008) Successful management of mild atopic dermatitis in adults with probiotics and emollients. Cent. Eur. J. Med., 3: 215–220.
- Kebary, K.M.K., Hussein, S.A. and Badawi, R.M. (1998) Improving viability of Bifidobacteria and their effect on frozen ice milk. Egypt. J. Dairy Sci., 26(2): 319–337.
- Kim, T.B., Song, S.H., Kang, S.C. and Oh, D.K. (2003) Quantitative comparison of lactose and glucose utilization in Bifidobacterium longum cultures. Biotechnol. Progr., 19: 672–675.
- Kim, W.S., Khunajakr, N. and Dunn. N.W. (1998) Effect of cold shock on protein synthesis and on cryotolerance of cells frozen for long periods in Lactococcus lactis. Cryobiology, 37: 86–91.
- Klijn, A., Mercenier, A. and Arigoni, F. (2005) Lessons from the genomes of bifidobacteria. FEMS Microbiol. Rev., 29: 491–509.
- Kołozyn‐Krajewskaa, D. and Dolatowski, Z.J. (2012) Probiotic meat products and human nutrition. Process. Biochem., 47: 1761–1772.
- Kourkoutas Y., Xolias V., Kallis M., Bezirtzoglou E. and Kanellaki M.(2005) Lactobacillus casei cell immobilization on fruit pieces for probiotic additive, fermented milk and lactic acid production. Proc. Biochem., 40(1): 411–416.
- Krasaekoopt, W., Bhandari, B. and Deeth, H.F. (2003) Evaluation of encapsulation techniques of probiotics for yoghurt. Int. Dairy J., 13: 3–13.
- Krishnakumar, V. and Gordon, I. R. (2001) Probiotics: challenges and opportunities. Dairy Ind. Int., 66: 38–40.
- Kullisaar, T., Songisepp, E., Mikelsaar, M., Zilmer, K., Vihalemm, T. and Zilmer, M. (2003) Antioxidative probiotic fermented goats’ milk decreases oxidative stress‐mediated atherogenicity in human subjects, Br. J. Nutr., 90: 449–456.
- Kullisaar, T., Songisepp, E., Aunapuu, M. et al. (2010) Complete glutathione system in probiotic L. fermentum ME‐3. Appl. Biochem. Microbiol., 46: 527–531.
- Kullisaar, T., Shepetova, J., Zilmer, K. et al. (2011) An antioxidant probiotic reduces postprandial lipemia and oxidative stress. Cent. Eur. J. Biol., 6: 32–40.
- Lacroix, C. and Yildirim, S. (2007) Fermentation technologies for the production of probiotics with high viability and functionality. Curr. Opin. Biotechnol., 18: 176–183.
- Ledeboer, A.M., Nauta, A., Sikkema, J. and Laulund, E. (1999) Technological aspects of making live, probiotic‐containing gut health foods. In: Halliwell, B. and Gutteridge, J.M.C. (eds) Free Radicals in Biology and Medicine. New York: Oxford University Press, pp. 1–7.
- Masco, L., Huys, G., de Brandt, E., Temmerman, R. and Swings, J. (2005) Culture‐dependent and culture‐independent qualitative analysis of probiotic products claimed to contain bifidobacteria. Int. J. Food Microbiol., 102: 221–230.
- Masco, L., Vanhoutte, T., Temmerman, R., Swings, J. and Huys, G. (2007) Evaluation of realtime PCR targeting the 16S rRNA and recA genes for the enumeration of bifidobacteria in probiotic products. Int. J. Food Microbiol., 113: 351–357.
- Matsuda, K., Tsuji, H., Asahara, T., Kado, Y. and Nomoto, K. (2007) Sensitive quantitative detection of commensal bacteria by rRNA‐targeted reverse transcription‐PCR. Appl. Environ. Microbiol., 73: 32–39.
- Mattila‐Sandholm, T., Myllarinen, P., Crittenden, R., Mogensen, G., Fonden, R. and Saarela. M. (2002) Technological challenges for future probiotic foods. Int. Dairy J., 12: 173–182.
- Maukonen, J., Alakomi, H.L., Nohynek, L. et al. (2006) Suitability of the fluorescent techniques for the enumeration of probiotic bacteria in commercial non‐dairy drinks and in pharmaceutical products. Food Res. Int., 39: 22–32.
- Meng, X.C., Stanton, C., Fitzgerald, G.F., Daly, C. and Ross, R.P. (2008) Anhydrobiotics: the challenges of drying probiotic cultures. Food Chem., 106: 1406–1416.
- Mikelsaar, M. and Zilmer, M. (2009) Lactobacillus fermentum ME‐3 an antimicrobial and antioxidative probiotic. Microb. Ecol. Health Dis., 21: 1–27.
- Mitsuoka, T. (1982) Recent trends in research on intestinal flora. Bifidobacteria Microflora 1: 3–24.
- Mortazavian, A., Razavi, S.H., Ehsani, M.R. and Sohrabvandi, S. (2007) Principles and methods of microencapsulation of probiotic microorganisms, Iran. J. Biotechnol., 5(1): 1–18.
- Mortazavian, A.M., Mohammadi, R. and Sohrabvandi, S. (2012) Delivery of probiotic microorganisms into gastrointestinal tract by food products. In: Brzozowski, T. (ed.) New Advances in Basic and Clinical Gastroenterology. InTech, pp. 122–146.
- Muller, J.A., Ross, R.P., Fitzgerald, G.F. and Stanton, C. (2009) Manufacture of probiotic bacteria. In: Charalampopoulos, D. and Rastall, R.A. (eds) Prebiotics and Probiotics: Science and Technology. Berlin: Springer, pp. 725–759.
- Nista, E.C., Candelli, M., Cremonini, F. et al. (2004) Bacillus clausii therapy to reduce side‐effects of anti‐Helicobacter pylori treatment: randomized, double‐blind, placebo controlled trial. Aliment. Pharmacol. Ther., 20: 1181–1188.
- Noriega, L., Gueimonde, M., Sanchez, B., Margolles, A. and de los Reyes‐Gavilan, C.G. (2004) Effect of the adaptation to high bile salts concentrations on glycosidic activity, survival at low pH and cross‐resistance to bile salts in Bifidobacterium. Int. J. Food Microbiol., 94: 79−86.
- O’Connell‐Motherway, M., van Sinderen, D., Morel‐Deville, F., Fitzgerald, G.F., Ehrlich, S.D. and Morel, P. (2000) Six putative two‐component regulatory systems isolated from Lactococcus lactis subsp. cremoris MG1363. Microbiology, 146: 935–947.
- Paul, E., Fages, J., Blanc, P., Goma, G. and Pareilleux, A. (1993) Survival of alginate‐entrapped cells of Azospirillum lipoferum during dehydration and storage in relation to water properties. Appl. Microbiol. Biotechnol., 40: 34–39.
- Piuri, M., Sanchez‐Rivas, C. and Ruzal, S.M. (2003) Adaptation to high salt in Lactobacillus: role of peptides and proteolytic enzymes. J. Appl. Microbiol., 95: 372–379.
- Playne, M.J., Bennet, L.E. and Smithers, G.W. (2003) Functional dairy foods and ingredients. Aust. J. Dairy Technol., 58: 242–264.
- Poolman, B., Blount, P., Folgering, J.H.A., Friesen, R.H.E., Moe, P. and van der Heide, T. (2002) How do membrane proteins sense water stress? Mol. Microbiol., 44: 889–902.
- Prasad, J., McJarrow, P. and Gopal, P. (2003) Heat and osmotic stress responses of probiotic Lactobacillus rhamnosus HN001 (DR20) in relation to viability after drying. Appl. Environ. Microbiol., 69: 917–925.
- Rastall, R.A. and Maitin, V. (2002) Prebiotics and synbiotics: towards the next generation. Curr. Opin. Biotechnol., 13: 490–498.
- Rau, B., Poch, B., Gansauge, F. et al. (2000) Pathophysiologic role of oxygen free radicals in acute pancreatitis: initiating event or mediator of tissue damage. Ann. Surg., 231: 352–360.
- Ridlon, J.M., Kang, D.J. and Hylemon, P.B. (2006) Bile salt bio transformations by human intestinal bacteria. J. Lipid Res., 47: 241–259.
- Ross, R.P., Desmond, C., Fitzgerald, G.F. and Stanton, C. (2005) Overcoming the technological hurdles in the development of probiotic foods. J. Appl. Microbiol., 98: 1410–1417.
- Roy, D. (2011) Probiotics. In: Butler, M., Webb, C., Moreira, A. et al. (eds) Comprehensive Biotechnology, vol. 4, 2nd edn. Amsterdam: Elsevier, pp. 591–600.
- Ruiz, L., Sanchez, B., Ruas‐Madiedo, P., de los Reyes‐Gavilan, C.G. and Margolles, A. (2007) Cell envelope changes in Bifidobacterium animalis ssp. lactis as a response to bile. FEMS Microbiol. Lett., 274: 316–322.
- Saarela, M., Rantala, M., Hallamaa, Nohynek, L., Virkajärvi, I. and Mättö, J. (2004) Stationary‐phase acid and heat treatments for improvement of the viability of probiotic lactobacilli and bifidobacteria. J. Appl. Microbiol., 96: 1205–1214.
- Salminen, S., Bouley, C., Boutron‐Ruault, M.C. et al. (1998) Functional food science and gastrointestinal physiology and function. Br. J. Nutr., 80(1): S147–S171.
- Sanchez, B., Ruiz, L., Gueimonde, M., Ruas‐Madiedo, P. and Margolles, A. (2012) Toward improving technological and functional properties of probiotics in foods. Trends Food Sci. Technol., 26: 56–63.
- Sanders, M.E., Walker, D.C., Walker, K.M., Aoyama, K. and Klaenhammer, T.R. (1996) Performance of commercial cultures in fluid milk applications. J. Dairy Sci., 79(6): 943–955.
- Santivarangkna, C., Kulozik, U. and Foerst, P. (2007) Alternative drying processes for the industrial preservation of lactic acid starter cultures. Biotechnol. Prog., 23(2): 302–315.
- Serrazanetti, D.I., Gottardi, D., Montanari C. and Gianotti, A. (2013) Dynamic stresses of lactic acid bacteria associated to fermentation processes. In: Kongo, M. (ed.) Lactic Acid Bacteria – R & D for Food, Health and Livestock Purposes. InTech, pp. 539–570.
- Shahidi, F. and Han, X.Q. (1993) Encapsulation of food ingredients. Crit. Rev. Food Sci. Nutr., 33: 501–547.
- Sieuwerts, S., de Bok, F.A.M., Hugenholtz, J. and van Hylckama Vlieg, J.E.T. (2008) Unraveling microbial interactions in food fermentations: from classical to genomics approaches. Appl. Environ. Microbiol., 74: 4997–5007.
- Songisepp, E., Kals, J., Kullisaar, T. et al. (2005) Evaluation of the functional efficacy of an antioxidative probiotic in healthy volunteers. Nutr. J., 4: 22.
- Stanton, C., Gardiner, G., Meehan, H. et al. (2001) Market potential for probiotics. Am. J. Clin. Nutr., 73: 476S–483S.
- Talwalkar, A. and Kailasapathy, K. (2004) The role of oxygen in the viability of probiotic bacteria with reference to L. acidophilus and Bifidobacterium spp. Curr. Issues Intest. Microbiol., 5: 1–8.
- Tanaka, H., Doesburg, K., Iwasaki, T. and Mierau, I. (1999) Screening of lactic acid bacteria for bile salt hydrolase activity. J. Dairy Sci., 82: 2530–2535.
- Taniguchi, K., Urasawa, T., Morita, Y., Greenberg, H.B. and Urasawa, S. (1987) Direct serotyping of human rotavirus in stools by an enzyme‐linked immunosorbent assay using serotype 1‐, 2‐, 3‐, and 4‐specific monoclonal antibodies to VP7. J. Infect. Dis., 155: 1159–1166.
- Teixeira, P., Castro, H., Mohacsi‐Farkas, C. and Kirby, R.J. (1997) Identification of sites of injury in Lactobacillus bulgaricus during heat stress. Appl. Microbiol., 83: 219–226.
- Van de Guchte, M., Serror, P., Chervaux, C., Smokvina, T., Erhlich, S.D. and Maguin, E. (2002) Stress responses in lactic acid bacteria. Antonie van Leeuwenhoek, 82: 187–216.
- Ventling, B.L. and Mistry, V.V. (1993) Growth characteristics of bifidobacteria in ultrafiltered milk. J. Dairy Sci., 76: 962–971.
- Vidhyalakshmi, R., Bhakyaraj, R. and Subhasree, R.S. (2009) Encapsulation “The future of probiotics”: a review. Adv. Biol. Res., 39: 96–103.
- Walker, D.C., Girgis, H.S. and Klaenhammer, T.R. (1999) The groESL chaperone operon of Lactobacillus johnsonii. Appl. Environ. Microbiol., 65(7): 3033–3041.
- Yousef, A.E. and Juneja, V.K. (2003) Microbial Stress Adaptation and Food Safety. Boca Raton: CRC Press.
- Zamora, L.M., Carretero, C. and Pares, D. (2006) Comparative survival rates of lactic acid bacteria isolated from blood, following spray‐drying and freeze‐drying. Food Sci. Technol. Int., 12: 77–84.