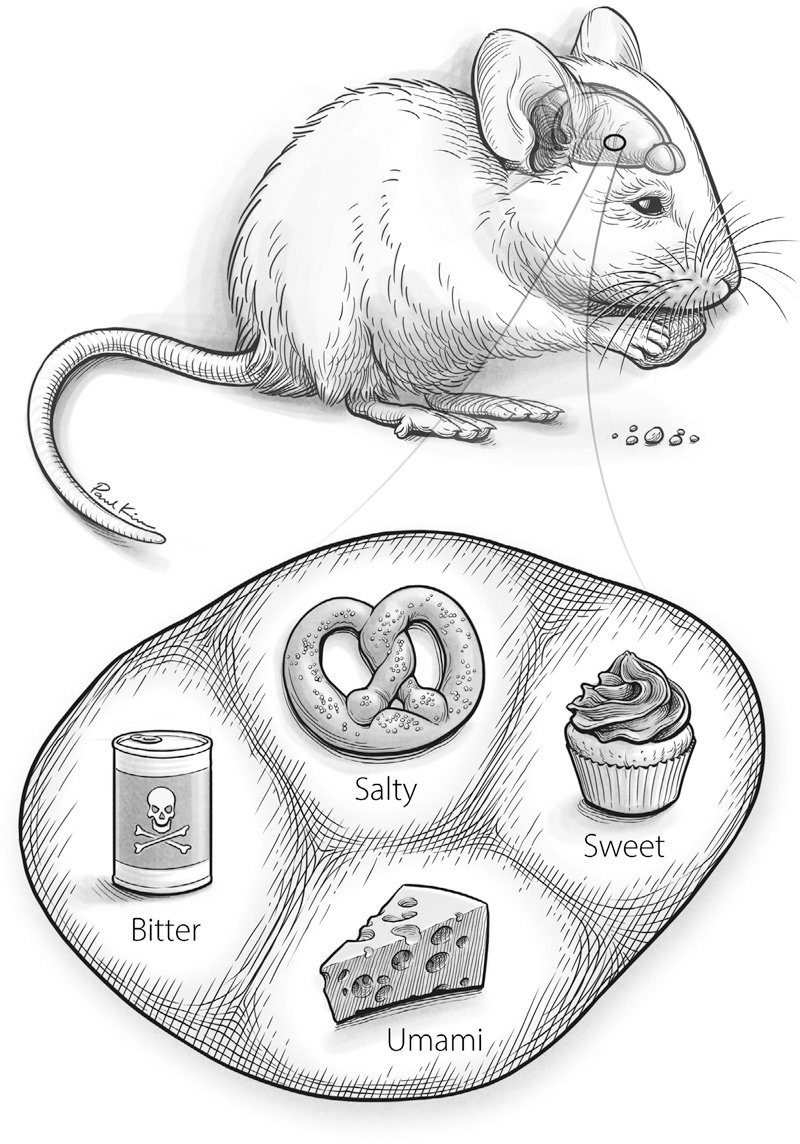
FIGURE 22. An illustration of the taste zones observed in the primary taste cortex of the mouse.
Paul Kim
TO SURVIVE AND MULTIPLY ON EARTH, all creatures must eat stuff, mate with stuff, and keep away from dangerous stuff. When you come down to it, life is really about stuff. And what is important to a creature is important to its brain.
Your sense of taste and sense of smell are tasked with determining what kind of stuff is in your mouth or near your nose, respectively. A closer look at these two senses and their representation in the brain reveals the specific and often unexpected ways in which they protect both animals and humans. These senses also demonstrate the limits to what brain maps can represent and show how something else—a neural code—helps carry the load when brain maps cannot.
It is easy to think of taste as a vehicle for experiencing pleasure. Each mealtime can be an opportunity to enjoy comforting, exciting, or indulgent flavors. But the basic purpose of taste is not to bring you pleasure. It is to keep you from dying.
Taste is important because, at the end of the day, you are a glorified sack of chemicals. Everyone is. When it comes to survival, what matters is the division between the vital stuff within your body and the myriad hazards outside it. Your body must be stocked with all of the necessary molecular building blocks and vital fuels required to keep you alive and yet free of common toxins that would do you harm. Although skin has a role in absorbing certain compounds, largely it acts as a fortress wall, surrounding you and keeping out intruders. Any city under siege faces a critical challenge: how to keep the enemy out while letting in food, water, and other supplies. Your body faces this challenge every day of your life.
All that you are, and all that allows you to continue to be, depends on the chemicals within you. Ingest the wrong compound—something poisonous, rotten, contaminated—and you will cease to be alive. Fail to ingest the right compounds day in and day out, and you will cease to be alive. Our world is filled with a vast array of compounds that you, a mouse, or a fruit fly might try to nibble. Thankfully, we don’t need to understand the difference between an amino acid and an alkaloid in order to choose between them at the table or the trough. Nature, or rather evolution, has given us a cheat sheet—a practical guide for ensuring that the right stuff goes in and the wrong stuff stays out.
Those who study the chemical senses distinguish between taste and flavor, terms that we tend to use interchangeably. A food’s flavor is the combined experience of its taste, its smell, and even its texture. The odors that a food gives off, its temperature, and its feel on the tongue all add a great deal of subtlety to the experience of its flavor. If you have ever tried to enjoy a fine meal despite a stuffy nose, you will have some idea of how very different flavor and taste can be.
The sense of taste begins with taste receptors that coat the tongue and other surfaces in the mouth. Taste receptors come in several different varieties, and each recognizes different types of chemical compounds in food. Each type of taste receptor is linked to one of two innate, or hardwired, reactions: swallow the food (and shovel more in) or eject the food from your mouth. Attraction or repulsion. In or out. Yes or no. Your entire sense of taste, and many hundreds of thousands of taste receptors bundled into thousands of taste buds, comes down to that one binary choice.
It might be surprising to learn that, for all the pleasure we derive from eating, only three types of taste receptors are known to drive our attraction to food. Sweet-taste receptors detect sugars and other carbohydrates—critical sources of energy for the brain and the rest of the body. They are sometimes fooled by other molecules that resemble sugars, which is why artificial sweeteners taste sweet to people even though they offer no nutrients or fuel. The umami (savory-taste) receptor detects free amino acids that indicate a food is high in protein. Amino acids are the building blocks of proteins, which are in turn the raw materials for pretty much everything that makes complex organisms function. Although your body can often recycle amino acids, it can’t manufacture many of them. So humans and other animals need to get these building blocks by eating foods that contain protein.
Saltiness reveals how delicate the balance of chemicals in your body truly is. Life on earth evolved in the sea, under conditions in which sodium salts (sea salt, or NaCl) were easy to come by. As a result, basic cellular functions supporting life on this planet depend on salt. In order for our bodies to function, we must all be a little bit salty inside. That is why hospitals hydrate patients using intravenous saline solutions that are, in essence, water mixed with a pinch of sodium salt. It is also why many other land-dwelling animals go to great lengths to ingest salt; in order for their bodies to function properly, they need to keep their insides salty too. Yet ingesting too much salt can be a problem. Overindulge in sodium, and over time, you could be on the path to problems with blood pressure and kidney function. And if your insides were to suddenly become extremely salty, you would have a medical emergency on your hands.
To survive this delicate balancing act, you possess two types of taste receptors for salt. One responds to low levels of sodium salts—the good kind of salt your body needs—and triggers an attractive response. Snack foods and restaurant fare tend to be salty because, at that level of saltiness, your attractive salt receptors send signals to your brain that make the food taste better, encouraging you to eat and buy more. This is the third and last type of taste receptor that leaves you wanting more.
Nature has invested a great deal more variety and creativity in making sure that you detect and reject certain foods. One example is a second type of taste receptor that detects sodium salts when they are overly abundant. These receptors also detect other types of salts in your food, such as lithium salts, which can be toxic. When activated, these receptors signal the brain to create an unpleasant taste sensation and trigger the urge to spit out whatever is in your mouth.
Another type of receptor, the sour-taste receptor, detects acids in your food. If microbes have beaten you to your meal, causing the food to ferment or spoil, the levels of acid in the food will be raised. When you take a bite, your taste receptors sense the acid and send a message to your brain, so that you experience a taste that is unexpectedly sour. Newborn babies naturally reject sour foods. As we get older, we may learn to appreciate a touch of sourness in certain foods, such as lemonade or sweet-and-sour sauce. Yet even for adults, if a food tastes uncharacteristically sour, this is a signal that it has gone bad and should not be eaten.
Finally, there is bitter taste. Your mouth and tongue are home to about thirty different varieties of bitter-taste receptors, compared to one or two varieties each for the other basic tastes. This panoply of bitter-taste receptors protects you from eating a range of noxious and poisonous substances. Young children universally reject bitter-tasting foods. As we age and become exposed to culturally accepted bitter foods, such as beer, coffee, and some vegetables, we may learn to appreciate a degree of bitterness. But a substance’s intensely bitter taste triggers the same reaction in human children and adults, not to mention dogs, rats, and a host of other animals. Each will make a facial expression indicating disgust and stick their tongue out of their mouth, as if to eject the offending substance.
A surprising fact about this type of reaction, and indeed everything about the sense of taste, is that it is arbitrary. Bitter and sweet tastes are not inherent properties of food. For instance, certain compounds called beta-glucopyranosides have a powerfully bitter taste to humans, whereas mice do not taste them at all. Yet when scientists created genetically altered mice whose tongues grew the human version of a bitter-taste receptor, those mice could taste the compound as bitter and rejected it. Likewise, the sweet-taste receptors of mice do not recognize aspartame, the artificial sweetener found in most diet sodas. To mice, aspartame has no taste, and for them diet sodas have far less appeal. But when scientists created mice with human versions of the sweet-taste receptors, the mice experienced a sweet taste from aspartame and feasted on it. By swapping part of the mouse’s sweet-taste receptor with one of its bitter-taste receptors, scientists even created a line of mice that devoured food that normal mice would reject as too bitter. Rather than having an aversion to the food, these mice sought it out.
Ultimately, there is nothing about aspartame that is inherently sweet. There is nothing about sugar that is inherently sweet. Sweetness is a kind of label, a category that our tongues and brains use to make swift, safe decisions about what we should ingest. The idiosyncrasies of receptors in your mouth create a small yet important set of taste categories. But, of course, it is your brain that must translate those categories into what you experience as taste. What happens to taste information when it finishes its journey from your mouth to your brain?
Sadly, much of what we have learned about the brain over the course of history has come from observing how people or creatures have been affected by brain damage. Nothing reveals the importance of an area of the brain better than its destruction. To see for yourself how one particular brain area contributes to the sense of taste, consider the story of a seventy-five-year-old woman I’ll call Mary.
Mary was cooking dinner when the right side of her body suddenly felt weak. She slid to the floor, where she lay confused, unable to speak or respond to what those around her were saying. A blood clot lodged in a major artery feeding her brain was blocking the flow of blood. Deprived of oxygen, the neurons in parts of her brain were shutting down and beginning to die.
Mary was taken to the hospital and received a treatment to dissolve the clot so that the artery could bring fresh oxygen to the suffocating cells. Fortunately, the treatment worked, restoring blood flow to her brain. Still, Mary’s stroke left lasting brain damage. On a medical scan, her doctors saw an ominous zone of darkness on the left side of her brain, inside a folded part of cortex that includes the primary taste cortex, where the sense of taste is mapped. This darkness indicated that Mary’s treatment came too late to save the neurons in this region of her brain. These cells were permanently damaged or dead.
Mary returned home and began to improve, although she still struggled with some everyday tasks. Only then, back at home and eating her usual foods, did she discover that something was terribly wrong with her sense of taste. Everything tasted like dirt. Despite having enjoyed the taste of ham, chicken, potatoes, and vegetables all her life, she could now no longer tolerate them. Wine and coffee too were no longer palatable. These foods still tasted like something, just not something she’d call food. Mary found that she had to force herself to eat. She stopped enjoying meals with loved ones and began to feel isolated. Six months after the stroke, she had lost fourteen pounds.
After some experimentation, Mary eventually discovered foods that she could tolerate. She found she liked tomato sauce with pasta or beef. She could drink tea in lieu of coffee. Her ability to enjoy sweet tastes was unaffected by the stroke, so she could still have desserts and chocolate. She adapted to her new palate and was able to stop shedding pounds, but she never regained her previous experience of tastes. Even a year after the stroke, she could not enjoy a dish of chicken and potatoes. These foods, once her favorites, now tasted like sawdust.
Scientists know less about the neural representation of taste than that of vision, touch, and hearing. For historical and anatomical reasons, far fewer research studies have focused on how the brain handles taste. But we do have some exciting clues. We know that taste information travels from the mouth to stations deep in the brain before arriving at a plot of cortex on either side of the brain, where the primary taste cortex resides. In humans, this cortex lies within the insula, a region of cortex buried within a prominent fold on each side of the brain. The brain damage that disrupted Mary’s sense of taste was in this region, within the insula in her left hemisphere. And when scientists have electrically stimulated neurons in this area of the insula in either hemisphere, patients reported experiencing nasty, metallic, or acidic taste sensations.
To learn about the layout of the primary taste cortex, it is best to start with what is known from animals. Neuroscientists generally turn to animals to learn about the fine-grained layout of brain areas. For obvious ethical reasons, scientists cannot use invasive methods to study the human brain up close unless there is a medical need for the procedure. Brain scanning technologies like functional MRI allow us to observe brain activity in humans without harming anyone. But these methods have poor spatial resolution, which means they detect a blurred signature of activity from lots of neurons at once. Compared to the direct methods neuroscientists can use to observe brain activity in animals, noninvasive scanning technologies like functional MRI are a bit like gazing at the brain without one’s glasses on.
When scientists have directly studied taste in animals, they have reported intriguing results. One landmark study of mice offers a particularly clear glimpse of a taste map in the creature’s primary taste cortex. (See Figure 22.) The scientists used a precise molecular technique to watch neurons in the living brain fire while they fed the anesthetized mice sweet-, umami-, salty-, sour-, and bitter-tasting chemical compounds. They discovered a map with zones, or neighborhoods, representing the different tastes. The zones formed a sort of elongated diamond shape, with bitter and sweet zones farthest apart, and salty and umami zones in between them. The scientists could not locate the sour zone and suspected that it was beyond the patch of tissue that they could view in their experiment.

FIGURE 22. An illustration of the taste zones observed in the primary taste cortex of the mouse.
Paul Kim
A second study showed how important the districts of this map were for the mouse’s experience of taste. Here, researchers used a different technique to make neurons in specific taste neighborhoods of the map more active by shining a laser directly on those regions of the exposed brain. Remarkably, they were able to do this while the mouse was awake and engaging in its normal behavior. While each mouse licked water from a spout, the scientists used the laser to trigger activity in the bitter-taste zone of its primary taste cortex. Although the mice were drinking only water, they reacted as though the water tasted powerfully bitter. They stuck out their tongue, gagged, and tried to wipe the offending taste out of their mouth with their paws. In contrast, when the scientists used the laser to stimulate each animal’s sweet-taste zone, the mice licked fiercely at the spout, as if feeding on syrup rather than water.
Despite these exciting findings, scientists are still wrestling with understanding the nature and layout of taste maps in mice and other rodents. Some studies have found that the taste districts of the map overlap and that the region is organized around taste pleasantness more than individual types of taste, like sweet or sour. Other work indicates that many of the neurons in the taste map actually respond to other properties of food, such as its aroma, texture, and temperature.
For all that remains unknown about the taste maps of animals, even less is known with certainty about the human taste map. Experiments attempting to study the organization of the human taste map by using functional MRI have yielded conflicting and ambiguous results. Some studies found that the human primary taste cortex features zones for tastes but that these zones overlap quite a bit. Other studies carried out with fancier techniques suggest something else entirely: the presence of taste representation in the absence of any map at all.
This might seem surprising, given how assiduously the brain represents many types of information using brain maps: for instance, the by-now-familiar example of how spatial information like distance and location within a brain map corresponds to information about important events unfolding around us. But although the brain is chock full of brain maps, it can represent information in another way: with a distributed code.
Representing information with a distributed code is entirely different from representing it with brain maps. In a brain map, neighboring neurons represent neighboring regions in space, frequency, time, and so forth. And brain maps essentially represent information using location, or where in that brain area the neurons are most active. In contrast, there is no consistent relationship between neighboring neurons in brain areas that use distributed codes. These areas represent information through the distributed pattern of activity across that entire region of the brain, rather than through the location of activity within it. This pattern of activity is a kind of code.
What do I mean by a code, and how does it differ from a map? Let’s imagine I’ve invited you to a party and need to send you directions to the venue. This is a problem of representation: I have information (your route to the venue) that I need to represent on paper or in an electronic message. If I do this well, you will understand the directions based on the message and make it to my party. I could send those directions in one of two ways. I could draw you a map that shows the route from your house to the venue. Or I could write out a verbal description of the streets you should take, where to turn, and so on.
If I opt for the second approach, I am using a code to represent and transmit the information. Language, whether spoken or written, is the quintessential example of a code. Consider how we form written words out of letters. Many written languages are based on an alphabet, or a small set of letter symbols. To make meaning out of such letters, I must combine them into words. It is only by combining these letters that I can create unique letter patterns, or words, that represent and mean something to you and to me.
In brain areas that use a distributed code, the activity of individual neurons plays the role that individual letters do in written languages with an alphabet. A single neuron can fire quickly in response to many different things, just as a single letter can be used to form many different words. For distributed codes, what matters is the specific set of neurons that are firing like mad at that moment. Information is contained in the pattern of activity across many neurons rather than in the activity of any single neuron.
Brain maps are a useful way for brains to represent information because they make representation and information processing efficient and compact. But what is the benefit to representing information with a code? In a word, that benefit is flexibility. Any given map has fixed dimensions and boundaries. In the case of brain maps, those could be surfaces of the body or regions of visual space. Every part of the map is assigned to representing something specific, which leaves no room in the map for representing new things, such as touch on an entirely new body part or vision from an eye newly installed in the back of your head. Likewise, if the venue for my party were moved to a new city that was beyond the boundaries of the original map I made for you, the old map would be of no help. I would have to draw an entirely new map to get you to my party. In short, maps do not handle new things well.
Codes do not have this problem. I can use the existing alphabet to create new words or combinations of words to convey new meaning. Venue moved? No problem! I can create new directions for you using the same set of letters, only in different combinations. This kind of flexibility is important for the brain, particularly for phenomena in which newness is common. Your brain can simply create new patterns of neural activity to represent new tastes, objects, or locales as you encounter them.
Although maps and codes are opposites in many ways, they are not in opposition. They operate jointly to support nearly everything you do. For example, you use frequency-based maps like A1 and distributed codes to convert sound-pressure waves detected at the ear into voices that you recognize (That’s my mom!) and words you comprehend (She’s calling me to come home!). Maps and distributed codes generally exist in separate areas of the brain that work together by sending signals back and forth. But maps and codes can also be combined in certain parts of the brain, particularly those that contain maps with zones, like the taste zones in the primary taste cortex. For example, neurons within a sweet-taste zone might use a distributed code to represent specific features of sweetness. Or neurons nestled in the no-man’s land between zones might represent new flavor features using a distributed code. This happy compromise marries the flexibility of codes with the benefits of maps. The human primary taste map may reflect just such a compromise. It will take more exploration on the part of intrepid scientists to know for sure.
As vital as your sense of taste may be, your sense of smell is by far the more impressive and mysterious of your chemical senses. When considered across the animal kingdom, it is nearly impossible to overstate the importance of scent. Sharks, snakes, mosquitoes, vultures, badgers, and hummingbirds are just a few of the creatures that follow their nose to find food. Scent can also signal social status, as it does among termites, which recognize their queen by her scented secretions. It drives reproduction in a stunning variety of ways: the spotted hyena wipes its pungent anal paste on grass to advertise its reproductive status, and the male frillfin goby, a marine fish, launches into an hour-long courtship routine whenever he gets a whiff of a fertile female’s ovarian secretions. Scent is also integral to parenting and bonding, enabling many newborn creatures to recognize their mother and drawing newborn mammals to her nipples to nurse. Albatrosses and other seafaring birds even find their way across the vast ocean using their sense of smell. In short, smell is essential for nearly all aspects of animal survival. But how do animals extract the information they need from odors, and what kinds of maps do their brains use to do this?
Olfaction, or the sense of smell, is a remarkable feat of molecular recognition. Consider the example of mouse olfaction. Embedded within the lining of the mouse’s nose are about ten million smell receptors made up of about a thousand different types. Any given airborne molecule might bind to more than one type of receptor, and any given type of receptor might bind to more than one kind of molecule. As a result, mice can detect and identify far more than one thousand odors, even though they have only a thousand receptor types.
When airborne molecules bind to the sensory receptors inside a creature’s nose, the attached neurons send a signal on to the brain. These signals travel directly to two conspicuous balls of brain stuff called the olfactory bulbs, which jut out in front of the brain in mice, humans, and other animals. The right and left olfactory bulbs each contain a detailed map of odor zones loosely organized around the structures of the molecules they represent, such as how long its carbon chain is or whether it is a carboxylic acid, a phenol, or an aliphatic ester. Although the chemical jargon might mean little to you, this structural information is the key to identifying what kind of stuff that molecule came from and therefore how it might be relevant to you. The map in your olfactory bulb takes the first step in this process by specifically representing information about which type of molecule has made its way into your nose.
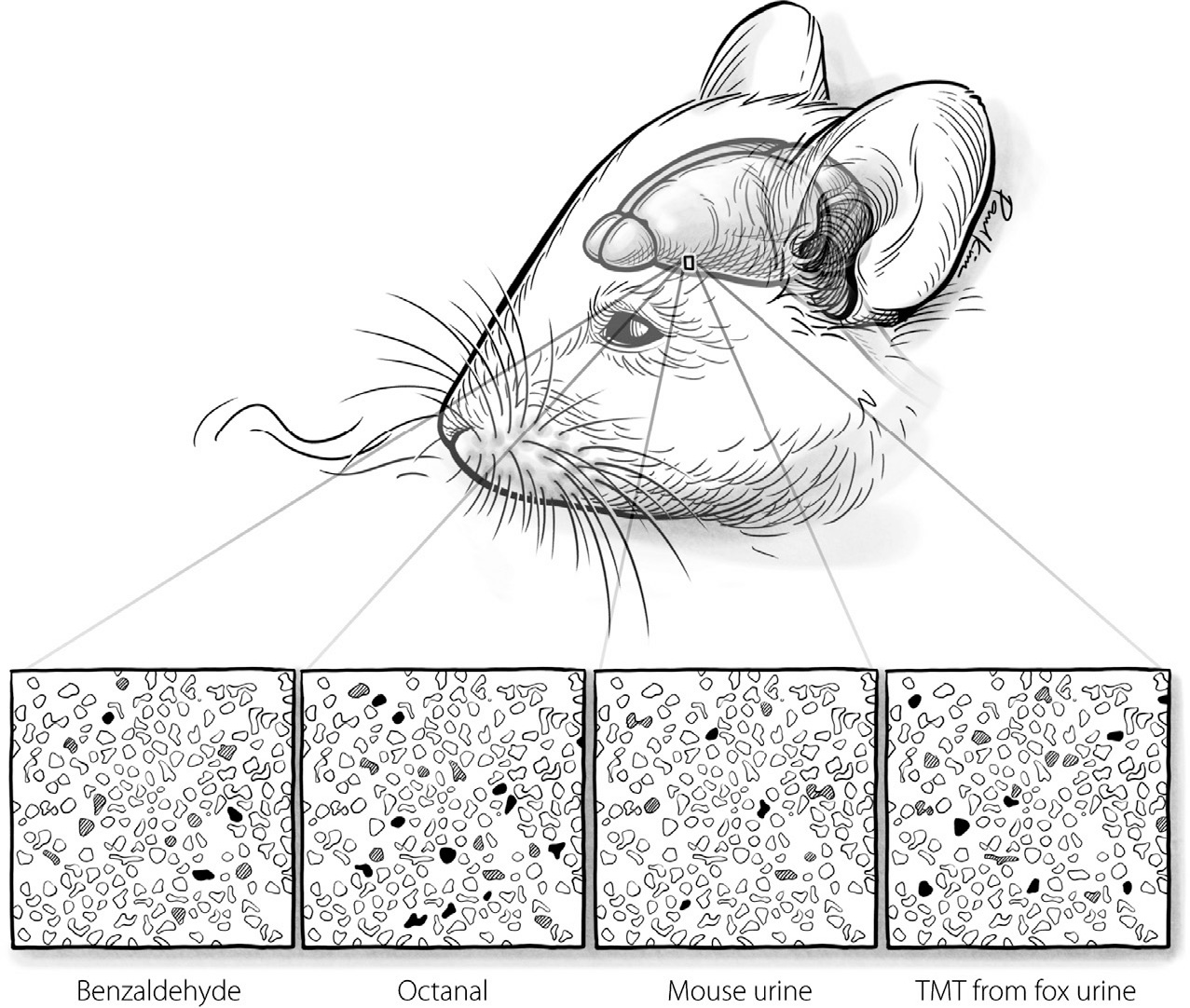
FIGURE 23. An illustration of a distributed code for odor in the piriform cortex.
Paul Kim
From the olfactory bulb, information about smell goes to several regions of the brain. Of these, the one scientists have studied most in rodents and humans is a region called the piriform cortex. Experiments have shown that this area plays a crucial role in learning new odors. And as you might imagine, given how poorly maps handle new stuff, the piriform cortex represents odor with a distributed code rather than a map. The illustration in Figure 23 shows this code in action, depicting how a set of neurons in the mouse piriform cortex collectively represents different odors through their different patterns of activity. The neurons with high firing rates are indicated with black; those with slightly heightened firing rates are shown in gray.
At this time we know more about the piriform cortex and its distributed code for odor than about other odor-processing regions of the brain. Still, scientists have found a handful of intriguing brain areas beyond the olfactory bulb that are organized into odor zones. One of the challenges in finding such maps is the complexity of olfaction and its truly overwhelming number of detected molecules and detecting receptor types. Above all, the challenge stems from the countless possible ways that odors could be grouped or related to one another in a map. In order for scientists to find an odor map in the brain, they first need to know which map dimensions or categories to search for. This is true for all brain maps, but it has proven a particular challenge for olfaction.
One approach to finding odor brain maps has been to study creatures with less complicated olfaction. For example, the channel catfish, a fish found in North American rivers and lakes, has only about a hundred different types of smell receptors that detect only a few types of molecules, including nucleotides, amino acids, and bile salts. Although nucleotide and amino acid molecules are structurally quite different, they are both found at high levels in living things, and they both mean the same thing to the channel catfish: food. In contrast, bile salts are created by the liver and then released in the feces or urine of other fishes. Like hyenas and their anal paste, catfish use these bile salts as social cues to learn about other members of their species that are nearby.
Scientists studying the catfish sense of smell first examined the map in the creature’s olfactory bulb. (See Figure 24.) There they found three separate zones for the three different types of molecules: nucleotides, amino acids, and bile salts. This fits with findings from other creatures: the olfactory bulb map is organized around the structures of odorous molecules. But the catfish’s olfactory bulbs send information about smells on to another part of the catfish brain, where the scientists found another odor map. This map contained only two major zones: one for bile salts and one for both nucleotides and amino acids.
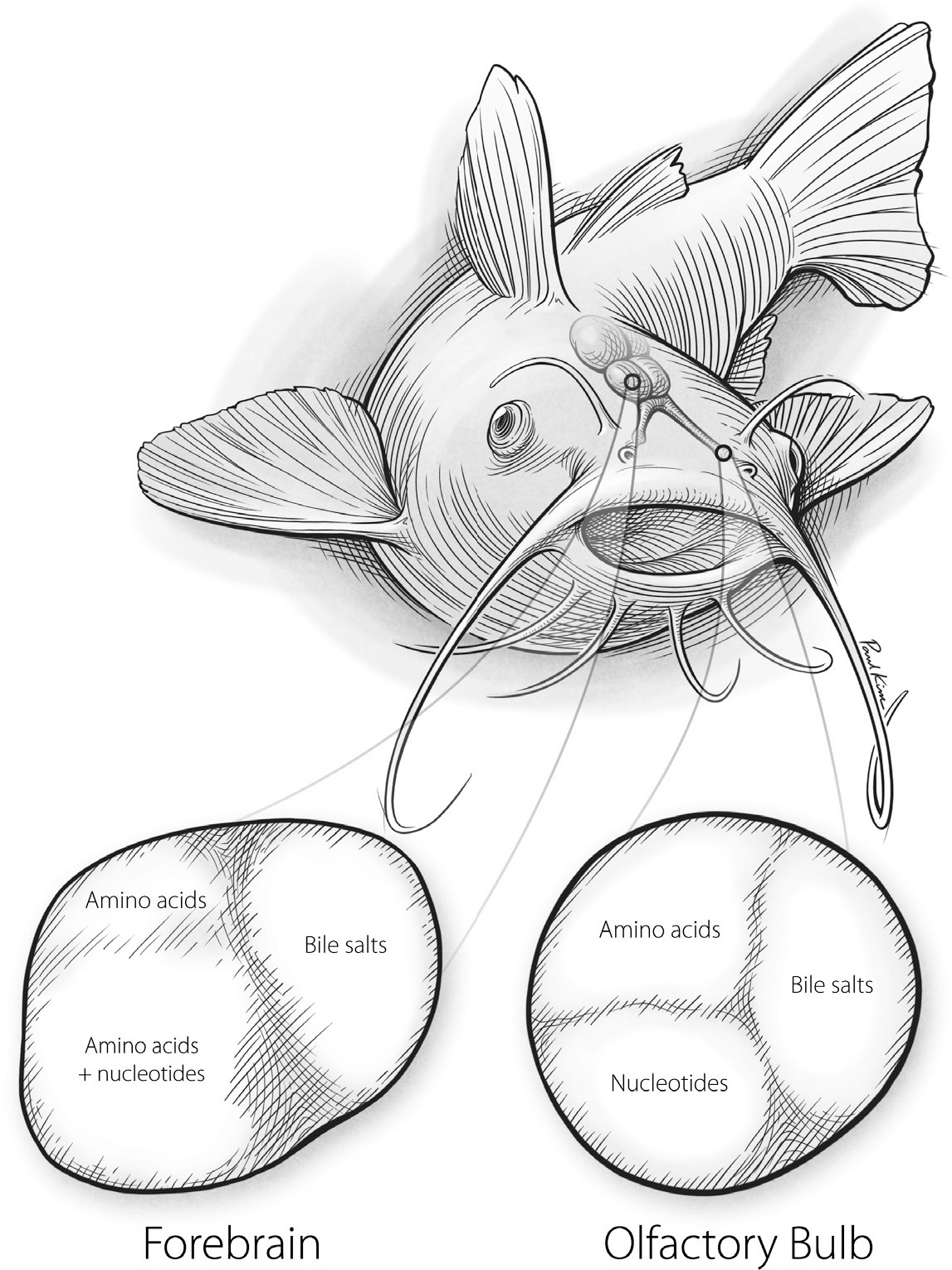
FIGURE 24. Odor maps in the olfactory bulb (right) and forebrain (left) of the channel catfish.
Paul Kim
The difference between these two odor maps is subtle but important. The map in the olfactory bulb is organized into odor zones based on molecular structure, an objective property of these compounds in the physical world. But the second map is organized into zones based on the significance of the odors to the animal. Whether a catfish smells amino acids or nucleotides, it will launch into the same type of feeding behavior. These two different types of molecules convey the same information and trigger the same behavior, whereas bile salts offer different information and call for different behaviors.
The second odor map of the channel catfish is a map of behavioral relevance. Only compounds that are important for the fish’s survival and procreation are awarded zones or districts in this map, and the grouping of these compounds is determined based on what the animal will need to do in response to them. This kind of map is powerful because it distills and classifies the stuff that is important to a creature. As you might imagine, such a map would be quite different for a mouse, a lion, a vulture, or you. Just as we can learn about the importance of the pony’s nostrils from its distorted touch map, we can learn about the importance of stuff to a catfish from its odor maps.
Odor maps that are important for driving innate, instinctual responses to smells have also been found in mice. If you place a drop of 2,3,5-trimethyl-3-thiazoline, a compound found in fox secretions, into a laboratory mouse’s cage, the mouse will freeze or scurry as far from the scent as possible. Although the lab animal has never encountered a fox, its brain knows that this is an odor to avoid. Mice instinctively avoid other scents, such as the musky bouquet of bobcat urine. In addition, there are odors that mice are instinctively attracted to, including peanut oil and 2-phenylethanol, a compound found in rose oil.
An area in the mouse brain located near the piriform cortex is responsible for these instinctive reactions to odors. It contains separate zones for predator odors to avoid, like bobcat urine, and odors to approach, like peanut oil. Using a clever technique, scientists were able to activate neurons in one zone or the other, without exposing the animal to actual odors. When scientists stimulated the predator-scent zone, the mice froze or scuttled away, just as they would if a predator were present. When the scientists stimulated the attractive-scent zone, the mice lingered, as if hoping for a snack. Another study found a nearby zone that responds to the smell of urine from mice of the opposite sex. This region seems to be involved in processing chemical signals that are relevant to mating.
There is still a lot of work to be done in uncovering the organization of the mouse’s odor maps, but these studies suggest at least three zones: one for predators, one for food, and one for mates. Each zone has clear behavioral relevance and appears to bridge the connection between which compounds the mouse detects and which action it makes in response.
These studies offer a glimpse of odor maps and the behavioral relevance of scent to catfish and mice. But what about humans? In order for scientists to find odor maps in humans, they need to know what kind of map zones or dimensions to look for. In animals, the layout of a creature’s odor maps is determined by how it uses odor information and, specifically, how that information leads to action related to reproduction or survival. Which raises this question: how do humans use smell to survive, if they do so at all?
My mother, Sally, lived her entire life without ever once smelling a thing. So far as anyone can tell, she was born without the capacity to detect odors of any kind. She wasn’t even sure that she could imagine what smelling was like. After all, how can people go about imagining a sense that they have never actually experienced?
Sally would talk about her missing sense of smell like it was a curiosity or a philosophical puzzle—but never a handicap. Its absence simply did not seem to affect her very much. Some of her acquaintances weren’t even aware that she lacked one of her five senses. By contrast, imagine not knowing that your friend couldn’t see or hear. Sally faced a few dangers because of her lack of smell; she could not smell a gas leak, could not smell smoke (although she could feel it with her eyes and nostrils when the smoke was concentrated), and could not tell that milk had spoiled until it poured from the carton in curdled chunks. But for the most part, Sally’s lack of smell made her seem stoic. She was unfazed by a reeking outhouse, just as she was unmoved by the smell of freshly baked bread wafting from a nearby bakery. In some ways, her missing sense seemed less a disability than a superpower. But how could that possibly be?
It’s important to distinguish between the experiences of people born without one of the five senses and those who lose it later in life. People who lose their sense of smell later in life, after they have come to associate foods, places, and experiences with certain scents, tend to find this loss extremely distressing. Yet aside from the substantial emotional impact, these individuals do not usually find themselves incapacitated. The loss does not leave them unable to navigate from place to place, fulfill the requirements of their jobs, or otherwise function independently. The same cannot be said for people who abruptly lose the sense of sight or hearing. The logical conclusion would seem to be that smell just isn’t that important for humans.
Until relatively recently, the scientific community endorsed the same conclusion. It was widely believed that humans have a paltry sense of smell compared to that of other creatures in the animal kingdom. Some proposed that the evolution of the human brain has been marked by a neural divestment in the sense of smell. To support this claim, they pointed to the olfactory bulbs. One thing that struck early anatomists is how small human olfactory bulbs are, relative to our large brain. As you can see in Figure 25, the olfactory bulbs of the mouse make up a much larger chunk of its total brain size. Scientists concluded that, as the brains of modern humans evolved, the growth of their olfactory bulbs was stunted and our ancestors’ ability to smell declined as a result. Some claimed that this happened because humans rely on reason rather than reflexive reactions to odors. Others suggested that our ancestors evolved to have brains that invested in vision at the expense of smell, making us super seers and pathetic smellers.
Despite these long-held notions, recent decades have brought a surprising revelation: humans are far better smellers than we ever imagined. Head-to-head tests of perception across species have shown that the human sense of smell is on par with that of the mouse. Although mice beat out humans at detecting certain odors, humans best mice on others. We also tie with dogs and rabbits in some odor matchups, and even surpass them in others. Moreover, studies that actually count neurons in the brains of various species suggest that mice, humans, and many other mammalian species all have about the same total number of neurons in their olfactory bulbs, regardless of the absolute or relative size of these structures.
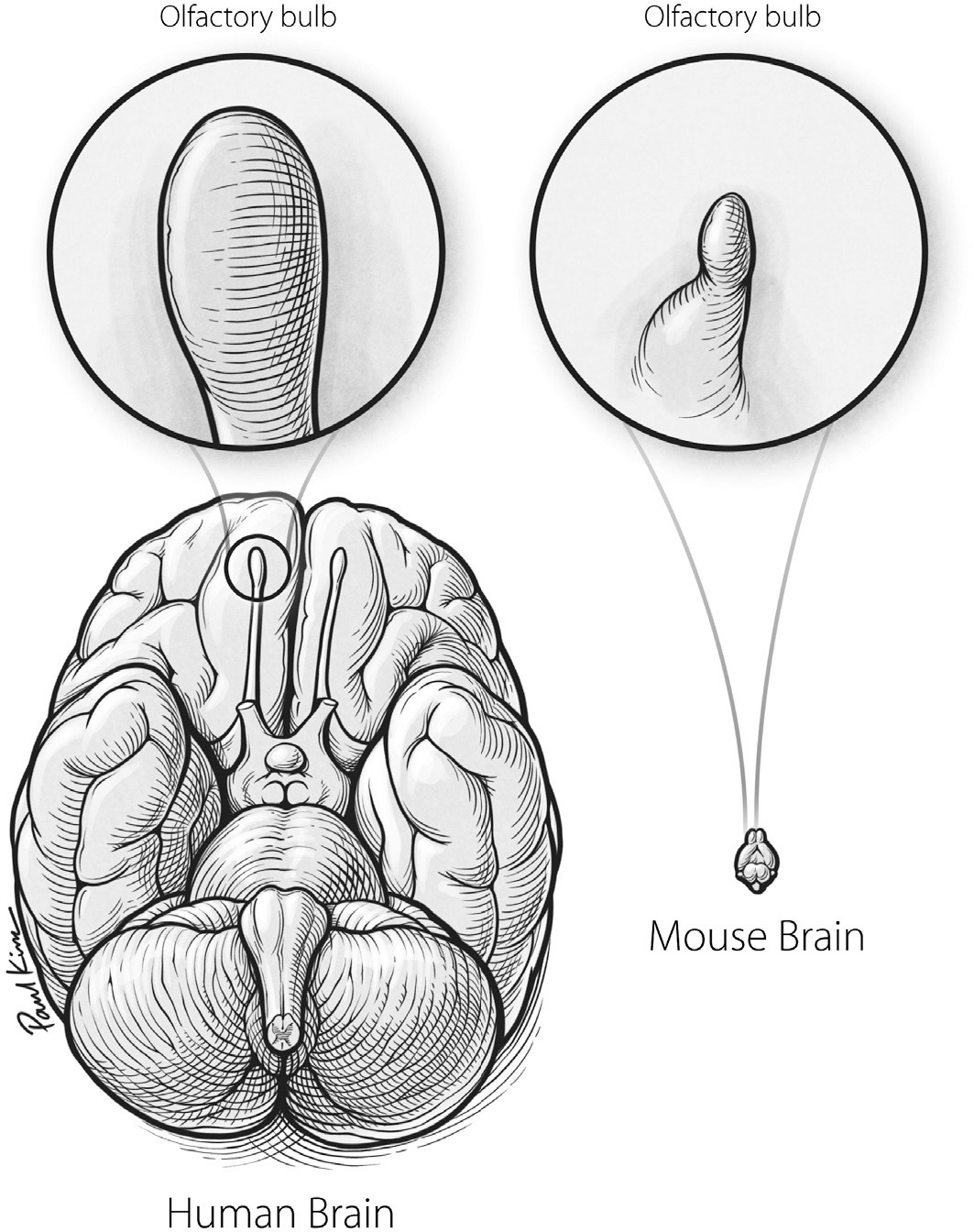
FIGURE 25. A comparison of olfactory bulb size (above) and total brain size (below) for humans and mice.
Paul Kim
Humans, like other creatures, are privy to a wealth of odors that inform us about stuff that is nearby. We can detect 1-octen-3-ol, a compound in mushrooms that yields their familiar scent. We can smell 2-isobutyl-3-methoxypyrazine from bell peppers, vanillin from vanilla, and eugenol from cloves and from wine that has soaked in oak barrels. We can enjoy the smell of geosmin, a compound produced by microorganisms in dirt, which gives us the earthy smell of soil after a summer rain. We can suffer the smell of compounds like trimethylamine from deteriorating fish carcasses and tetrahydropyridine from rotting meats and vegetables. The world of scent is filled with rich detail about the objects and physical processes taking place around us. But does this information drive our daily life-or-death decisions? Based on how well people manage after losing their sense of smell, the answer would seem to be no.
But there is a missing variable in this equation. Nearly all of our studies of human olfaction, like the vast majority of psychology and neuroscience research on humans, has been carried out on people from a handful of Western cultures with their own idiosyncratic way of life. In fact, there is good reason to believe that culture and way of life matter a great deal when it comes to olfaction. Westerners don’t tend to use the nose for finding food, but this is not true of people from all cultures and was almost certainly not true of our distant human ancestors. People from hunter-gatherer societies rely more on odor to glean information about their environment and have clear categories for describing smells. Among the Jahai, a group of hunter-gatherers living on the Malay Peninsula, the word cƞes is used to describe a variety of odors, including those of gasoline, bat droppings and bat caves, smoke, millipedes, wild ginger root, leaf of gingerwort, and wild mango wood. Mushrooms, deadwood, stale food, fur, and feathers are pɂus. When scientists asked members of the Jahai and a group of Americans to identify scents familiar to Westerners, the Jahai blew the Americans out of the water. Likewise a hunter-gatherer society, the Semaq Beri, easily bested their neighbors, the agricultural Semelai peoples, at odor naming, even though the two groups spoke related languages. In other words, smell is discussed, used, and conceived of differently in different cultures, which are shaped by different ways of life. If an individual, like my mother, without a sense of smell were born into a hunter-gatherer culture, she might have been severely handicapped. And just as the behavioral relevance of smell can differ between cultures, so too might the layout of odor maps in the brain.
The long debate over our human capacity to smell presupposes that olfaction must be a means of gathering information that we can consciously perceive and report. But the dirty little secret about olfaction—in Western cultures and elsewhere—is that it works much of its magic without our ever knowing it. In fact, the value of smell for informing us about the stuff around us may pale in comparison to its value as a delivery service for secret messages.
You deliver such messages all the time, although you don’t realize it. Humans are home to myriad glands in the skin that harbor bacteria. You don’t need to be sweaty from exercise to give off body odors; small amounts of these secretions are oozing out of your glands pretty much continually. People may not notice or comment on your body odor, but that does not mean that you don’t have one or that they are not detecting it, at least subconsciously. A growing number of studies show that the body odors we give off contain tons of information about ourselves—about our gender, age, physical health, emotional state, and fertility. Although scientists don’t yet know exactly which compounds in the odors convey these specific details, they can tell that these cues exist because of their effect on the people who smell them.
For example, when others smell the odors that you give off when you are fearful or anxious, they can quite literally smell your fear, even if they do not consciously realize it. And smelling your fearful sweat will make them more likely to detect threats in their environment. It can even influence their performance in high-stress situations, making people more likely to bungle a procedure or exam. Findings like these suggest that our bodies are constantly sending messages to one another via scent—messages that people do not consciously send nor consciously receive, even though they alert us to potential dangers (illness or threat) or opportunities (a fertile potential mate) and influence our behavior.
Communicating fear is just one way that humans influence one another through scent. Body odor also communicates identity; newborn infants recognize their mother’s scent, and family members learn to recognize their infant’s scent soon after birth. Body odor also causes young women living in close quarters to synchronize their menstrual cycles. The scent of human tears reduces human males’ sexual attraction to females. And people unconsciously tend to sniff their fingers after shaking hands with a stranger, although what they are sniffing for, and why, remains an intriguing mystery.
Scientists are only just beginning to discover how we actually use smell to guide our interactions and behavior. They know even less about the role that culture and lifestyle play in this process. And so perhaps it should come as no surprise that scientists have yet to discover human brain maps for odor beyond those in our olfactory bulbs. Of course, we may have failed to find them because there are simply none to find. But until scientists have a better understanding of how humans actually use odor, universally and in specific different cultures, they will have little chance of unearthing maps that make it possible.