Without our sensory receptors we would be isolated from the world and each other. Our five sensory systems respond to specific stimuli—light, sound, touch, airborne chemicals (smell), and solubilized molecules (taste). The first three are the most important for us to interact with the world and with each other, as the story of Helen Keller illustrates.
At 19 months of age, Helen lost both vision and hearing because of a devastating illness. As Helen grew, she was often depressed, and even angry, having frequent temper tantrums. This changed when her teacher, Annie Sullivan, hired to work with Helen when she was seven, managed to communicate with her through the sense of touch. Annie first taught Helen the manual finger alphabet by tapping on the palm of her hand. Initially, Helen had little understanding of what the various letter taps meant, but the breakthrough came one day when Annie and Helen were in the family pump house. As water from the spout rushed over one of Helen’s hands, Annie spelled w-a-t-e-r in the palm of the other hand. The sensation of water rushing over one hand, feeling the letters spelled out in the other, was transforming for Helen; she instantly understood and wanted to know the names of everything she touched. In a few hours, she learned the names of thirty objects. Within 4 months, Helen had a vocabulary of 400 words. She learned language much as a child learns spoken language, but she did so amazingly fast. By the end of their first year together, Annie was spelling into Annie’s hand stories from the Iliad and Odyssey.
Together, Helen and Annie achieved remarkable things, including Helen earning a college degree from Harvard’s sister school Radcliffe in 1904, the first blind and deaf person ever to earn a college degree.
—Adapted from Dorothy Herman, Helen Keller (Chicago, IL: University of Chicago. Press, 1999)
All neurons have characteristics of sensory receptors in that they possess specialized membrane proteins that respond selectively to specific chemicals—the neurotransmitters or neuromodulators. The effects of neurotransmitters on neurons, directly producing potential changes across the cells’ membrane, are similar to the effects of stimuli on touch, auditory, and some taste receptors. Olfactory receptors, photoreceptors, and certain taste receptors respond to stimuli as neurons respond to neuromodulators: enzyme cascades are activated, resulting in alterations of second-messenger levels and excitation of the cell.
This chapter describes examples of these two basic types of receptors: directly gated receptor cells and receptor cells gated by second messengers. In directly gated receptors, such as touch receptors, deformation of membrane channels themselves or of surrounding membrane results directly in the opening of the channels and in the generation of receptor potentials. Activation of olfactory or photoreceptors, in contrast, leads to activation of enzyme cascades by membrane receptors and to changes in second-messenger levels. Alterations in second-messenger levels result in the generation of receptor potentials.
Mechanoreceptors: Touch and Hearing
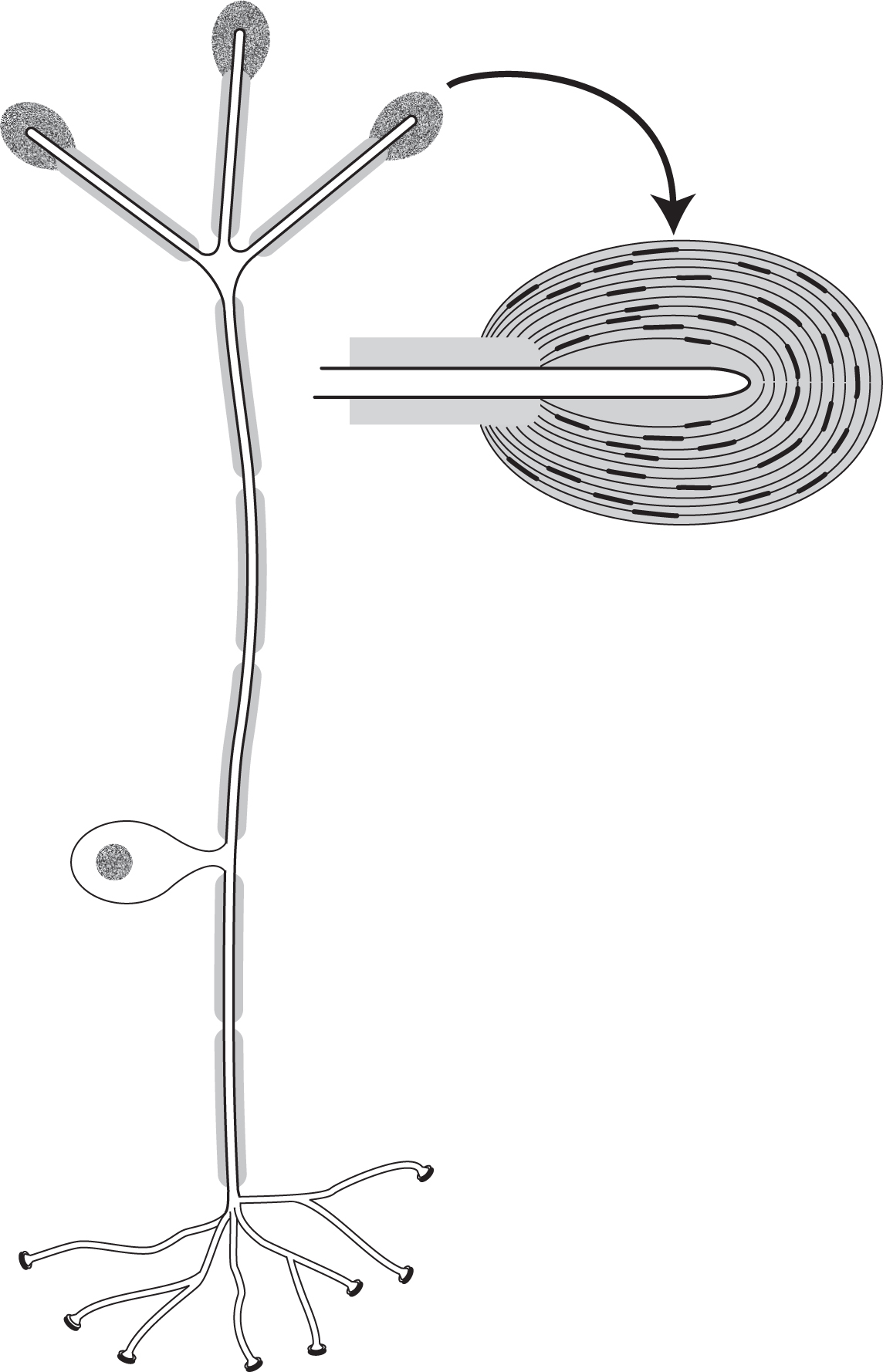
A great variety of receptors found in the skin and other tissues respond to mechanical stimuli, including touch, pressure, and vibration. Some mechanoreceptors are even found in muscles, and they signal muscle stretching and tension. Our model is the Pacinian corpuscle, a large pressure receptor found in the skin, muscle, joints, and tendons. The Pacinian corpuscle has been studied extensively because of its large size, so we know more about it than any other touch or pressure receptor, all of which are thought to work much like the Pacinian corpuscle. Under the microscope the corpuscle looks like a slice of onion, with many concentric layers of flattened nonneural (epithelial) cells surrounding a bare nerve ending. The nerve ending becomes myelinated upon emerging from the corpuscle. This corpuscle and a length of nerve fiber can easily be excised from tissue and kept functioning for some time; hence, they are convenient for study.
Figure 4.1 also shows what a Pacinian corpuscle receptor cell looks like. It is monopolar in nature—that is, only one process comes from the cell body. This process divides into two myelinated processes: one goes to the sensory endings, and the other to the spinal cord. Action potentials generated in the sensory endings are propagated directly to terminals in the spinal cord. The cell bodies of sensory cells innervating the limbs and trunk lie just adjacent to the spinal cord in structures called dorsal root ganglia. With a preparation consisting of the corpuscle and a short length of nerve hooked with a wire, it is easy to stimulate neural activity with a fine needle or stylus (see Figure 4.2). Compression of the corpuscle by a tiny amount, just a fraction of a micrometer (0.2–0.5 µm), induces a small receptor potential. With more compression, provided by a stronger stimulus, a larger receptor potential is generated. A depolarization of 10–15 millivolts or so generates an action potential superimposed on the receptor potential.

FIGURE 4.1 A typical pressure receptor. The neuron is monopolar; a short process from the cell body divides into two, with one myelinated process extending to the sensory endings (Pacinian corpuscles) and the other to the cell terminals. A Pacinian corpuscle (enlargement on right) consists of a bare nerve ending surrounded by a capsule of flattened nonneural cells.
FIGURE 4.2 When a Pacinian capsule is depressed with a stylus (a), depolarizing receptor potentials are evoked in the nerve that, if large enough, generate an action potential (b). (c) An S-shaped relation typically exists between receptor voltage (V) and stimulus strength.
Action potentials can be blocked by the drug tetrodotoxin, but the receptor potential is not. This is because tetrodotoxin blocks voltage-gated Na+ channels but not channels underlying receptor or synaptic potentials. By bathing the preparation in a solution containing tetrodotoxin, one can study the receptor potential in isolation, without the intrusion of action potentials. Intensity-response relations for the receptor potential can then be worked out (e.g., Figure 4.2c). Many types of receptors have similar S-shaped intensity-response relations. How is this receptor potential generated? Compression of the myelinated fiber coming from the capsule yields no response unless a large distortion of the membrane (10–15 micrometers) is induced. Then a change in membrane potential is recorded that probably represents damage to the nerve. (This is analogous, probably, to the excitation of the ulnar nerve when you hit your elbow on a hard object, that is, when you hit your crazy or funny bone.) Removing the onion-like capsule does not affect the response either. Indeed, when a preparation is stripped of the capsule, small deformations all over the bare nerve ending generate small receptor potentials. These small receptor potentials summate and produce a larger receptor potential. If the first node of the myelinated part of the fiber is mechanically blocked (by applying pressure to the node), no action potentials are generated but receptor potentials are still recorded.
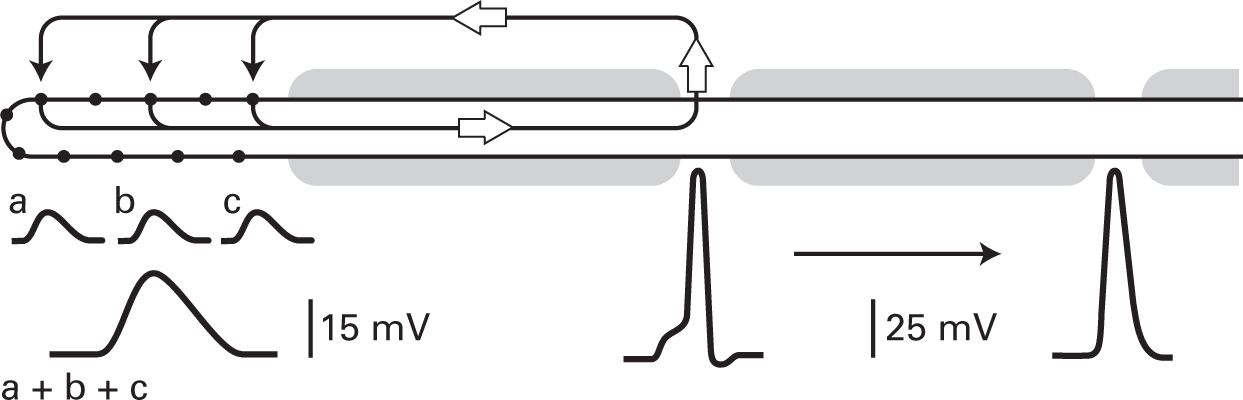
FIGURE 4.3 When the Pacinian capsule is removed, leaving a bare nerve ending, deformations of the membrane (arrows) result in small depolarizing potentials (a, b, and c) that can summate. Current (open arrows) entering the fiber through the mechanosensitive channels (filled circles) results in depolarization of the membrane at the first node, where voltage-gated channels are present, resulting in the generation of action potentials, which then travel down the fiber.
The experimental results suggest the following model. Contained within the membrane of the bare nerve ending are specialized channels whose conductance is altered when they or the surrounding membrane is deformed. At rest, few if any of the channels are open, but when the membrane is stretched, the channels change configuration and allow ions to cross the membrane. Because of the conductance change, net positive charge accumulates inside the cell, depolarizing the membrane at the first node in the fiber’s myelinated part. Here voltage-sensitive channels are located, and with sufficient depolarization, action potentials are generated that propagate down the fiber. Figure 4.3 is a diagram of the Pacinian corpuscle based on this model. Positive ions enter the bare nerve ending where the membrane is deformed and flow internally down the fiber. This depolarizes the first node in the fiber’s myelinated part and generates action potentials that travel down the cell.
What ions generate the receptor potential? This question can be answered by altering ion concentrations in the extracellular solution. Changes of Na+ most affect the receptor response; hence, the channels are mainly permeable to Na+, as is the case for channels that give rise to excitatory synaptic potentials (see Figure 2.9).
The Pacinian corpuscle also dramatically illustrates an important property of all receptors: adaptation. With a sustained stimulus, all receptor potentials decline. They may do so rapidly or slowly, completely or partially. Sensory adaptation is a distortion of the real world in the sense that receptors do not provide a faithful representation of the stimuli impinging on the organism. Even so, sensory receptor adaptation can have significant advantages; it is unlikely, for example, that we could wear clothes without being severely distracted by the continuous response of our touch receptors if they did not rapidly adapt.
Three types of adapting receptors are distinguished: fast, medium, and slow. Fast-adapting or phasic receptors respond only to a change in stimulus level. Pacinian corpuscles are fast-adapting receptors, as are other touch receptors and olfactory receptors. Figure 4.4a shows the response of a Pacinian corpuscle to a prolonged stimulus. Upon application of pressure to the receptor it rapidly depolarizes, but within 10 milliseconds or so the voltage decays to zero even though the pressure is maintained. The Pacinian corpuscle displays another feature of many phasic receptors in that it responds at the offset of the stimulus as well as at its onset.
FIGURE 4.4 Sensory receptor adaptation. (a) Fast-adapting receptors respond only for a short time after a stimulus is applied. With fast-adapting receptors, a response at stimulus offset following a prolonged stimulus is often observed. (b) Medium-adapting receptor also decline in response amplitude over time, but more slowly. (c) Slow-adapting receptors show an initial decline in response amplitude, but then plateau and the response lasts as long as the stimulus is maintained.
Medium-adapting receptors decline much more gradually in potential during the presentation of a long stimulus. If the stimulus is sufficiently prolonged, the response can decay almost completely to baseline, as illustrated in Figure 4.4b. Taste and hearing receptors are of this type. Photoreceptors and deep pressure receptors are slow-adapting or tonic receptors (Figure 4.4c). Photoreceptors respond to a prolonged stimulus in two stages, an initial transient potential that decays to a smaller plateau potential, which is maintained for as long as the stimulus continues. Although these receptors respond for as long as the stimulus is applied, as with other receptors they give a maximal response at stimulus onset and a smaller response thereafter. All receptors, even tonic ones, respond best to changes of stimulus level.
The sensory receptors for hearing are called hair cells, and they, too, are mechanoreceptors. Hair cells, like the Pacinian corpuscle, have channels that open when the channels themselves or the surrounding membrane are stretched or deformed. These channels are found at the tips of hairlike projections that extend from the top surface of the cell, and the hairs are connected directly by fine filaments that run between adjacent “hairs.”
When sound impinges on the fluid-filled inner ear, as shown schematically in Figure 4.5, the hair cells move relative to the tectorial membrane to which the hairs are attached. The hairs bend, increasing tension on the filaments and causing the channels to open, which allows positive ions to cross the membrane. The fluid surrounding the hairs in the inner ear is high in K+; indeed, K+ is higher outside the hairs than inside. Thus, K+ flows into the hair cell and depolarizes it when the channels are opened.

FIGURE 4.5 (a) Representation of hair cells in the inner ear. When sound impinges on the ear, the tectoral membrane moves relative to the basilar membrane (see Figure 4.6b), causing the hairs on the cell to bend. This causes the fine filaments connecting the hairs to stretch, thus opening channels on the hairs, allowing K+ to flow into the cell and causing the cell to depolarize. (b) An intracellular recording from a hair cell shows a change in membrane potential that closely matches the tone stimulus. When the cell depolarizes, transmitter is released from the hair cell, activating the auditory nerves that contact the hair cells.
Action potentials are not generated by hair cells. Rather, the graded receptor potential leads to the release of transmitter from the cell’s synapses; it is the second-order cells that first generate action potentials in the auditory system. A similar situation is found in the visual system. Indeed, in the vertebrate retina, action potentials are first generated mainly by third-order cells. That is, in the vertebrate retina excitation and synapse activation is via graded potentials in both the receptor and the second-order cells.
Hair cells serving hearing are found in a complex structure, the cochlea, situated in the inner ear (Figure 4.6). The cochlea is a rigid, coiled structure, encased in bone, that gets narrower as it goes from base to apex—hence, it resembles a snail. Indeed, the word cochlea derives from the Greek word for snail. A cross section through the cochlea shows that it consists of three fluid-filled compartments (Figure 4.6b). The hair cells are in the central compartment, termed the scalia media, in a structure called the organ of Corti that sits in the basilar membrane. The other two compartments are in continuity with each other at the apex of the cochlea, whereas at the base of the cochlea they are in contact with two membrane-covered holes called the oval and round windows.
When sound impinges on the ear, the eardrum vibrates. Three small bones transmit the vibration to the oval membrane, which induces vibration of the fluid in the upper and lower chambers and of the round window. Both the tectorial and basilar membranes flex in response to the fluid movements but differentially, resulting in bending of the hairs on the hair cells; this is key to excitation of the hair cells.
If the cochlea were unrolled, it would be about 32 millimeters long. Although the cochlea narrows as it goes from base to apex, the opposite is the case for the basilar membrane: it is five times wider at the apex than at the base. Furthermore, its rigidity decreases from base to apex about a hundredfold. At the apex, the basilar membrane is much more flexible than at the base. The result is that the region of maximal vibration along the basilar membrane depends on sound frequency. In this way place coding is established along the basilar membrane that determines pitch. In other words, we can detect pitch—different tones—by which hair cells along the basilar membrane are maximally activated.

FIGURE 4.6 (a) The cochlea in the inner ear. Sound impinging on the ear drum causes vibrations that are transmitted to the oval window of the cochlea via three small bones: the malleus, incus, and stapes. (b) A cross section of the cochlea illustrating its three chambers: the scala vestibuli (SV), scala media (SM), and scala tympani (ST). The organ of Corti sits in the scala media and consists of hair cells, which are embedded in the basilar membrane and the overlying tectoral membrane. (See Figure 4.5 for a closer view.) The auditory nerve innervates the hair cells.
Second-Messenger Receptors: Olfactory and Visual
The olfactory and taste systems have many features in common. Olfaction is specialized to respond to airborne molecules whereas taste detects solubilized molecules. A variety of taste receptors are known that respond to different-tasting substances. Salts and some amino acids interact directly with channels on the receptor cells, resulting in the depolarization and activation of the taste cells, much as excitatory neurotransmitters activate neurons. Other substances, including sugars and bitter compounds, activate G-protein-coupled receptor molecules on the receptor cells that link to either adenylate cyclase (sugars) or other synthetic enzymes (bitter substances). Depolarization of these taste receptor cells results ultimately from activation of G-protein-related molecules. All olfactory receptors appear to be activated via second-messenger pathways, and they will be our model. In some olfactory receptor cells a cAMP pathway is activated, whereas in others a different second messenger system is activated. Different types of odors preferentially activate one or the other pathway. Fruity odors, for example, such as essence of lemon, activate primarily the cAMP pathway, whereas offensive odors, such as those found in sweat, activate another pathway.
The olfactory receptors are found in the olfactory epithelium that lines the interior of the nose (see Figure 4.7a). The receptor cells are characterized by cilia that extend from a prominent apical dendrite into a thick layer of mucus that lines the nasal cavity. The mucus is produced by cells and glands that sit under the olfactory epithelium. Supporting cells are also present in the epithelium. Odorants entering the nose become dissolved in the mucus and are detected by their presence in the mucosa.
The odorant receptor molecules are found on the cilia. Application of an odorant to the cilia will cause a depolarization of the receptor cell, whereas odorant applied to the cell body causes little or no response. Intracellular recordings from olfactory receptors show that in response to odorants applied to the cilia, a very large depolarizing receptor potential, up to 50 millivolts in amplitude, is generated that passively spreads down the dendrite and into the cell body. Action potentials are generated where the axon comes off the cell and propagate along the olfactory nerve axons into the olfactory bulbs, which sit above the nasal cavity below the brain (Figure 4.7b). The olfactory nerve axons end in specialized spherical structures called glomeruli, which I discuss further below.

FIGURE 4.7 (a) The olfactory epithelium is located in the nasal cavity just below the olfactory bulb. (b) The olfactory receptors, which lie in the olfactory epithelium, extend their axons into glomeruli in the olfactory bulb, where they innervate the dendrites of mitral cells. The mitral cells carry the olfactory signal to the olfactory cortex. Each glomerulus is odor specific; that is, receptors that express the same odorant receptor molecules project to the same glomerulus.
The way olfactory receptor cells are activated is as follows. An odorant molecule activates a G-protein-related receptor molecule that activates either adenylate cyclase or another synthetic enzyme, resulting in increased levels of cAMP (Figure 4.8) or another second messenger within the cell. cAMP interacts directly with a channel in the membrane—no phosphorylation is involved—that allows both Na+ and Ca2+ ions to cross the membrane. The influx of Na+ depolarizes the cell, whereas the Ca2+ ions (which by themselves will also cause some depolarization) interact with a membrane channel that permits Cl− to leave the cell. Cl− leaving the cell adds to the depolarization by reducing the negative charge within the cell, and thus the Ca2+-activated Cl− current acts as an amplifying mechanism, explaining the large receptor potentials that can be generated in olfactory receptor cells.

FIGURE 4.8 (a) An olfactory receptor cell. Cilia extend from an apical dendrite on the receptor cell into a mucus layer that lines the nasal cavity. (b) Olfactory transduction occurs in the cilia. An odorant interacts with a receptor linked to a G-protein, which in turn is linked to an enzyme (in this case, adenylate cyclase). The cAMP generated by adenylate cyclase opens channels in the membrane that allow Na+ and Ca2+ to enter the cell. The Na+ depolarizes the cell, whereas the Ca2+ activates a Cl− channel, which promotes Cl− efflux from the cell, leading to further depolarization.
Humans can discriminate as many as 10,000 odors. How does this happen? It turns out that many different odorant receptor molecules are found in the olfactory epithelium, almost four hundred in humans and two to three times more in other animals. Each of these receptor molecules is coded by a separate but related gene; they make up the largest family of related genes known. Furthermore, it appears that only one or a few of these genes are expressed in a particular olfactory receptor cell, which means that the olfactory epithelium has a large number of distinct receptor cells. How are the coding and discrimination of different odors managed?
One receptor cell will respond to more than one odor, but each cell responds best to a specific odor. In other words, olfactory receptors have “tuning curves”—they respond best to a specific odor, less well to other odors. In this way they are similar to hair and photoreceptor cells, which respond maximally to one frequency of sound or wavelength of light and less well to others. The surprise in the olfactory system is how many different receptors there are; in contrast, in the visual system only three or four different receptors are required to discriminate all the colors in the spectrum.
How are the responses of the various olfactory neurons sorted out? Receptor cells of the same type—that express the same odorant receptor molecules—innervate separate structures (termed glomeruli) in the olfactory bulb (Figure 4.7b). In mice, each glomerulus contains the dendrites of up to one hundred glomerular (mitral) cells. Impinging on these dendrites are as many as 25,000 olfactory receptor axons, all of the same type. The mitral cell axons from a single glomerulus thus send to the rest of the brain quite specific odorant information.
Most vertebrate eyes have two types of light-sensitive photoreceptor cells, called rod photoreceptors and cone photoreceptors. Rods mediate dim-light vision, whereas cones function in brighter light and mediate color vision. Usually there is just one type of rod but several types of cones in vertebrate eyes. Humans, for example, have three types of cones: one that responds best to red-yellow light, another to green light, and a third to blue light. Rods and cones are elongated cells with a specialized outer segment region and an inner segment and synaptic terminal (Figure 4.9a). The outer segments consist of numerous membrane infoldings, usually pinched off from the outer membrane in rods, but still connected to the outer membrane in cones.

FIGURE 4.9 (a) In both rod and cone photoreceptors, light-sensitive visual pigments are found in membranous disks in the outer segments of the cells. (b) A portion of cone outer segment of a lizard. The lizard cone outer segments are long, and over a short portion of their length the cone shape is not obvious.
Photoreceptors “see” because they contain abundant light-sensitive molecules, the visual pigments, in the membranes of the cell’s outer segment (Figure 4.9b). Light is captured (absorbed) by these molecules, and this leads to excitation of the photoreceptor cell. The light-sensitive molecules are called pigments because they absorb certain wavelengths of visible light and hence have color. The visual pigment in rods, called rhodopsin, absorbs blue-green light best and captures red and blue light less well; because it lets red and blue light escape, it appears purple to us. If one removes the retina from an eye of a dark-adapted animal that contains abundant rods (and most animals, including ourselves, have many more rods than cones), the entire retina has a reddish purple hue. Indeed, the original name for the rod pigment was visual purple.
Light does two things when captured by the visual pigment molecule. First, it activates the molecule and excites the cell; second, it breaks down the molecule into component parts. All visual pigments consist of a large protein to which is bound a slightly modified form of vitamin A: vitamin A aldehyde, or retinal. When bound together, the molecule is sensitive to light in the visible range of the spectrum—between deep blue and far red light or between 400 and 700 nanometers. When split, the two components of the molecule absorb mainly ultraviolet light, which is invisible to us. The breakdown or bleaching of the visual pigment molecules in the light inactivates or desensitizes the photoreceptors. So when you go from bright light into a dark theater, it takes many minutes for your eyes to adjust. What you are waiting for is the resynthesis of the visual pigments in the photoreceptor cells—a process known as dark adaptation—which restores the light sensitivity of the photoreceptors. After bright-light adaptation, cones dark-adapt in 5–6 minutes, but rods require up to 30 minutes to complete dark adaptation.
Individuals deficient in vitamin A are less sensitive to light than are well-nourished individuals. This condition is known as night blindness because it is most obvious at night. Yet both rods and cones are affected in vitamin A deficiency—both have visual pigments that require vitamin A, and thus with vitamin A deficiency both are less sensitive to light. What differs between the rod visual pigment and the three cone pigments are the proteins. Differences in these proteins give the visual pigment molecules somewhat different properties, including their color sensitivity, that is, the wavelength of light they best absorb.
The genes coding for the rod and cone visual pigment proteins thus are different. Defects or alterations in these genes lead to significant visual abnormalities. Color-blind individuals either have lost a gene or have a defective gene for one or another of the cone visual pigment proteins. Red-blind individuals are missing or have an altered red-sensitive visual pigment gene, green-blind individuals are missing or have an altered green-sensitive pigment gene, and blue-blind individuals are missing or have an altered blue pigment gene. The genes for the red- and green-sensitive pigments are on the X-chromosome. Since males have just one X-chromosome and females have two X-chromosomes, red and green color blindness is much more common in males than in females. This is because even with only one good chromosome, color vision will be normal; the photoreceptor cell can still make a normal pigment. Since females have two X-chromosomes, they can have one defective gene and one good gene and have normal color vision. Because males have only one X-chromosome, if that chromosome has a defective color pigment gene, the individual will be color-blind. Red-green color blindness is thus described as sex-linked.
It is important to note that most individuals we call color-blind still see colors. That is, if a person is unable to make one of the three cone visual pigments because of a defective gene (which is by far the most common situation), he or she still has two other cone types—cones sensitive to green and blue, red and blue, or red and green. With two cone types, color discriminations can be made, although such individuals cannot distinguish colors as well as a normal person with all three cone types. The color blindness exhibited by Jonathan I., described at the beginning of Chapter 7, was caused by a deficit in color vision processing in the brain, not by an alteration in his cones. He was totally color-blind; he could make no color discriminations at all.
Alterations in the gene for the rod pigment, rhodopsin, can lead to a disease called retinitis pigmentosa. People with this disease start off life with normal vision, but then the rods degenerate, first in the periphery of the retina and eventually throughout the retina. As the rods die, patients first lose the ability to see well in dim light, but eventually the cones die too, for reasons unknown, and all vision is lost. Why the rods die gradually, over the course of many years, is also not known. People with this genetic disorder usually begin to notice diminished visual sensitivity in their twenties or thirties. Complete blindness may come in the fifties or sixties.
When a photon of light is absorbed by one of these light-sensitive molecules in a photoreceptor, it initiates a series of chemical reactions termed phototransduction, (Figure 4.10). A light-activated visual pigment molecule first interacts with and activates a G-protein (called transducin) that then activates an enzyme called phosphodiesterase (PDE). The second messenger in vertebrate photoreceptors is, however, cyclic guanosine monophosphate (cGMP), a molecule similar to cAMP but with guanosine rather adenosine in its structure. Interestingly, cGMP levels are maintained at a high level in vertebrate photoreceptors when in the dark. The cGMP interacts with channels in the membrane that allow Na+ and Ca2+ to cross the membrane. The PDE activated by light breaks down the cGMP, thus closing the membrane channels, resulting in the cell’s membrane potential becoming more negative—the cell hyperpolarizes, the opposite of what happens in an olfactory receptor when it captures an odorant molecule. In a sense, then, darkness is the stimulus for vertebrate photoreceptors, and light turns them off. But it is the change of membrane potential in both cases that is important and leads to excitation of the system.
Why do vertebrate photoreceptors behave this way? We do not know. Another question is why do photoreceptors use a second-messenger system, and here we do have an answer: for amplification. That is, one activated visual pigment molecule can activate many transducin molecules, and one transducin molecule can activate several PDE molecules. And an activated PDE can break down many cGMP molecules. Thus, the amplification is considerable when a single visual pigment molecule absorbs a photon of light, explaining the extraordinary sensitivity of a photoreceptor responding to a single photon of light.
A final question is the role of Ca2+ in the process. As noted above, the channels in the photoreceptors activated by cGMP allow both Na+ and Ca2+ to enter the cell. Na+ is much more abundant outside cells than is Ca2+ and is mainly responsible for the change in membrane potential when the cell absorbs a photon of light. Ca2+, on the other hand, although present in lower amounts than Na+, does inhibit the enzyme that makes cGMP. Thus, when the cGMP levels fall in the light, resulting in Na+ and Ca2+ levels falling in the cell, the inhibition of cGMP synthesis also decreases, allowing an increase in cGMP levels, partially countering the effect of light. This results in a partial reopening of the Na+/Ca2+ channels, explaining adaptation of the photoreceptor response This is why the photoreceptor response to light is initially larger than the subsequent sustained plateau responses even though the light impinging on the cell remains the same for the duration of the light stimulus. (see Figure 4.4c).

FIGURE 4.10 A simplified scheme of the phototransduction process in the photoreceptor outer segment. Light-activated rhodopsin (Rh*) activates transducin (T) a G-protein, which in turn activates the enzyme phosphodiesterase (PDE). PDE breaks down cGMP to an inactive product (GMP); in the absence of cGMP, which opens channels in the outer-segment membrane, the channels close, and the cell hyperpolarizes (becomes more negative inside) because the positively charged Na+ ions no longer can enter the cell. Also, Ca2+ levels in the cell decrease, allowing guanylyl cyclase (GC), normally inhibited by Ca2+, to increase the synthesis of cGMP from guanosine triphosphate, an energy-rich molecule similar to ATP but with adenosine replaced by guanosine. With more cGMP available, more channels open, countering the effects of light. Ca2+ thus plays a role in photoreceptor adaptation.