… And God said, “Let there be ultraviolet, visible and infrared radiation.”
Well, that's not an exact quote, but it certainly was a good decision. The Lord's Lightbulb, the sun, is the source of not only light, but all the energy we use on Earth, with the exception of energy from nuclear reactors, which humans invented in 1942, and Earth's own deep-down heat energy, which we are only now beginning to tap for practical purposes.
But the most apparent role that Old Sol plays—the only one, in fact, that most people ever think about—is that it provides the light we see by, the purifying light of day that brightens and illuminates all of Earth.
When any light—solar or artificial—strikes an object, some of it bounces off (is reflected), some of it is absorbed and transformed into heat, and some of it may even go straight through, as in the case—fortunately—of air, water and glass.
This chapter is a biography of light—what it is made of, where it comes from and goes to at its incredible speed of 186,000 miles per second (3 million kilometers per second), and how it can entertain us, trick us and burn us. As we follow this path of enlightenment we'll have occasion to play in the snow, go to the movies, watch television with a magnifying glass, cool ourselves with an electric fan, fool around with mirrors and even eat some candy that makes sparks in the dark.
Brighter Than Bright
Those brilliant Day-Glo colors—they're unreal! How can they be so much brighter than anything else? They look as if they're actually generating their own light.
They are.
In a Day-Glo-colored object there's a chemical that takes invisible ultraviolet radiation out of the daylight and converts it into visible light of the same color as the object. Thus, the object is not only reflecting its normal amount of colored light, but is actively emitting some light of the same color, which makes it look “extra-colored” and up to four times brighter.
The Day-Glo Color Corporation of Cleveland is only one manufacturer of what are called daylight fluorescent pigments. As the self-proclaimed world's largest supplier, it makes a dozen different colors, from aurora pink to saturn yellow. It sells the pigments to companies that put them and similar dyes into everything from orange safety vests and traffic cones to yellow tennis and golf balls and highlighting pens.
What's going on is fluorescence, a natural process by which certain kinds of molecules absorb radiation of one energy and re-emit it as radiation of a lower energy. The molecules in the pigment are absorbing ultraviolet radiation, a kind of short-wavelength radiation that human eyes can't see, and re-emitting it as a longer-wavelength light that human eyes can see. The radiation is, in effect, shifted from invisible to visible.
How do molecules absorb and re-emit radiation? Molecules contain lots of electrons that have certain specific amounts of energy characteristic of the particular molecule. But these electrons are always willing to take on certain amounts of extra energy from outside. (For more on this point, meet me in the Nitpicker's Corner.)
A molecule of a typical pigment may contain hundreds of swirling electrons of various energies. When a bullet of ultraviolet radiation (Techspeak: a photon;) hits such a molecule, it may kick some of those electrons up to higher energies. (Techspeak: The electrons become excited; honest, that's what scientists say.) But they can hold on to their overabundance of energy for only a few billionths of a second (a few nanoseconds) before spitting it back out as radiation again—usually as several photons of lower energies or longer wavelengths. It's sort of like spitting out buckshot after stopping a bullet.
Now the “buckshot” radiation, having somewhat less energy than ultraviolet radiation, falls into the region of radiations that human eyes can see: colored light. The net result is that the pigment molecule has absorbed invisible radiation and re-emitted it as visible radiation.
As long as the pigment molecules are being exposed to ultraviolet radiation—and daylight contains lots of it—they will be absorbing it and emitting light of a visible color. If the pigment happens to be orange to begin with and the emitted light is also orange, the dyed object will be an unnatural super-orange—“oranger” than you think it has any right to be.
TRY IT
Shine an ultraviolet lamp—a so-called black light lamp—on a Day-Glo fluorescent object, such as a paper with a few streaks of fluorescent highlighter on it or, if you're the type who wears them, your Day-Glo-printed T-shirt. The fluorescent dye will glow very much brighter than it does in daylight because the lamp puts out much more ultraviolet radiation. If you don't want to buy an ultraviolet lightbulb, take your streaked paper or T-shirt into one of those tacky stores that sell tasteless gifts and fluorescent posters, and use their black light for free.
By the way, if you use a fluorescent yellow highlighter on your books or notes, remember that it is brightest in daylight, which contains plenty of ultraviolet. Ordinary household incandescent lightbulbs give off very little ultraviolet light; moreover, their light is somewhat yellowish, and that washes out the yellow highlighter color. So when reviewing your highlighted book passages or notes by lamplight the night before an exam or a presentation, you may find to your chagrin that your highlighting is all but invisible. It's safer to use the stronger highlighter colors: orange, green or blue, whether fluorescent or not.

In my work as a professor, just about the only excuse I haven't heard from a student who did poorly on an exam is that the highlighting on his notes disappeared.
You Didn't Ask, but …
Why does a white shirt glow brightly under “black light”?
It's the same fluorescence phenomenon as the Day-Glo colors. Most laundry detergents contain “brighteners” that absorb ultraviolet radiation from daylight and re-emit the energy as a bluish light that makes the shirt look “whiter and brighter.”
Moreover, the blue cancels out any yellowish cast. When stimulated by an ultraviolet lamp, which is even richer in ultraviolet radiation than daylight is, the fluorescence becomes bright enough to see as an actual glow-in-the-dark luminescence.
You Didn't Ask This Either, but …
How do those luminous light sticks work?
You mean those plastic rods full of liquid chemicals that are made by Omniglow and other companies and are sold at street fairs, festivals and concerts and that start glowing with green, yellow or blue light when you bend them, and that gradually lose their light after an hour or so? Never heard of them.
Okay, seriously.
By now you know that a fluorescent dye needs to be stimulated by absorbing energy before it can re-emit that energy as light. But the stimulating energy need not be visible light or ultraviolet radiation; it can also be heat, electrical or chemical energy. In the case of the light sticks, the stimulating energy is chemical. When you bend the stick, you break a thin glass capsule containing a chemical, usually hydrogen peroxide, that reacts with another chemical in the tube. The reaction gives off energy, which is taken up by a fluorescent dye and re-emitted as light. As the chemical reaction gradually plays itself out because the chemicals are used up, the light fades.
NITPICKER'S CORNER
At several places in this book I talk about a substance's absorbing certain colors or wavelengths of light. You may be wondering how molecules actually absorb light, and what determines which wavelengths they absorb. If that problem is not exactly keeping you awake at night, Nitpicker's Corners are designed to be skippable.
A molecule has custody of all the electrons that belong to the atoms that it is made of. (Molecules are nothing but atoms glued together.) But electrons, and for that matter all subatomic particles, have a peculiar property: They can have only certain amounts of energy and no others. (Techspeak: The electrons' energies are quantized.) For example, the electrons in a certain kind of molecule can have energies A, B, C or D, etc., but never A-and-a-half or C-and-two-thirds. They can change their energies up or down among the values A, B, C, D—that is, from A to B or from D to C and so on—but they can never have values anywhere in between. Nobody can give you a reason for this; that's just the way it is. When you get down to things smaller than an atom, it's a different world from the one we see every day up here in big-land.
Now inasmuch as each unique substance is made up of its own unique molecules, it will have its own unique collection of electrons with their own unique sets of allowable energies. When light energy falls upon the substance, its electrons will absorb only those energies that correspond to its allowed energy jumps from A up to B or C, etc; it will reject and reflect the rest. This means that the substance is actually picking out the light energies (wavelengths) that it prefers, leaving the others to bounce back as reflected light.
And that's why every colored substance has its own color: the color of those wavelengths that it cannot absorb and that it reflects back for our eyes to see.
Snow White and the Seven Hues
Why is snow white? It's made of water, and water is colorless. So how come it turns white just by freezing?
First, we have to look at what “white” is.
You've heard people say dozens of times that white light is the presence of all colors. But other people tell you that white isn't a color at all, that it's the absence of color. You use bleach to remove all color from your laundry and make it white, don't you? So how can white be both all colors and no color?
The answer is that these two groups of well-meaning people are talking about two different things: white light and white objects.
White light, as it comes to us from the sun, is indeed a mixture of all possible colors—all visible wavelengths. Because we “grew up” as a species with sunlight as our natural, neutral, everyday light, we named it “white,” a word that has its origins in an Indo-European word meaning “bright” or “gleaming,” with no color implications at all. So white light is colorless light—to human eyes.
But in 1666 Sir Isaac Newton discovered that this neutral light can be broken down into a rainbow of component colors, simply by passing it through a triangular chunk of glass—a triangular prism. He then proved to himself that all these colors were indeed present in the original white light by recombining them: He projected overlapping rainbows onto a wall and saw that they combined to form white light.
Newton thought it would be a nice idea to divide the entire rainbow or spectrum of colors (and he invented the word “spectrum” for the purpose) into seven categories that would be analogous to the seven musical tones in an octave. For his color categories, he chose red, orange, yellow, green, blue, indigo and violet. Unfortunately, more than three centuries later we are still being taught in school that these are “the seven colors of the rainbow”—even though nobody seems to know what “indigo” is. Sir Isaac had to fudge a bit to eke out seven color names.
In reality, there are an infinite number of colors—both visible and invisible to human eyes—in the sun's light, just as there are an infinite number of possible musical tones. Change the wavelength of light or sound by an infinitesimal amount and you've got a brand-new color or tone, irrespective of whether humans can detect the difference. For example, there are dozens of different hues that we lump under the term “red,” limited only by our eyes' ability to distinguish them. It is said that the human eye can distinguish as many as 350,000 different hues. (Whose eye? I wonder.)
A white object, as distinguished from white light, is white because when white light falls upon it, it reflects all those zillions of colors back to our eyes equally, without changing the composition of the mixture at all. Its molecules just don't happen to be absorbers of visible light, so it appears to be the same “color” as the light that fell upon it: what we choose to call “white.” The object contributes no color of its own.
But colored objects are indeed contributing colors of their own. Their molecules are selectively absorbing and retaining certain of the sunlight's colors, reflecting back the others as an altered mixture.
Think of an actor on the stage, wearing a red cape over a white shirt. If you shine a red spotlight on him, he will appear red all over, the shirt as well as the cape. That's because the only light that any part of his costume can reflect back to us is red. No part of him can reflect back any green or blue light because it simply isn't receiving any.
Now shine a white spotlight on him. The red cape is still red, because that's the nature of the dye in it; that particular chemical was chosen because it absorbs all other colors in the white spotlight, reflecting back only the red. But the white shirt doesn't absorb any of the colors in the spotlight; it has no red dye in it (until the actor surreptitiously applies some in the stabbing scene). The shirt just sends the whole mixture of spotlight colors back to us, looking just as white as when they came out of the can.
Now let's get back to the snow before it all melts.
Snow is white, you now know, because its molecules reflect back to us all the colors in the sunlight. It doesn't selectively absorb any particular colors.
“But wait a minute,” you're thinking, “neither does liquid water; it's made of the same H2O molecules. So why isn't liquid water as white as the driven snow?”
Because liquid water is a poor reflector. When light hits it squarely, almost all of it goes straight in—penetrates—rather than bouncing back. In other words, liquid water is transparent. And if practically no light bounces back, it can't display much color, not even whiteness.
Snow, on the other hand, is an excellent reflector of light—whatever kind of light hits it. You want green snow? Hey, Sammy! Turn on the green lights! It's an excellent reflector because, unlike liquid water, which passively allows light to penetrate it, snow consists of zillions of ice crystals, each one a tiny jewel with dozens of sparkling facets that reflect light like mirrors. All of this white light bouncing back to our eyes, with its full complement of original colors intact, is what makes snow appear even whiter than the actor's sweaty shirt.
You Didn't Ask, but …
What is black? Is it a color?
A black surface is one whose molecules are absorbing all visible wavelengths of the light that is falling upon it, and reflecting virtually none of it back. So black isn't really a color, because we define a color in terms of the specific combination of light wavelengths that reflect back into our eyes.
But, of course, you can see a black object, so it must be reflecting some light back to our eyes. Hey, who's perfect? The light that a black object is reflecting comes from the fact that its surface has a small but unavoidable amount of shininess to it. So it reflects back some of the light that hits it at a glancing angle. That's why there are “light black” and “dark black” objects, depending on the microscopic glossiness of their surfaces. Go to a hardware store and look at the black paints; they're all equally black, but they'll range in reflectivity all the way from flat to glossy.
NITPICKER'S CORNER
I said that when light hits liquid water squarely, most of it goes straight in without being reflected. The emphasis was on that word “squarely.” As I'm sure you've observed, the surface of water can be a very good reflector of light that is hitting it obliquely—at a glancing angle. When the sun is low over a lake, for example, its reflection on the water can be almost blinding.
It's the same with snowflakes. Yes, they're made of transparent crystals of ice, but because there are zillions of them, all with complicated shapes, scattered helter-skelter with their smooth reflecting facets facing in all directions, the light is almost invariably striking the facets obliquely and being reflected. That's why snow is such a good reflector—so good, in fact, that skiers and other masochists who like to frolic in frigid weather have to wear very dark glasses to avoid “snow blindness.”
A final point. I let you get away with thinking that liquid water is colorless. It's almost colorless, but not quite.
School Colors
In science class they told us that the primary colors are blue, green and red. But in art class they told us that the primary colors are blue, yellow and red. Why can't artists and scientists agree?
Because they think of color differently.
Scientists describe objectively what Nature provides. They therefore think of color as a fundamental characteristic of light itself. To a scientist, light of different colors is radiation of different wavelengths. Artists, on the other hand, create their own interpretations of Nature. They therefore tend to think of color subjectively, as something to be manipulated with paints and dyes, rather than accepting light in its natural state.
Why, then, do these two camps have to use different primary colors—trios of colors that can be combined in different amounts to produce all other colors? In a nutshell, it's a matter of the primary colors of light versus the primary colors of pigments. As we'll see, they can be called the additive primaries and the subtractive primaries, respectively.
Light-minded (not light-headed) scientists claim that they can make light of any perceived color by combining blue, green and red light of various intensities. On the other hand, pigment-minded artists claim that they can tint an object any color by combining blue, yellow and red pigments in various amounts. And they're both right, because there is a fundamental difference between the color of light and the color of an object.
Colored light is a certain color because it is made up of a mixture of light waves of various wavelengths. The different-colored components add together to produce the net color. It happens that, because of the way our eyes work, blue, green and red light contain all the necessary wavelengths that need to be mixed in order to produce any perceived color. So blue, green and red are called the primary colors of light. (Understood: for human eyes.)
A colored object, on the other hand, is a certain color because of the wavelengths that it absorbs from the light that is falling on it. In other words, it subtracts certain wavelengths from the light and reflects the rest back to us as the color that we see. Various mixtures of blue, yellow and red pigments are capable of absorbing almost any combination of wavelengths. So blue, yellow and red are considered the primary colors for mixing paints and dyes. (But see below for a little hedging about these three colors.)
The light-based system of primary colors is called additive because different combinations of wavelengths add together to produce different colors of light. The pigment-based system of primary colors is called subtractive because different combinations of wavelengths are absorbed or removed from light to produce different colors of paints and dyes.
Let's look at the light primaries first, then the primaries for objects or pigments.
Light: The human eye—even an artist's eye—works on the additive principle. It has three kinds of color-sensitive cells (so-called cone cells) on the retina: One is most sensitive to blue light, one to green and one to red. Our perception of various colors depends on the relative degrees of stimulation of these three types of cells by the incoming light; the brain adds them together to produce sensations of various colors. That's why scientists—human chauvinists that they are—chose blue, green and red as the primary colors of light. (Muskrat scientists undoubtedly use a different set of primary colors.) Our eyes react only to stimulations of those three color receptors, so they are all we need in order to produce all humanly discernible hues. And that's why there are three, and only three, “primary” or fundamental colors of light.
Note that each kind of cone cell is not sensitive exclusively to pure blue, green or red light; each one is sensitive to a lesser degree to the other colors as well. That's why we can see pure yellow light, even though we don't have any yellow-sensitive cone cells. The yellow light slightly stimulates both the green and red cells, and our brains perceive that combination as yellow.
Your color TV and computer screens take advantage of the three-color idiosyncrasy of human vision. They contain blue, green and red phosphors (chemicals that glow when stimulated by electrons), glowing with varying brightnesses. The glows all add together to produce the colors that we perceive.
TRY IT
Look at the picture on your color TV or computer screen with a magnifying glass. You'll see that it's made up of tiny blue, green and red rectangles—no other colors—that are being stimulated to glow with varying brightness. Your eye blends them all together because the individual rectangles are too small to see at normal viewing distance. Added together in this way, the primary-colored rectangles make the hundreds of different hues that you perceive.
Pigments: The color film in your camera, on the other hand, makes its colors by the artist's subtractive system. It contains three layers of dyes that absorb or filter out blue, green and red. And the absorbers or filters that best absorb blue, green and red happen to be yellow, red and blue, respectively. So yellow, red and blue are the three subtractive colors in color film.
But are these color-film filters the same old yellow, red and blue colors that your art teacher told you are the artist's subtractive primary colors? Sort of, but not exactly.
Here's the hedging that I promised you: The three colors that are really best at absorbing the blue, green and red that our cones are most sensitive to are yellow, a purplish red called magenta and a greenish blue called cyan. Yellow, magenta and cyan are therefore the three real primary subtractive colors that are used in concocting the entire spectrum of ink, photography and paint colors.

All artists, from kindergarten kids with crayons to the subtlest watercolorists, could create their entire palettes by mixing various amounts of yellow, magenta and cyan. But it's a lot easier to buy paints and crayons already blended.
Let There Be Fluorescence!
How do fluorescent lamps make so much light without a lot of heat? And when one burns out, can I replace it with any tube that fits, or are there different kinds?
Fluorescent lights were invented for one purpose: to confuse you. I'm glad to see that they're doing their job.
When an ordinary incandescent lightbulb burns out you can just screw in a new one with the help of a certain number of friends, depending on your vocation or ethnicity. But when a fluorescent light burns out you look at the tube to find out what kind to replace it with and you see markings that look something like “F20CW-T12.” If you replace it with the “F15W-A10” that you saw in the store, will it explode when you turn it on?
Cheer up. 'Tis better to light a candle and read this book than to curse the darkness.
First, let's decipher those hieroglyphics on the tube. They're a secret code that divulges everything about the bulb. Not to you, the poor consumer, of course, but to the people who make and sell them, who apparently have a need to appear smarter than you are.
I'm going to tell you how the secret code works. (I suppose that now they'll have to kill me.)
Any given fluorescent tube is either straight, U-shaped or circular in shape; it has a certain wattage; it gives off a certain color of light; and it has a certain diameter. The letters and numbers on the tube give this information in that order: shape, wattage, color, diameter. The only trouble is that you have to know how this information is coded.
For shape, it's a U or a C for U-shaped or circular, and no letter at all if it's straight. Then comes the wattage: 4, 5, 8, 13, 15, 20, 30, 40 or whatever. (The wattage is generally lower than for comparable light-producing incandescents, because fluorescent lighting is from two to four times more efficient.) Then comes the color code: W for white, CW for cool white, WW for warm white, plus abbreviations for other exotic colors that we needn't bother with. Last comes the tube's diameter, but it is given—would you believe?—in eighths of an inch: T8 means a tube that is eight-eighths of an inch in diameter, which any sane human being would call one inch. A T12 tube is twelve-eighths or one-and-a-half inches in diameter, and so on.
Pop Quiz: Describe the properties of an F40CWT10 fluorescent bulb. (Answer at the end of this section.)
Oh, I forgot to tell you: The codes always begin with an F for “fluorescent,” presumably to keep you from trying to screw them into an ordinary lamp socket. (How many idiots does it take to screw a fluorescent tube into an incandescent lamp socket?)
As an alert consumer, you may have noticed that you can't replace an 18-inch-long tube with one that is 24 inches long. The manufacturers graciously give you enough credit to make that decision on your own, so you won't find a length code on the tubes.
Okay, now. How do the things work?
You know that ordinary incandescent lamps, including halogen lamps, make light by electrically heating a filament to white heat. The outside of the lamp bulb can get up to temperatures of several hundred degrees. Fluorescent lamps work on an entirely different principle.
The fluorescent tube is filled with a small amount of inert gas (usually argon) plus a drop's worth of mercury. At each end is a small filament that is heated by the electric current so that it emits electrons. (You don't know why a hot filament emits electrons? Go stand in the corner. The Nitpicker's Corner, that is.)
The electrons emitted from the filaments fly through the gas in the tube to get from one filament to the other, and in the process they collide with mercury atoms, which have been vaporized by the filaments' heat. The mercury atoms absorb the collision energy and spit it out again as light energy. But we can't see that light because it's in the ultraviolet region of wavelengths, so it has to be converted into light that humans can see. This is accomplished by that white coating on the inside of the tube. It consists of chemicals (calcium and strontium phosphates and silicates) that absorb ultraviolet light and re-emit it as visible light; this wavelength-shifting process is called fluorescence.
Fluorescent lamps are cooler than incandescent lamps because they have only those two little mildly heated filaments at the ends and the fluorescence process itself doesn't produce any heat. But they're hard to start, because the filaments' electrons first have to blast their way through the gas in the entire length of the tube. That requires several hundred volts of push, but our household voltage is only 115 volts. So something has to provide an initial voltage kick to the electrons.
That's what the starter does—or the ballast. And here's where it gets really confusing, because there are several kinds of fluorescent lamp systems and circuits. Some have ballasts, those heavy little iron transformers, while others have starters, those little aluminum cans. And some have both. Fuhgeddaboudit. You don't hafta know.
What to do when your fixture of unknown breed won't light up? First, replace the tube with one that has identical code numbers on it. You can't even substitute a different wattage, as you can with incandescent bulbs; that can cause dangerous overheating of the ballast, which was designed for the other wattage. The only freedom you have is to swap a cool white for a warm white or vice versa, or to substitute one of the many other “deluxe” colors. If your fixture has one of those little starter cans in it, you may as well replace that too; they're cheap and they simply twist in and out of the socket.
If you're still in the dark, both literally and figuratively, buy a whole new fixture.
Oh, and an F40CWT10 is a 40-watt, cool white, one-and-a-quarter-inch straight fluorescent tube.
You Didn't Ask, but …
Why do small fluorescent tubes cost so much more than the big four-foot-long “shop lights”?
You can buy the common, 48-inch shop-light tube in home centers for a couple of dollars, whereas a small, thin fluorescent tube for under the kitchen cabinet might cost up to five times that amount. The answer is that the four-footers, used by the thousands in schools, factories and office buildings, vastly outsell the smaller, more specialized tubes and are mass-produced at a much lower unit cost. It's a classic textbook case of supply and demand.
NITPICKER'S CORNER
Why do the filaments in a fluorescent tube emit electrons when they're heated?
Almost anything will emit electrons if heated hot enough. Atoms contain negatively charged electrons, which are held on to with various degrees of strength, depending on which atoms we're talking about. Metal atoms hold on to their electrons very loosely. When you heat a metal, some of those electrons gain enough energy to detach themselves completely from their atoms and go flying off.
In a fluorescent tube there are two filaments, one at each end, both getting hot because of their resistance to the flow of a sixty-cycle alternating electric current (a current that continually reverses its direction). At any given instant, one filament is negatively charged with respect to the other, but a hundred-twentieth (half a sixtieth) of a second later it becomes positively charged with respect to the other. At any instant, the electrons from the negative filament are attracted to the positive filament, and the only way they can get there is to plow through the intervening mercury vapor in the tube, making it emit ultraviolet radiation.
Star Light, Star Bright, Which Bulb Should I Use Tonight?
What's so special about halogen lightbulbs?
They contain a gas called a halogen, which makes them brighter, whiter, more efficient and longer-lasting. And, of course, much more expensive.
A halogen lamp is a variation on the standard incandescent, as opposed to fluorescent, lamp. An incandescent lamp contains a tungsten filament enclosed in a glass bulb filled with gas. An electric current heats the filament to incandescence—a white-hot glow. It may look very bright, but in reality only 10 to 12 percent of the energy it emits is visible light; about 70 percent of it is invisible infrared radiation, which heats, rather than illuminates.
In a regular bulb, the gas inside is an inert (unreactive) one such as argon or krypton with some added nitrogen. These inert gases keep the tungsten from oxidizing, or “burning up,” as it would in air. Some smaller bulbs solve the problem by being completely evacuated; there's practically no gas inside at all.
In a halogen bulb, the gas is usually iodine or occasionally bromine, two highly reactive chemical elements in the family that chemists call halogens. They perform a two-step chemical dance that makes the filament last twice as long. But first, we have to understand how the standard bulb works.
The filament is a coil of thin tungsten wire. Tungsten is used because it has the highest melting point of all metals—6200 degrees Fahrenheit (3400 degrees Celsius)—and it stays strong even at white-hot temperatures of 4500 degrees Fahrenheit (2500 degrees Celsius) or higher. Moreover, it has the lowest vapor pressure of all metals, meaning that it evaporates less than any others. Yes, even metals evaporate a few atoms now and then, but so slowly that we never notice it except at very high temperatures. (Never fear; your gold jewelry isn't going to dry up.)
When it is white-hot, even tungsten will evaporate enough so that the filament gets thinner and thinner as the bulb burns, until it finally breaks apart and interrupts the electric circuit. That's when your bulb burns out. For some time before this disaster strikes, you can see the evaporated tungsten as a dark coating on the inside of the glass, where it has condensed because of the glass's relatively low temperature. This darkening, of course, progressively cuts down on the amount of light that the bulb puts out as it ages.
Sometimes a bulb's filament will have developed such a thin spot that it will blow out suddenly when you turn on the switch. The blue flash that you see is an electric arc, leaping across the widening gap as the thin spot evaporates completely under the heat stress of the power surge.
Tip: When a bulb burns out, try tapping or shaking it gently while the power is on. Sometimes you can get the broken ends close enough together so that an arc will flow between them and weld them back together, rewarding you with perhaps an hour or so of life-after-death experience.
What halogen-filled bulbs do is to cut down the evaporation rate of the tungsten in a very interesting way. First, the iodine vapor reacts with the evaporated tungsten atoms before they can condense out on the glass and converts them to tungsten iodide, a gaseous chemical compound. The molecules of tungsten iodide then float around inside the bulb until they happen to encounter the white-hot filament, whereupon the high temperature breaks them back down again into iodine vapor and metallic tungsten, which deposits itself back on the filament. The released iodine is then free to apprehend and deliver more tungsten atoms, and the cycle continues, with the iodine atoms continually capturing evaporated tungsten atoms and returning them to the filament. This recycling process approximately doubles the life of the filament, and hence of the bulb.
The halogen process allows the lamp to be operated at a much higher temperature without excessive deterioration of the filament, and that makes a brighter, whiter light. In fact, the temperature of the bulb's inside wall has to be high—above about 480 degrees Fahrenheit (250 degrees Celsius)—to keep the tungsten atoms from condensing on it before the iodine vapor can grab them.
Halogen bulbs are made of quartz, which withstands much higher temperatures—and is more expensive—than ordinary glass. They are usually tube-shaped and closely surround the filaments to stay hot. In fact, tungsten lamps burn so hot that they can be a fire hazard if used too close to flammable materials such as curtains.
You Didn't Ask, but …
Why don't lightbulbs last longer than they do?
Lightbulbs are very carefully engineered to last for a certain length of time. A suspicious person might be tempted to say that they are carefully engineered to burn out after a certain length of time. There is no reason that a lightbulb couldn't be designed to last almost indefinitely. But you probably wouldn't like it.
As with most devices, there is a trade-off among several conflicting considerations. More than anything, the life of a bulb depends on the running temperature of the filament. For a given wattage (the amount of electric power consumption), the higher the temperature and light output, the shorter the lifetime.
“Long-life” lightbulbs have filaments that are designed to glow at a lower temperature. But the lower temperature doesn't produce as much light. Also, since higher temperatures produce a bluer, whiter light, the long-life bulbs can have a slightly yellowish cast by comparison.
Long-life bulbs achieve their lower temperatures by using a filament that allows less electrical current to pass through. Less current flow makes less heat and less light, so you get not only a yellower light, but less of it. If you buy long-life bulbs, you have to buy a higher wattage than usual to get the amount of light you expect from a normal bulb.
By law, the packaging of standard lightbulbs must tell you the number of hours they are intended to last and the amount of light they put out in all directions: the number of lumens. Compare the numbers of hours and lumens on a long-life package with the numbers on a regular package of comparable wattage. If you're willing to put up with the lesser amount of light and higher price for the convenience of not having to change the bulb for a longer period of time, buy the long-lifer.
On the other hand, if you're a compulsive discount shopper for standard lightbulbs, take your calculator to the store. For a given wattage, you want the most light for the longest time at the lowest price. Divide the price in cents by the number of lumens, and then divide the result by the number of hours of expected lifetime. The smallest number is the best bargain.
And speaking of saving money, a dimmer switch reduces the voltage applied to the bulb, which reduces the current flowing through the filament, which reduces the temperature, which reduces the evaporation of tungsten, which considerably increases the lifetime of the bulb. The next best thing to turning out the lights when you leave a room is to dim them.
Mirror, Mirror, on the Wall, How Come You Don't Invert at All?
When I look in the mirror and raise my right hand, my image raises its left hand. And yet both our heads are still on top. Why does a mirror reverse things right to left, but not top to bottom?
This is one of those loaded questions that can drive you crazy because the question itself is misleading. It starts with a mistaken assertion and asks us to carry on our reasoning from that point. But you can't pursue the road to truth if somebody starts you off in the wrong direction.
A mirror does not reverse things right to left. It reverses things front to back; it reverses in and out.
Read that again.
And think about it.
All a mirror can do is reverse a direction. It can't rotate anything. It's you that imagines yourself rotated. The mirror didn't do it.
Stand in front of a full-length mirror. Let's name the person in the mirror Egami. Now how do you think Egami got that way, with his left arm toward your right and his right arm toward your left? I'll bet you seven years of bad luck that you think Egami got that way by your turning around—by your rotating half a turn, executing an about-face. That's why you think right and left have been reversed. You did it yourself, by turning yourself around—in your imagination.
But that's not what the mirror did.
All the mirror did by taking its incoming light and shooting it back at you was to reverse the direction of the light. Egami is simply you with your “toward” and “away” directions reversed. You are, of course, in the habit of looking away from yourself, but Egami is looking toward you; if you're facing north, Egami is facing south. And whenever a person is facing in the opposite direction from you and looking toward you, his left arm will be on your right, no? What's so unusual about that? No rotations or right-to-left swaps are needed.
Notice that the words “up,” “down,” “top” and “bottom” appear nowhere in the foregoing. They're completely irrelevant to two people who are facing each other. “Up” and “down” mean exactly the same thing to both of them. Unless, of course, one of them is standing on his head.
How can we get one of them to stand on his head? Easy. Hold the mirror high above your head and parallel to the floor. Or else put the mirror on the floor and stand near (not on!) it. Egami is now standing on his head, isn't he? Which proves that the mirror reverses only its in and out directions, which from your current viewpoint just happens to be up and down.
You can see the same up-down reversal in the mirrorlike surface of a small, calm lake or pond. Look at the reflection of the trees on the other side. They're upside down, aren't they?
And by the way, I've referred to Egami with masculine pronouns to avoid “him-or-her”-ing all over the place in an explanation that you may think is already complex enough. If you're female, please don't think I'm saying that mirrors reverse gender. (And don't wear a skirt when you put that mirror on the floor.)
Oh, the name? If you haven't yet figured it out, Egami is “image,” reversed from right to left.
You Didn't Ask, but …
When I look into the bowl of a shiny spoon, my image is both reversed right to left and inverted upside down. How does it do that?
I just finished explaining that the image isn't really reversed right to left, so let's put that aside. But indeed, how about the upside-down inversion?
The spoon's inner surface is concave—that is, it is hollow like a cave. (That's a good way to remember the distinction between con cave and con vex.) When you look into the spoon, you'll notice that the top part is shaped so that it reflects its light slightly downward, like a mirror held high. At the same time, the bottom part is shaped so that it reflects its light slightly upward, like a mirror on the floor. These “high” and “low” reflectors give you a stand-on-the-head image, exactly as the above-your-head and on-the-floor mirrors did in the preceding explanation.
Mirror, Mirror, on the Wall, Who's the Sharpest One of All?
I'm nearsighted. When I look in the bathroom mirror without my glasses on I can see my beautiful face quite clearly, but everything else in the room is blurred. Shouldn't everything be equally clear, because all the images in the mirror are equally close to my eyes?
The distance from your eyes to the mirror is irrelevant. It's the distance from your eyes to a given object that counts, just as it would when you look at it without the mirror.
The light reflected from an object has to get to your eyes somehow, or else you wouldn't see it. The light coming from things behind your back would never get to your eyes if the mirror weren't there to turn it around. That's all the mirror does: It takes light that would have passed you by and shoots it back at your eyes.
Suppose you're facing the mirror and looking at an object behind you. Instead of coming straight from the object to your eyes, the light has to pass you by, go to the mirror and then come back to your eyes. That's a greater distance than if you had been facing the object, so it is even blurrier than if you had turned around and looked at it directly. The image of your beautiful face is also blurrier than if you were looking at it from the position of the mirror. The light has to go from your beautiful face to the mirror and back to your beautiful eyes—twice as far as if you were looking at your beautiful face from the position of the mirror.
This is all based on the fact that the farther away an object is, the fuzzier it will appear to nearsighted eyes. That's generally true, and here's why.
Nearsighted eyes are good at focusing light rays that are diverging, radiating out in all directions, as they are from a nearby object. But nearsighted eyes are not so good at focusing light rays that are more or less parallel, as they are from a distant object. It's not that near and distant objects are shooting their light out differently; every object reflects light in many directions. (Remember how we drew a shining sun in kindergarten, with all those rays coming out in all directions?) But when you're far away from an object, your eyes are intercepting only a small fraction of those “all directions” rays. It's as if all the rays are now coming from the same, severely limited direction, like a bundle of parallel sticks, all pointing from the object straight at you. And that's the situation—focusing parallel rays—that nearsighted eyes can't handle well, so the object is blurred.
They Went … Which-a-way?
In western movies, why do the stagecoach wheels sometimes turn backward?
This is the only remaining artificiality in today's remarkable, computer-driven movie effects, which can make anything imaginable look real, no matter how bizarre—except, ironically, an old-fashioned stagecoach wheel. You can also see the effect with automobile wheels, in those television commercials that show the cars speeding along an open road.
If you watch carefully, you'll see that the wheels go backward only some of the time; at other times they look as if they're rolling forward rather slowly, and at still other times they seem to stop entirely, making the coach look like a sleigh. It's all a matter of timing—the speed of the rolling wheel compared with the speed of the camera's pictures.
A movie camera takes a series of still pictures at the rate of 24 per second, or 24 “frames per second.” Fortunately for Hollywood, our slow human brains can't assimilate so many separate pictures and we perceive them as all run together, as if the objects in them were progressing smoothly from one position to the next. (Actually, it's our eyes that can't separate the images if they come too close together: Our brains are fast enough. But it still takes my brain more than an hour—if then—to understand what's going on in some movies.)
Let's say that one of the wagon wheel's spokes is painted red. And let's say that when the camera snaps picture number one, the red spoke is pointing straight up, at the twelve o'clock position. Depending on the speed of rotation of the wheel, when picture number two is snapped a twenty-fourth of a second later, the red spoke might happen to be caught in the one o'clock position—even if it had made a couple of complete turns in the interim. That makes it look as if it had moved to the right, or clockwise. Or, it might happen to be caught in the eleven o'clock position, making it look as if it had moved to the left, or counterclockwise. As the camera continues to take its 24 pictures per second, the red spoke—together with the rest of the wheel—will look as if it is moving continuously, either clockwise or counterclockwise.
For extra credit, as we professors like to say, can you figure out how fast the wheel appears to be rotating in this example? (The answer can be found at the end of this section.)
So depending on the number of spokes in the wheel and the actual rotational speed of the wheel compared with the 24 frames per second at which the film was shot, the wheel can appear to be moving forward or backward or—when the spoke speed just happens to be synchronized with the camera's shooting speed—not moving at all. This last is a highly specific coincidence, so it doesn't happen often. But if you look closely, you can see the wheel “stop” briefly as it passes from “forward” motion, when the spoke is slightly ahead of the camera clicks, to “backward” motion, when it is slightly behind.
In reality, of course, the wheels don't have one red spoke; they all look alike. Any spoke is a double for any other. Therefore, any spoke at all might be in the one o'clock or eleven o'clock position when the camera's shutter clicks, and it will still look as if the wheel is turning right or left.
When the wheel is going fast enough, the spokes are moving too fast for the camera's shutter speed to stop their motion. They therefore degenerate into a blur, and the whole effect of backward or forward motion disappears.
You can see exactly the same effects in movies that depict a later mode of transportation: propeller-driven airplanes. When the plane's engine is started, the propeller looks as if it is alternating between the clockwise and counterclockwise directions. As its speed increases, the blades pass through successive “slightly ahead” and “slightly behind” positions with respect to when the camera clicks. As their speed becomes fast enough, the blades become a blur.
Want to see the same effects at home, but you don't have a stagecoach or airplane handy? Try this.
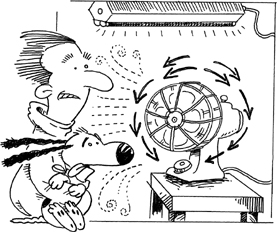
TRY IT
If you have a portable electric fan, take it into a room that is illuminated with fluorescent light. When you turn the fan on and it speeds up, the blades will appear to be rotating first in one direction and then the other. That's because fluorescent lights flicker on and off 120 times a second (yes, 120; visit the Nitpicker's Corner), and that's five times faster than a projected movie, so we are unaware of the flickering. The “on” flickers are what you see by, so it's just the same as if you were being presented with a series of rapid frames in a movie theater.
NITPICKER'S CORNER
Fluorescent lights run on alternating current (AC). That means that the electricity flows in one direction for half the time and in the opposite direction the rest of the time. In the U.S., the AC frequency is sixty cycles per second, meaning that one full cycle takes a sixtieth of a second.
Let's say that the current is “positive” for the first half-cycle and “negative” for the other half-cycle. That means that it is “positive” for a hundred-twentieth of a second (half of a sixtieth) and then is “negative” for the next hundred-twentieth of a second, and so on. Thus, there are two current surges (albeit in opposite directions) during each sixtieth of a second, for a total of 120 surges per second. A fluorescent light is “on” only during the current surges, so you might say that it behaves like a movie camera that is snapping 120 pictures per second.
You Didn't Ask, but …
Why did everybody look as if they were moving so fast in movies from the early days of the last century (the twentieth, that is)?
Photographic film wasn't as sensitive as it is today, so the exposures had to be longer and therefore further apart in time. The cameras shot only 16 pictures per second, rather than 24. In that longer amount of time between pictures, the people moved farther, so in a second's worth of pictures they seem to have covered more distance. More distance per second equals faster.
Answer to the extra-credit question: There are twelve positions on the clock and the camera is catching the red spoke at the next position every twenty-fourth of a second. The spoke therefore makes one full revolution in twelve twenty-fourths of a second, or half a second. One revolution per half-second is two revolutions per second, or 120 revolutions per minute (rpm).
Damned Spot!
Why does the wet spot on a fabric look darker?
I'll assume that you're in the dining room, concerned about soup on your necktie, although you may have noticed this phenomenon in other rooms under different circumstances.
We see an object because light is coming from that object and entering our eyes. The more light coming from the object, the brighter it appears. And of course, the reverse is also true: An object that is sending less light to our eyes appears darker. So our job is to explain why there is less light coming from the wet spot.
Where does an object get the light that it sends to our eyes? If it is not inherently luminous, like the sun, a lightbulb or Rudolph's nose, then it must be reflecting some of the light that it receives from elsewhere. But nothing reflects all of the light that falls upon it; every substance absorbs some light and returns, or reflects, the rest. So the wet spot must be reflecting less light because for some reason it is absorbing more.
Let's take a highly magnified look at the wet fabric as it would be seen by an incoming ray of light.
A fabric is a latticework of interwoven fibers. When it gets wet and soaks up water by capillary action, the spaces between the fibers become filled with water. Many of the incoming rays of light will then be falling upon a water surface instead of striking a fiber.
Now when a ray of light enters a water surface at an angle—and by sheer statistics most of the rays will be hitting the water at an angle, rather than perfectly perpendicular to its surface—a funny thing happens: The ray changes direction. (Techspeak: It is refracted. Why does it change direction? Meet me in the Nitpicker's Corner.) Instead of continuing through the water in the direction in which it entered, the light ray veers away from the surface and plunges into the watery depths at an even steeper angle than its entry angle. This steeper angle of penetration means that the light ray penetrates deeper into the depths of the fabric, where it has an increased chance of being absorbed, never to be seen again. Thus, there is more “lost light” inside a wet spot than in a dry one, there is less light reflected and the spot appears darker.
Similar goings-on explain why wet rocks, leaves and grass appear to be more intensely colored when they're wet—why the countryside looks “fresher” after a rain. These objects have colors in the first place because they absorb certain wavelengths of light from the multicolored daylight and reflect the rest back to our eyes. When they are coated with a film of water, the incident light rays are refracted deeper into their microscopically rough surfaces. The refracted light then bounces back and forth off these surfaces, which provides them with many more opportunities for their absorbable wavelengths to be absorbed. The remaining reflected light is thus even more depleted in these absorbed wavelengths than it ordinarily would be, and it therefore looks more intensely colored.
NITPICKER'S CORNER
Why is light “bent” when it enters water?
Whenever a scientist has to explain something about light, he or she has the choice of explaining it on the basis of light waves or light particles (Techspeak: photons), because light behaves as if it were both or either a particle and/or a wave. Explaining refraction on the basis of light's being a wave would require my drawing a diagram and using such terms as “wave front” and “phase velocity,” which would make this look too much like (heaven forbid) a science book. So I'll take the easy way out and talk about refraction as if the light ray were a photon bullet.
Better yet, an arrow.
If you stand at the edge of a swimming pool (DO NOT TRY THIS AT HOME!) and shoot an arrow into the water at an angle—not straight down—you won't be surprised to observe that the arrow loses speed as it enters the water and swerves downward, away from the surface. That's because the arrow must travel more slowly in water than in air, and the drag slows down its forward speed. Well, the same thing happens if the arrow is a stream of photons. As they enter the water they slow down and change their direction to a steeper angle than the one at which they entered. The light stream has been refracted. (Note that if you had shot straight down, the arrow would have been slowed, but its direction wouldn't have been changed. It's the same with light; if it enters the water perpendicular to the surface, its direction isn't changed.) Did I say that light is slowed down when it enters the water? Yes, indeed. But isn't the speed of light always the same? Indeed, no.
When people talk about “the speed of light” as being 186,000 miles per second (3 million kilometers per second), they should always be careful to add “in a vacuum.” Because when light enters a transparent medium it slows down, and different transparent media slow it down to different degrees. The speed of light in water, for example, is only three-quarters as fast as it is in air. And that slowing down leads to the bending of the light when it enters water from air.
The bending —refraction— of light is even greater when it enters glass from air, because the speed of light in various types of glass is only 50 or 60 percent of its speed in air. Which is just great, because that allows us to use specially shaped pieces of glass—lenses—to really bend light a lot and make all sorts of clever gadgets such as telescopes, microscopes and eyeglasses.
Spurn That Burn
My dermatologist told me that a sunscreen lotion labeled SPF 30 does not block out twice as much harmful radiation as one labeled SPF 15. What gives?
Your doctor is correct. The SPF numbers aren't sun -filtering factors—they're sun -protecting factors. SPF stands for “sun protection factor.” The numbers are not telling you how much radiation they block out, but how much time you can spend in the sun before your skin turns red, a condition doctors call erythema. And that's quite another matter.
With an SPF 15 on you, you can stay out in the sun fifteen times longer than with bare skin. With an SPF 30, you can stay out thirty times longer than with bare skin. That's twice as long as with an SPF 15. And yet an SPF 30 blocks out only about 3 percent more of the harmful radiations than an SPF 15 does!
I'm well aware that the foregoing is probably the most confusing paragraph you have ever read outside of an IRS publication. But I'll show you that it's all quite logical.
First, though, what are those menacing radiations that rain down upon us from our life-giving star? The sun's atoms, being as hot as they are (about 9800 degrees Fahrenheit or 5400 degrees Celsius at the sun's surface), are continually giving off radiations of almost every energy … uh, under the sun, ranging from radio waves to X rays. The dangerous X rays are pretty much filtered out by Earth's atmosphere, while the sun's radio waves are substantially less harmful than those emanating from a hard-rock radio station. That leaves only visible light and two types of invisible radiations: infrared, which warms us but doesn't burn us, and ultraviolet. This last one is the villain.
Ultraviolet (UV) radiation is usually subdivided into three regions of energy, which scientists have imaginatively labeled A, B and C. We can eliminate ultraviolet C (abbreviated UVC) from our fears, because it is absorbed by the atmosphere's ozone layer, which, though threatened by human activities, is still pretty much up there. So the only things we have to worry about at the beach besides our paunches and cellulite are UVA and UVB, which can cause not only sunburn, but permanent skin damage and cancer.
Sunscreens are a mixture of active chemicals in a cosmetically appealing base. The molecules of any chemical selectively absorb radiations of specific energies. The sunscreen chemicals have prodigious appetites for absorbing ultraviolet radiation, even when in extremely thin layers on the skin. On the labels of sunscreen containers, you'll see UVA absorbers such as avobenzone or Parsol; UVB absorbers such as octyl methoxycinnamate and other cinnamates, homosalate, octyl salicylate and padimate O; and double-threat UVA-UVB absorbers such as oxybenzone and other benzophenones. A chemical called PABA used to be popular, but it irritated some people's skin and is no longer used.
Okay, chemistry class dismissed. But I thought you'd like to be able to interpret the ingredient lists on the product labels.
Not to worry about the names, however. Most products are carefully balanced witches' brews of chemicals designed to absorb the entire range of harmful UV energies. But remember that they are tested primarily for burn prevention, whereas research continues to find certain UV energies to be worse than others at causing premature skin aging or cancer. It's best to choose a “broad spectrum” sunscreen to cover both your back and your bets.
Now back to those tricky SPF numbers. It's all in the arithmetic. Watch me. Nothing up my sleeve.
Suppose that Brand X sunscreen cuts out half—50 percent—of the burn-producing UV rays. Obviously, you could stay out twice as long as usual without burning. If you'd ordinarily burn in one hour with no protection, you could stay out for two hours. In other words, the SPF is 2.
Now suppose that Brand Y cuts out 75 percent of the UV rays, which means that you're being exposed to only 25 percent of the burning rays instead of 100 percent. You'd be able to stay out four times as long as with no protection, wouldn't you? (100 ÷ 25 = 4.) The SPF then is 4. Brand Y cuts out only 25 percent more of the UV rays than Brand X does, yet its SPF is twice as high: 4 instead of 2!
I won't go through the algebraic derivation (do I hear a release of bated breath?), but if you want to figure out the percent of absorbed burning rays from an SPF number, here's how: subtract 1 from the SPF, multiply by 100, and divide the result by the SPF. For example, for an SPF of 20: 20 ‒1 = 19; times 100 = 1,900; divided by 20 = 95 percent absorption.
In that way, you can figure out that an SPF of 15 absorbs 93.3 percent of the UV rays, while a twice-as-big SPF of 30 absorbs 96.7 percent, only 3.4 percent more.
You see that by paying more money for a higher-SPF product, you're blocking only a small amount of additional radiation. It's a classic case of diminishing returns. Even if you're a creamy-skinned redhead whose skin tends to match your hair after an hour in the sun, you don't really need an SPF of more than, say, 30. What makes you think you're going to be outdoors for more than thirty hours, anyway? The sun does have a habit of setting, you know.
BAR BET
A sunscreen rated at SPF 30 allows you to stay out in the sun twice as long as an SPF 15, yet it cuts out only about 3 percent more of the sunburning rays.
Wrong, Wrong, Wrong!
Those “light windmills” that we see spinning around in the windows of novelty stores: What makes them work?
They're called radiometers and are generally supposed to illustrate that light has pressure. But they don't. If a machine could be a con artist, this gadget would take the cake.
You've seen them. They look like a lightbulb on a stand. Inside the bulb, which has had most of the air pumped out, are four thin, metal vanes, mounted like a pinwheel on a low-friction pivot. One side of each vane is shiny (or sometimes white), while the other side is black. The shiny side of one vane faces the black side of the next, and so on. When exposed to sunlight, the vanes spin merrily around, away from their black sides and in the direction of their shiny sides.
People have been trying to find out what makes the radiometer turn ever since 1873, when it was invented by Sir William Crookes (1832–1919). He thought it was pressure from the light, which was somehow pushing harder on the black surfaces than on the shiny surfaces. Sir William, who was a smart man but was wrong about the light-pressure effect, launched a scientific quest that hasn't stopped yet. Even today's encyclopedias give a popular, but demonstrably wrong, explanation of how Crookes's radiometer works.
Warning: You are about to encounter one of only two places in this whole book (I hope) in which the answer to a question will be somewhat less than satisfying. The best current explanation of the radiometer, which I promise I will give you at the end, is a bit hard to swallow and is still being doubted by some scientists, including me. The other less-than-satisfying explanation is why the shower curtain is sucked inward during your shower. (For that one.)
First, let's debunk some of the obviously wrong radiometer explanations that are circulating as recklessly as a radiometer in hell.
Radiation pressure
Light, as everyone knows, is electromagnetic radiation. And electromagnetic radiation, as you either know or can quickly find out, is a stream of tiny packages of energy called photons. Photons act like little bullets, insofar as when they hit something, they can have a physical impact. For example, light photons can actually knock electrons out of many solid substances. That's called the photoelectric effect, and the photon explanation that I just gave you won Albert Einstein a Nobel Prize. (He explained it in a little more detail than I just did.)
So, one might think, it's the stream of photon bullets hitting the radiometer's vanes that spins them around, just like when you—DO NOT TRY THIS AT HOME!—shoot a machine gun at a weather vane. While radiation pressure does indeed exist, we now know that it is much too weak to be pushing those vanes around. Moreover, radiation pressure should make the radiometer turn the other way!
Here's why. Light is absorbed by black surfaces and reflected by shiny surfaces. The black surfaces of the vanes simply swallow the photons, whereas the shiny surfaces spit them right back out again, getting a backward recoil kick just as a gun gets when spitting out a bullet. That would make the vanes spin away from their shiny sides and toward their black sides—just the opposite of what we see happening.
Gas pressure
This appears to be the best-loved of all the wrong explanations. It is dispensed by the Encyclopædia Britannica and other encyclopedias, as well as by many science teachers.
The story goes that the black surfaces of the vanes absorb more light energy than the shiny sides do and are thereby slightly warmer. (Correct so far.) The air adjacent to the black sides—there's still a small amount of air in the bulb—is warmed by this energy (still correct), which makes the air pressure higher on the black sides (wrong!). This supposedly increased pressure pushes on the black sides, making the vanes move toward their shiny sides.
But let's ask the following question: When the air is heated, which indeed makes its molecules move faster, why should those faster-moving molecules dash themselves against the vanes any more often than they dart off in any other direction? There can be no net directional force from the molecules' motion. Putting it another way, the air's pressure can't increase, because it is not confined. It is free to expand and relieve any incipient pressure anywhere it likes within the bulb, so there is no more reason for it to expand against the vanes than in any other direction. Thus, there is no net vane-pushing force caused by the warmer air.
Outgassing
Some conspiracy theorists would have us believe that the black coating on the vanes contains adsorbed (surface-bound) gases, and that when the black sides are heated by absorbing light, those gas molecules are expelled, sort of like popcorn from a frying pan. The leaping gas molecules would exert a force on the black surface, just as a basketball player exerts a force on the court floor when he jumps, and this force pushes the vanes around. But if this were true, the radiometer would eventually wear out as all the adsorbed gases were released.
Photoelectric effect
What if the photons of light are ejecting electrons from the black sides of the vanes and, in departing, the electrons give a backward kick to the vanes? No cigar on that one, either, because you can make radiometer vanes out of materials that don't exhibit the photoelectric effect; their electrons are held too tightly for visible light to be able to knock them out. Also, the photoelectric effect would still occur even if the bulb were completely evacuated, but the radiometer won't work without some air in the bulb.
Convection currents
The heated black surface sets up air currents by convection, and the moving air blows the vanes around. The only trouble with this one is that nobody can invent any air currents that blow mainly in one direction: against the black sides of the vanes.
The best scoop
In 1881, a British mechanical engineer named Osborne Reynolds (1842–1912) published a paper that explained the radiometer in a way that many scientists now grudgingly accept. The reason it isn't more widely known is probably that it isn't easy to describe or to understand. But here goes.
It has something to do with the temperature difference between the warmer air adjacent to the black sides of the vanes (due to their energy-absorbing nature) and the cooler air adjacent to the shiny sides. Apparently, when this air flows out to the edges of the vanes, the warmer, faster molecules strike the edges at a more oblique angle than the cooler molecules do, and that pushes the vanes in a direction away from the black sides. Exactly why this should be true is buried in complex mathematics, which I shall not attempt to decipher for you (or me). I confess that it's hard for me to believe that it's the edges of those skinny vanes, rather than their broad surfaces, that push them around. But that's what Mr. Reynolds says, and none of the other explanations stands up under close examination.
I warned you, didn't I?
You Didn't Ask, but …
If scientists today can unravel the mysteries of life itself, why can't they explain the simple little radiometer after more than a hundred years of trying?
The main answer is that they haven't really been trying. There has been no vast federal program to inject billions of dollars into radiometer research as there have been for the Manhattan (atomic bomb) Project, the space program, genetic research and other health-related enterprises. Not that money alone can solve a scientific problem, but scientists are like everybody else: They tend to do what they get rewarded for, and nobody is going to get a research grant, a promotion or a Nobel Prize for figuring out how a toy works.
Window, Window, in the Wall, How Come You Block No Light at All?
Why are air, water and glass transparent, when practically no other materials are?
Well, what does “transparent” mean? It means that any light being reflected in our direction from an object outside a glass window, for example, can pass right through the glass unobstructed and come out the other side, where our eyes can deal with it. We therefore see the object through the window. That's why people who have little regard for the English language use the term “see-thru” (invariably spelled that way) instead of the perfectly good word transparent.
In general, when a traveling ray of light encounters a new substance, it may be reflected backward from the surface or it may penetrate the surface and be absorbed. If it manages to escape both of these fates, it can continue traveling through the medium; it will be transmitted. So our job is to explain why air, water and glass don't reflect and/or absorb very much of the light they receive. Almost all other substances—except some waterlike liquids and glasslike plastics—absorb some of the light and reflect most of it, leaving practically none to be transmitted.
Let's get air out of the way first. Under ordinary conditions, the spaces between air molecules are around ten times bigger than the molecules themselves. So air is almost completely empty space, containing virtually nothing that could interfere with the passage of light except for a very occasional molecule. Ditto for all gases.
Water and glass are quite a different ball game, however, because their molecules are very close together—close enough to do a fair amount of reflecting. Remember the glare from that pond's surface or from that car's windshield on a sunny day? So even from the most transparent liquid or solid substances, some light is reflected. It depends on the angle at which the light hits the surface.
Of the light rays that do succeed in penetrating air, water or glass, very, very few of them are absorbed; almost all the light gets through. Molecules absorb light because their electrons have certain preferred energies, and by taking on the extra energy of a light particle (a photon), they can reach another, higher one of their preferred energies. It happens that none of the molecules in air, water or glass can absorb and “use” any of the energies in visible light; the energies that they can absorb are certain radiations that humans can't see, such as ultraviolet and infrared radiations. Ditto for alcohol, kerosene and other familiar transparent liquids. So if very little light is absorbed and the angle isn't right for reflecting, almost all of the light will go straight through by default.
NITPICKER'S CORNER
There are, of course, colored glasses, liquids and even gases. What's going on there is that they selectively absorb some of the wavelengths or energies in white (or colorless) light and transmit only those that they can't “use.” The transmitted light therefore has a different composition of wavelengths from white light and hence a perceived color.
You Didn't Ask, but …
Why is a mirror such a good reflector?
Mirrors are the best reflectors of light that human ingenuity has been able to devise. Notice, however, that the light is reflected only from the backing of the mirror, after passing through the front layer of transparent glass.
What is there about the backing that makes it such a good reflector? It's a thin, smooth layer of silver metal. All metals are shiny, or reflective, because their atoms are held together by a sea of loose, swarming electrons that have no affiliation with any particular atoms. (That's why metals conduct electricity so well—because electricity is just a movement of electrons.) The swarm of footloose electrons in the silver, belonging as they do to no particular atoms, have no particular preference for absorbing any specific wavelengths of light, so they reject and reflect back all wavelengths.
Of course, a sheet of shiny silver metal would make a fine mirror without the glass, but it would quickly tarnish.
A Light Bite
Why do WintOGreen Life Savers make flashes of light?
Your question may sound silly to those who haven't heard about it before, but chomping on those little candies really does make flashes of light. It may not help you at all to know that the phenomenon is called triboluminescence, but there, I've said it and done my duty as a scientist.
Life Savers, it will not surprise you to know, are little more than donut-shaped crystals of sugar. Certain crystals, including cane sugar, have long been known to exhibit this property of tri … whatever. In fact, way back in 1605, the English philosopher Sir Francis Bacon (1561–1626) reported that when he chopped up blocks of sugar in the dark (sugar was sold in big blocks and candlelight was dim), he observed flashes of “a very vivid but exceedingly short-lived splendour.” Mineralogists have long known that certain mineral crystals also give off light when subjected to sudden shock.
Here's what's going on.
A crystal is an orderly, geometric arrangement of atoms, all bound together into a sort of three-dimensional lattice-work structure. Examples that you may be familiar with are sugar (sucrose), salt (sodium chloride), quartz (silica) and diamond (an overpriced form of carbon). It has been found that crystals whose molecular arrangements are not symmetrical—that is, whose molecules are not situated identically in two opposite directions—are the best flashers.
When such a crystal is cracked open, the atoms are torn apart from one another and some of their electrons are torn off in the process. Crystal fragment A may wind up with more electrons than it deserves, while crystal fragment B may not have enough. As they begin to separate, the extra electrons on fragment A are attracted strongly back to where they belong, and they zap across the widening air gap between A and B, exactly like a bolt of lightning zapping through the air between a cloud and the ground.
These miniature lightning bolts make tiny blue flashes because the air's molecules are energized by the swift rush of electrons through them, following which they throw off their extra energy in the form of light. Hard, fracturing whacks on the crystal can therefore produce weak flashes of light.
That's all that happens in most triboluminescent crystals. But in the case of WintOGreen Life Savers, that's not all that happens. There's an almost instantaneous second step that makes the light much brighter.
Much of the “lightning” that the electron-zapped air gives off is invisible to humans; it is ultraviolet radiation, which is of higher energy than visible light. But WintOGreen Life Savers contain a chemical called methyl salicylate, also known as oil of wintergreen; it's the flavor in the leaves of the wintergreen plant, a small, creeping evergreen sometimes known as teaberry. This chemical has the property of being fluorescent. That is, its molecules absorb the ultra-violet radiation and re-emit it as visible light. It's that visible light that is mainly what you see when someone chomps a WintOGreen Life Saver in a dark closet.
Can't wait to try it?
TRY IT
Take a roll of WintOGreen Life Savers into a dark closet with a hand mirror or a close friend. (If you're already in the closet, so much the better.) Make sure the closet is completely dark; wait until nighttime and plug the crack under the door with a towel if necessary. Think pure thoughts for about ten minutes, while your eyes become thoroughly dark-adapted. Now pop a Life Saver into your mouth and, in spite of what your mother taught you, quickly crunch it noisily between your teeth with your mouth open. Your mirror or your friend will see surprisingly bright flashes of light inside your mouth. Baby dragons are trained on WintOGreen Life Savers.
You may also want to play around with sugar cubes. In the closet, clash them glancingly against each other, as if striking a match. You'll see the miniature lightning flashes, but they won't be brightened by the fluorescence of wintergreen's methyl salicylate.

