NOTE-BY-NOTE COOKING? Trust me: I always use words with due regard for what they mean—but I don’t deny myself the luxury of metaphor. In the phrase “note-by-note cooking,” the noun that is modified is cooking. In French, the word cuisine denotes a room, the kitchen, but it refers above all to an activity, cooking, well described by the title of a book I wrote a few years ago that was published in English under the title Cooking: The Quintessential Art. The original title is more revealing: La cuisine: C’est de l’amour, de l’art, de la technique. By this I mean that the cook’s primary purpose is to show love (which may or may not be wholly sincere—though the reader will have guessed that I myself favor honesty in matters of the heart) to his or her guests. Beyond that, the cook seeks to make something good, a work of art in the best case, and to make it well, according to some standard of technical excellence.
Let’s begin with the simplest part, the technical aspect. Culinary activity physically assumes the form of a series of operations: cutting, heating, grinding, filtering, evaporating, assembling, melting, blending, emulsifying, mixing, expanding—exactly the same things, as it happens, that are done in the marvelous field of chemistry. There is nothing in the least surprising about this: chemistry, like cooking, has a technical component. But this component is almost an incidental detail. Chemistry, though it involves technique, is not merely a matter of “doing.” It is also a science, a search for the underlying mechanisms of phenomena whose aim is to acquire knowledge. What does it mean to say, then, that cooking has a technical component? It means that cooking is not solely a matter of technique, as most people suppose. There is much, much more to it than that.
If cooking were only a matter of technique, it would be a very sad thing indeed, for a machine is all that would be needed to cut, heat, grind, filter, evaporate, assemble, and so on. Worse still, machines often outperform human beings from the technical point of view. But cooking is able to transcend mere doing by virtue of the fact that it also has an artistic aspect. It aims at making something good, which is to say dishes that are fine and beautiful things to eat. Technique is a means, not an end. And so we are agreed that cooking is both a technical and an artistic activity. But that is by no means all it is. Even the most beautiful, the most splendid meals are not always good because we partake of something more than food when we eat. We partake also of the love shown to us not only by the cook, but also by those with whom we share a meal. It is often (and rightly) said that if you sup with the devil, you need a long spoon. This is why expensive business meals are apt to be so unsatisfying, whereas a simple snack with good friends can be one of the loveliest experiences there is. We do not eat the way animals do. We are social creatures, endowed above all with the power of speech, and it is the act of gathering together (some food lovers even speak of “communion”), in which eating is combined with conversation, that accounts for the happiness of so many moments we spend at the table. This is what La Varenne meant when he said that jolly meals are the best ones of all.
My use of the term
love to describe the third aspect of cooking (in reality, the first in order of importance) is no doubt inappropriate, at least some of the time, for we do not all look at it in the same way. For some people, love really is love, but for others it is power or money or something else. The command “Eat your vegetables!”—unfondly remembered by many of us from childhood—is an example of the power exerted over us by our parents, not always benevolently, when we are young. When we are older, doctors and nutritionists order us to eat in ways that they consider to be healthy. The word
love may seem a bit naive, I grant you, but I use it deliberately, even if it is clearer, more reasonable, to speak of cooking as having a social aspect. And obviously I don’t mean to suggest that we should want to eat in ways that are unhealthy. We are right to prefer that cooking be done by trained professionals rather than run the risk of being poisoned by self-taught amateurs. But in that case we put ourselves on the other side of the cooking line, where the waiters are loading up their trays with the dishes that they are about to bring out to us in the dining room.
So much, then, for the “cooking” part of “note-by-note cooking.” The “note-by-note” part is a bit trickier. We will need to examine it with some care.
A NONOBVIOUS PIECE OF OBVIOUSNESS
It is curious that the value of note-by-note cooking is not thought to be plain on its face, but instead becomes apparent only after one has thought about it for a while (which says a great deal about what is or is not obvious to us, but that’s another story). And yet we shall see that note-by-note cooking altogether naturally recommends itself to cooks and gourmets alike.
We cannot avoid talking in the first place about the love of food, for our subject is cooking. If we love wild mushrooms, for example, we are naturally saddened by the thought that the season for them will eventually come to an end and they will become vanishingly scarce. It makes us melancholy to see the forest floor fill up with water and then freeze, to see the ground covered by dead and decomposing leaves, a sad brown carpet on which our marvelous specimens are nowhere to be found. The only way to lift our spirits is to buy imported fresh mushrooms or to use products that have been preserved in one or more ways, ancient or modern, from dried mushrooms to canned. Many kinds of food are preserved, of course (candied fruits, pickled vegetables, conserved meats, and so on), to say nothing of the great variety of frozen foods available to us today. Nevertheless what is scarce is expensive, and we do not always feel wealthy enough to buy everything we would like.
Then one evening a friend comes over for dinner—a true friend, as it happens, since he solves our problem. He tells us about 1-octen-3-ol, a compound that is found in many mushrooms. We listen to him with an open mind because gastronomy is part of what makes us human: by helping us to go beyond our animal instincts, it has taught us not to rule anything out without subjecting it first to thorough examination. Gastronomy teaches curiosity, an essential element of both the arts and the sciences. The enlightened food lover in us therefore seeks to know more about this compound with the strange name. We get hold of a sample, smell it, and discover that 1-octen-3-ol (or octenol, for short) has a truly marvelous fragrance, very similar to the ones that we love and miss so much. To be sure, it is not quite the same as the odor of a chanterelle or a cèpe or a black trumpet. No, the scent is familiar and novel at the same time: in addition to the smell of mushroom, there are notes of undergrowth, the undergrowth of a deep and wet forest. What should we do? Should we be content with only a memory—the memory of our favorite mushrooms? Should we go on living, in other words, in the realm of the mind? Or should we embrace the sensual appreciation of good food and get ourselves into the kitchen?
The true gourmet, once tempted, cannot resist. Quickly he grows accustomed to cooking with this new product, just as he would get used to cooking with a new spice (spices, by the way, contain a great many flavorful compounds, most of which we are unaware of). Having come to feel comfortable using this first compound, the enlightened food lover now goes on to acquaint himself with a second one, limonene, which has a wonderfully fresh scent that calls to mind the fragrance of citrus fruits such as lemon and orange. And then a third one, sotolon, which, in addition to notes of walnut, curry, and fenugreek, has an odor reminiscent of the famous “yellow wine” of the Jura in France. And a fourth, arginine, which, although it has no odor, yet has a very distinctive flavor.
The gourmet’s scruples melt away in the face of so many intriguing sensations. Familiar products such as rose water, orange blossom water, nutmeg, parsley, and ginger are soon joined in his kitchen pantry by a variety of chemical compounds in liquid or powdered form. Before long the pleasure he derives from cooking, which inevitably comes before the pleasure he takes in dining with others, leads him to mix two of these novel culinary preparations, then three of them, then four. Fruits, vegetables, meats, and fish eventually disappear, giving way to pure compounds, which are skillfully combined to create dishes of a new kind. And there it is—note-by-note cooking!
THE HISTORY OF NOTE-BY-NOTE COOKING
The just-so story I have just imagined is not so very different from the actual history of the creation of note-by-note cooking. It all began in 1994, when I was rushing to finish an article for Scientific American entitled “Chemistry and Physics in the Kitchen” with my dear friend Nicholas Kurti. He was fifty years older than I was, but we were like two children for whom the difference in age counted for nothing next to our desire to do wonderful things and, even better, to do them together. Nicholas was a remarkable physicist. He had had an eventful life, leaving Budapest, where as a young man he had contemplated a career as a concert pianist, for Paris, then moving to Berlin, and finally to Oxford after being driven out of Germany by the Nazis. He is remembered in particular for the discovery of nuclear adiabatic demagnetization, a technique he used to achieve the lowest temperatures ever recorded, only fractionally above absolute zero (−273.15ºC)—the temperature below which it will never be possible to go, for it is at that point that atoms cease to move or change position.
Six years earlier Nicholas and I had created the scientific discipline that came to be known as “molecular gastronomy,” taking advantage of every possible opportunity to work together. When I was asked to set up an institution of higher learning in France dedicated to food research, I enlisted Nicholas’s assistance as cofounder. When Nicholas was asked to write scientific notes for a cookbook, he sought my aid. Thus it was that we came to collaborate on the article for Scientific American.
For such articles, there was one invariable rule: I wrote the first draft, which Nicholas then edited. It was an efficient division of labor. For the
Scientific American piece we had agreed on an outline in advance, so the writing went quickly. It remained only for me to come up with a conclusion. But what could I say in conclusion when the adventure of molecular gastronomy had scarcely begun? At the time my own work had less to do with octenol than with another volatile compound, paraethylphenol, which in very weak concentrations contributes to the smell of leather given off by vintage Burgundy wines and, in only slightly higher concentrations, transforms an ordinary whiskey into a peated beverage. Since I was busy investigating the properties of paraethylphenol in my lab, it occurred to me to tell our readers what I had found out so far. One thing led to another, altogether naturally and logically it seemed to me, with the result that the idea of note-by-note cooking was born. Nicholas was willing to indulge me—partly because the story I had to tell was not entirely uninteresting, but mainly because we had an urgent deadline to meet! Even so, and even though I was a chemist, I worried that I was pushing things a bit too far. Was I really suggesting that we should add compounds to these marvelous works of art known as wines? Indeed, no sooner had the article appeared than oenophiles rose up in protest. If this sort of thing were permitted, they said, the world of wine would be fatally corrupted. The myths of Pandora and Prometheus combined to raise the specter of a future in which science is used to encourage first the doctoring and adulteration of wines and then the marketing of them by every known means of fraud and dishonesty.

Are wine lovers’ fears justified? We will never be able to reply to this question, or to any other question worth asking, if we do not take care to speak as precisely as possible. Adulteration—making wine impure by adding alien ingredients—is against the law. The modification of wine is not in and of itself a serious matter. What is a serious matter, from the legal point of view, is giving the name
wine to something that plainly is nothing of the sort. By definition, wine is a product that can be obtained only through the fermentation of grape juice. From this it follows that adulteration is improper. How is it, then, that we have come to accept the idea, common today, that adulteration may be a source of improvement? I fear, alas, that “sophisticated” women are at least in part to blame because their love of cosmetics makes them victims of adulteration (the literal meaning of the French word
sophistiqué). In our culture, however, women who apply makeup are considered beautiful. Surely this is why the idea of adulteration has gradually acquired a meliorative connotation, without due regard for what words mean.

The fact remains that doctoring and adulteration, particularly of wines, are unlawful activities. This, as I say, is because the law does not authorize use of the word wine to designate anything other than fermented grape juice. The addition of sulfur is permitted so that the wine will keep longer, but nothing else. And yet, in my view, even the use of preservatives should be opposed. There is nothing wrong, of course, with making wine-based products (as indeed the ancients did by adding honey and various aromatics), but in that case we are no longer dealing with wine; we are dealing instead with a product that is made from wine and that must therefore be labeled as such. The same is true of bread, which by law can be made only from water, flour, yeast (and, again, a very restricted number of additives): bread must be bread—not cake or any bread-based preparation. New preparations need new names. There is no reason to debase the names we have been using correctly all these years.
Note-by-note cooking, I am pleased to say, has nothing to do with any of this. It is not a method for altering or adulterating or doctoring foods. It is a method for making foods out of compounds.
NOTE-BY-NOTE COOKING IS NOT “CHEMISTRY”
From the very beginning, then, there was adamant opposition to note-by-note cooking on the ground that putting “chemistry” into cooking would be a terrible thing. Why the scare quotes? Because chemistry will never be a part of cooking! It is an impossibility. Chemistry is an activity that seeks to acquire knowledge: it seeks to understand how atoms are arranged in matter and how they can be rearranged in the course of reactions. Chemical reactions? No, reactions. Atomic reactions, perhaps, because atomic reactions are what produce molecular compounds—but not chemical reactions. The expression chemical reaction, like chemical product, should be used only very seldom, as we shall soon see.
First, however, let me emphasize that however shocking the claim that chemistry will never have a place in cooking may seem, it is nevertheless perfectly true. It is not a piece of linguistic legerdemain palmed off by a chemist looking to defend the parochial interests of his profession. To see why, let’s begin by taking a closer look at the class of substances known as compounds—substances so poorly understood that they are commonly confused with molecules, products, chemical products, synthetic compounds, and who knows what else. The easiest way to do this is to step into the kitchen. Put a piece of beef sirloin in a hot oven, say 200ºC (almost 400ºF). After a few minutes, the surface of the meat will have turned brown because compounds in meat that are heated to more than 100ºC (212ºF) react and are transformed. Are these chemical compounds? No, they are compounds. Water is a compound because it is made of very small objects, constantly in motion, that are called molecules (in this case, water molecules). If water is a compound, it is not because it is composed of water molecules, but because water molecules, all of them identical, are composed of atoms of different sorts. In a water molecule, for example, there are two atoms of one sort, hydrogen, and one atom of another sort, oxygen. What are atoms? Let us content ourselves for the moment with saying that they are objects smaller than molecules since molecules are composed of atoms.
Molecules make up the main part of living matter and therefore of our bodies as human beings and of our environment. In wines, for example, the principal molecular compounds are water, ethanol, and tartaric acid. In the starches obtained from potatoes and rice, the compounds are amylose and amylopectin (not to be confused with the pectin found in jams and jellies). In oils, the compounds are triglycerides. An egg white is composed chiefly (90 percent) of a single compound, water, along with smaller amounts of various other compounds known as proteins. There is nothing in the least “chemical” about these compounds. The confusion arises in part from a logical error concerning the relation between a whole and its parts. Referring to the train of officials following a head of state, for example, as a “presidential procession” is an error of this type because the procession could be presidential only if it itself were the president. Similarly, a compound is a compound. It becomes a chemical compound only when it is studied by chemists.
The mistake in talking about “chemical compounds” involves a deeper confusion, however, between so-called natural and synthetic compounds. The difference between them will become clear at once if we look more closely at what water is made of. A drop of water that falls from the sky is composed of molecules, each of which has two hydrogen atoms and one oxygen atom. These molecules (all of them of natural origin because the sky is a part of nature, not a product of human activity) are in every respect identical to those that a chemist would obtain by making two gases—dihydrogen (one used to say simply “hydrogen,” but there was a risk of ambiguity, which modern chemistry has since dispelled) and dioxygen (formerly called “oxygen”)—react: a flame or a spark that comes into contact with a mixture of these two gases produces an explosion, with the result that the atoms of the two gases are reorganized into water molecules (if this makes you nervous, rest assured that chemists today know how to synthesize water without the gas exploding). Whether it is water that rains down from the sky or water that has been synthesized by means of a reaction between dihydrogen and dioxygen, it is made up of molecules, and each of these molecules consists of a unique combination of three atoms, two of them hydrogen and one oxygen. The physical, chemical, sensory, and nutritional properties are entirely the same for the two sorts of water. And yet one of the two has been synthesized, the other not.
The same thing is true of acetic acid, the compound mainly responsible for the acidity of vinegar, and of vanillin, the principal compound of the smell of vanilla. Here again one finds molecules that are wholly identical to artificial molecules made in a laboratory. In these and many other cases, the operations carried out by the chemist are indistinguishable from what a cook does when he cooks. Why should we be bothered then?
Chemists also know how to make compounds that are not found in nature. By rearranging natural compounds, they gradually learned to construct compounds that had not been previously identified. This does not mean that such compounds do not in fact exist, only that they are not known to exist in nature, at least not for the moment, and that there is no real point trying to find out whether they do already exist, unless perhaps to settle trademark or copyright disputes, for example. This, at any rate, is what most chemists would tell you. Many nonchemists fear that fabricating novel compounds would only lead to unforeseen troubles. But once again it needs to be pointed out that cooks do exactly the same thing when they impart flavor to food by cooking it: the heating causes compounds to appear that initially were not present and that in many cases do not (so far as we know) exist in nature. No one seems to think this poses a problem.
Whether the nonchemists are right or not, we all can agree that a synthetic compound (one should really speak instead of a “synthesized” compound or of a compound that has been made “by synthesis”) is a substance produced by human beings through the reorganization of matter brought about by a reactive process. Again, rather than speak of a chemical reaction, we should refer instead to a rearrangement of atoms—a slightly more cumbersome phrase, I admit, but how much more accurate! At all events it should be clear that chemistry will never be found in cooking. At worst (or at best, depending on your point of view) there will be—and already are—synthesized compounds in addition to so-called natural compounds.
NATURAL FOODS? IMPOSSIBLE!
In distinguishing between compounds of natural origin and compounds that have been synthesized in a laboratory, I passed too quickly over the idea of nature and what it is supposed to signify. In an era of self-righteous environmentalism, these things are inevitably a crucial part of the debate that note-by-note cooking has helped to inaugurate in the world of food.
Whole treatises have been devoted to the subject. All of them begin from a true premise—namely, that a thing is natural rather than artificial by virtue of the fact that it has not been transformed in any way by human intervention. An exploding volcano is a natural phenomenon, for example, whereas a musical melody is an artificial creation. From this it follows that no prepared food is natural, for prepared foods, by definition, are the result of human preparation and therefore artificial from the very start.
Moreover, most traditional (as opposed to note-by-note) dishes are made from animal and vegetable products that themselves are artificial since they have been modified to one degree or another by human activity. Just as it takes a gardener to grow carrots, a breeder and a butcher are needed to bring a leg of lamb to our kitchen.

In other words, because even the ingredients used in traditional cooking are not natural, foods are still less natural than one might at first be inclined to suppose. (Indeed, a scale of artificiality could be devised to measure the degrees of separation between a product and pure naturalness.) Complicating matters still further, modern culinary ingredients are typically parts of animals or vegetables that themselves are the result of a long process of selection. Consider, for example, how great a distance separates the natural wild carrot, a hard and fibrous stick, from the artificial cultivated carrot, bright orange and swollen with sugars. Our foods are not natural—and they never were, no matter how stridently natural-foods advocates and others may insist on it.
This distinction has now become an issue of public policy, at least in the European Union, where regulatory authorities are being pressed by various interest groups (government agencies, food companies, consumer groups, ordinary citizens) to provide a rigorous definition of what constitutes a “natural” food. Evidently this is an absurdity, for the reasons I have just given. The same absurdity will present itself later, in
chapter 4, in connection with odorant compounds. A most unwelcome regulation has recently authorized the use of the term
aroma to designate various combinations of odorant compounds, whereas plainly it should be restricted to the scent or fragrance of aromatic plants—aromatics, as they have long and rightly been known. Companies that prepare and market such mixtures (some of which are terrific, by the way) should not be granted a monopoly on the term
aroma. We mustn’t yield to demagogy or commercial pressures, even if they have the force of law: if a law commands us to believe that 2 + 2 = 5, it is our duty to oppose it.

Conversely, it must be recognized that the term
artificial is derived from
art. The artificial is something quite remarkable, far superior to the natural, because it has been designed by human beings and realized through their own labor. Our clothing, our homes, our computers, our medicines and protective cosmetics—all of these and many more things are artificial. Without them, most of us would have been dead a long time ago. To be sure, not all things made by human hands are good—or, shall we say, equally good. A bomb, for example, is a lethal device; rape, murder, and other violent crimes are abhorrent. There are, of course, grades of quality, just as there is a difference in quality between the drawing made by a child who has not yet learned how to hold a pencil and a drawing by Dürer, who spent a lifetime mastering his art. But even if we may agree that not all artificial things are equally good, isn’t it up to us to try to improve them, to make them better?
People who talk of a “golden age” of food—an age when ingredients were natural, when cooking was better than it is now, and so on—are perpetuating a myth, a childish illusion that we are loath to abandon. Someone recently wrote me that because she had been raised in the countryside, her parents and grandparents made sure she had a wholesome diet. I do not doubt that she was raised in the countryside, if she tells me she was, but I am rather skeptical about the expertise of parents and grandparents in this matter. What did French peasants really know about diet in the early twentieth century? Can we really say that their own diet was wholesome, considering how many people in rural villages in France died at a relatively young age in those days? Without modern advances in epidemiology and nutritional science, we would still be consuming enormous quantities of smoked products that cause cancers of the digestive system; we would still be adding cinnabar (mercuric sulfide, a horribly toxic substance) to pissala, the paste traditionally prepared in Nice from anchovies and salt; we would still be sweetening wines, as the Romans did, with intoxicating lead oxides. When it comes to diet, the wisdom of the ancients may be doubted.
Ought we not, in fact, take the view that the ancients are not to be listened to at all, no more in matters of cooking, science, or art than in matters of nutrition, and that it was the cumulative labor of generations that led to an increase in knowledge and humane learning? The primitive understanding of pictorial perspective in Egypt, under the pharaohs, when figures were shown in profile, underwent no fundamental modification until Leon Battista Alberti published his treatise On Painting in the mid–fifteenth century. Plato, one of the greatest of all philosophers, attempted to justify slavery. The classical doctrine of the inferiority of certain human races continued to be taught as late as the twentieth century. Surely there can be no good reason not to free ourselves at long last from this idea that everything that is ancient is good?
The question whether the artificial is better than the natural (or vice versa) makes no sense because it is too general. We need to recognize that what is naturally good—Caesar’s mushroom (
Amanita caesarea), for example—is good, but what is naturally bad—the death-cap mushroom (
Amanita phalloides), for example—is bad; likewise, that what is artificially good—Bach’s
Sonata for Solo Flute, for example—is good, but what is artificially bad—a very cancerous compound, for example—is bad. Moreover, even though nothing is good or bad in an absolute sense, there must nevertheless be an apt correspondence between a thing and its function. A knife, useful when used to prepare food, may be an instrument of evil when used to commit a crime.
Why do some people believe that the artificial is unequivocally bad? No doubt because we are animals who have evolved over time, on the one hand, and because not all the things we make are equally good, on the other. There is no disputing the first claim: we are indeed animals, and as animals we have been “coded” by billions of years of biological evolution. If a newborn child (no less than a newborn monkey) smiles when sugar is rubbed on its lips, for example, it is because its animal instinct tells it that sugar is a source of energy. This is not surprising since primates coevolved with plants: the sweet fruits produced by plants attracted primates, and primates dispersed those plants’ seeds in turn. As for the second claim, that not everything that is artificial is equally good, nothing could be easier than to multiply examples beyond the ones I have already given.
Take the case of vanilla, vanillin, and the various extracts and preparations that are sold in grocery stores. To begin with, vanillin is a compound and must not be confused with vanilla, a fermented pod. Keep in mind, too, that vanilla is not in the least natural. It is the result of an elaborate manufacturing process in which the pod (or capsule) of an orchid of the genus Vanilla is subjected to a series of procedures: scalding, sweating, drying, conditioning (during which it is kept in closed boxes and cured for five to six months), and grading. The capsule turns brown only during the sweating stage, when an enzymatic reaction occurs, and it releases its aroma (the proper term in this case since vanilla is an aromatic plant) only during the drying and conditioning phases, when the moisture content is reduced.
Much of the flavor of vanilla in its processed form is due to the compound vanillin, but the richness of the flavor, its mellowness, results from the presence of many other compounds as well. Vanillin is only one of the compounds found in vanilla. It accounts for a part of the flavor of the fermented pod, but not all of it. Moreover, it is false to speak of
the flavor of vanilla because no two neighboring pods have exactly the same flavor. Just because trained tasters cannot tell the difference when they are asked to compare vanilla and vanillin, should we allow the two things to be conflated? No, a thousand times no! That would be a lie—and all the more so since vanillin can be extracted from lignin, a compound found in wood, which is much less expensive than the plant from which vanilla is harvested. Is vanillin somehow better than vanilla, or is it less good? There is no clear answer, for “better” in this case is a matter of individual preference. However it is correct to say that vanillin is vanillin (whether it is natural or synthetic) and that vanilla is vanilla (a quite artificial product, considering the number of steps required to make it).
It must be admitted that not all preparations that give off a scent of vanilla are equally good. Some, prepared by gifted artists who have spared no expense, are very fine; others, in which vanillin has been dissolved in an ordinary caramel, for example, are unmemorable. Whether the vanillin is natural or synthetic, however, is of no importance whatsoever. We all can agree, I think, that what matters is the final result.
COMPOUNDS AND MOLECULES
Compounds are evidently a stumbling block for many people. They will feel more at ease with the idea of note-by-note cooking once they understand what compounds are, because compounds are at the heart of note-by-note cooking. The way forward will be to take a closer look at the notion of molecular structure. Let’s start by considering some unfamiliar solid object. No, wait, let’s start with water—because no one argues about it (except, of course, gourmets, who are also lovers of wine). Better yet, let’s start with a glass of water. There is something wondrous about even this, for water is liquid, whereas the glass that contains it is solid. Water is transparent, whereas many physical objects are opaque. Water is odorless, whereas many flowers have a smell. Water is—
Hold it right there! We don’t care about water in and of itself, only about using it to illuminate a fundamental fact of the physical world. Let’s confine our attention for the moment to the water that’s in this particular glass and divide it into two parts: each part is likewise water. Divide each part again. We still have some water, less than before in each fraction, of course, but it’s still the same: still colorless, still transparent, still liquid (at least at room temperature in the temperate zones of the world). Now go on dividing again and again and again. So long as we divide fewer than eighty-three times, we will still have some water: less and less each time, until it can no longer be seen without specialized equipment, but water just the same.
Then, suddenly, at the eighty-fourth division, the water is no longer water, but another form of matter. It is at this point that we go beyond the smallest part that can be called water: the molecule. There is no need for the moment to go further, to the level of atoms, the constitutive elements of molecules. For the moment, it is enough to keep in mind that a “pure” substance is made of identical molecules. Water is made of a great many water molecules. Indeed, there are hundreds of thousands of millions of billions of them in a single glass.
The same is true for ordinary sugar, which chemists call “sucrose.” Divide a lump of sugar in two, and each of the halves is still sugar; divide it again and again, and you still have sugar—until the moment when, to the taste, the sensation of sweetness finally disappears, even for an ideal palate. The last stage of division in which the tiniest bit of sweetness can still be recognized, assuming a faculty of perception that is extraordinarily sharper than our own, is that of the molecule. The same goes for salt, for acetic acid, for ethanol, for limonene, for citric acid, for hydroxyproline, and so on.
Salt and sugar are very familiar to us, but what about the other items in this list? Unlike salt and sugar, they are not used in pure form in traditional cooking. Instead they are mixed with other compounds. I have already mentioned acetic acid. It is the principal compound of vinegar, the thing that gives vinegar the main part of its acidity. Once again, the best thing will be to start from what we know. The known in this case is white vinegar (vinegar, that is, whose color has been removed by distillation or filtration). In this liquid, just as in water, there are molecules. Nine out of ten of them are water molecules; the others are mainly (though not exclusively) acetic acid molecules, without which white vinegar would have little to recommend it. Molecules of other compounds are present in such small quantities, in fact, that for present purposes we may as well think of acetic acid as being composed simply of water molecules and acetic acid molecules.
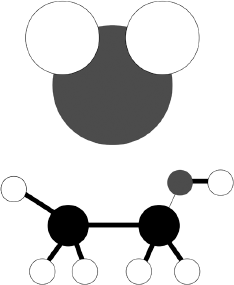
These various molecules display both similarities and differences. Let’s start with the similarities. All molecules (more precisely, all molecules that make up compounds) are formed of atoms. We may think of atoms as little billiard balls of different sizes and colors. Not that atoms really are billiard balls or that they really have colors. I use the image of billiard balls of different sizes to make it easier to picture them and colors to make it easier to tell the different atoms apart. These atoms have names: hydrogen, oxygen, carbon, and so on. There’s nothing very complicated about any of this. There exist in nature only about a hundred distinct atoms, known as “elements.”
A water molecule contains only three atoms, two of hydrogen and one of oxygen. Again, to make things easier to visualize, we may think of molecules as resembling Mickey Mouse heads. No offense is intended to chemistry by this comparison, of course; it is meant only as a way of getting our minds around an unfamiliar idea. (Besides, chemistry couldn’t possibly take offense—it’s a science, not a person!) In an ethanol molecule, unlike a water molecule, a carbon atom is attached to three hydrogen atoms and to one other carbon atom, which in turn is attached to two hydrogen atoms and one oxygen atom, which is itself attached to a hydrogen atom—rather like a small dog. And so on. As I say, there’s nothing very complicated about any of this. It’s simply a matter of attaching billiard balls—atoms of various sorts—to one another in order to make different molecules.
Here it occurs to me once more that words often get in the way. Because thought is inseparable from language, we have a hard time thinking without the right words. In chemistry, there is no way to avoid having to learn a few basic terms: molecule, compound, substance, matter, and so on. Not the least of the reasons why nonchemists are liable to be disconcerted by the word compound is that it designates a category, which is to say an abstract concept. And yet just as we are in the habit of designating every tree that belongs to the category of oaks by the word oak, we can do the same thing with different kinds of molecules—but with this difference: we do not call an acetic acid molecule “acetic acid”; we call it an “acetic acid molecule,” which has the advantage of allowing us to distinguish between a particular object and the class of such objects.
It is true, of course, that few of us have ever seen acetic acid or citric acid or hydroxyproline or indeed any other compound in its pure form. But this is nothing to be concerned about. In their pure form, these compounds are generally powders, crystals, or liquids. They are typically white, but not always. Beta-carotene, for example, one of the compounds that contributes to the color of carrots, is bright orange; pure chlorophyll a, which is present in most green vegetables, is—you guessed it—green. And so on.
FROM MOLECULAR COOKING TO NOTE-BY-NOTE COOKING
We will have a better idea of what note-by-note cooking involves if we place it in its historical context. For this purpose, we need to proceed in much the same way as painters do, adding to our canvas little touches of color that gradually combine to form a coherent image. To begin with, a few definitions that I have come to realize are indispensable, not least in trying to dispel the persistent confusion between cooking and gastronomy:

Cooking is an activity that consists in preparing foods in the form of dishes.

Gastronomy is the intelligent knowledge of whatever concerns man’s nourishment, as Jean-Anthelme Brillat-Savarin put it in
The Physiology of Taste (1825)—a definition that has not been improved upon since. Brillat-Savarin popularized the term
gastronomy, which had been introduced by the poet Joseph Berchoux a few years earlier, at the turn of the nineteenth century.

Molecular gastronomy, as more and more people are beginning to realize, is a branch of physical chemistry that explores the results of culinary transformations. In the extreme case, it has no interest in the activity of cooking itself and looks upon the chemical art embodied by cooking solely as an occasion to discover novel phenomena and mechanisms.

Molecular cooking is a way of cooking that makes use of new utensils, ingredients, and methods. Here the word
new is to be understood as referring to anything not found in the cooking of, say, Paul Bocuse. Molecular cooking was created in the early 1980s, when I had the idea of adapting for use in the kitchen all (or almost all) of the devices and materials found in a chemistry laboratory: rotary evaporators, ultrasound probes, liquid hydrogen, separating funnels, filter pumps, and so on. What began as an eccentric culinary tendency in France has by now become a global phenomenon: not a day goes by without news of yet another chef who has been converted to this style of innovation. It is evidently the result of applying science to technology. Just the same, one must hope that molecular cooking will soon die out—though not, of course, molecular gastronomy! The obsolescence of molecular cooking would be proof that a decisive technological transition has been accomplished—that the activity of cooking has at last been modernized.

Note-by-note cooking was first imagined in the mid-1990s. The following passage occurs near the end of the
Scientific American article I mentioned earlier:
The manufacturers of wines and spirits are typically forbidden by law to improve the taste of their products by adding sugar or other chemicals.
Nevertheless, if the consumer wants to use the results of chemical research to enhance the qualities of inferior wines or spirits, should he or she not be encourged to do so? A few drops of vanilla extract may wonderfully enrich the flavor of a bottle of cheap whiskey.
This kind of experiment can be extended to a large number of beverages and dishes. Perhaps in the cookbooks of the future, recipes will include such directions as “add to your bouillon two drops of a 0.001 percent solution of benzylmercaptan in pure alcohol.”
The idea wasn’t crazy in the least. Cooks have always modified the flavor of their dishes by adding herbs and spices, which are nature’s way of packaging mixtures of aromatic molecules. At all events, as I say, Nicholas Kurti approved this passage, although I myself considered it to be a piece of extravagant audacity. What? Chemicals in foods? Today I would state the matter differently—mainly, of course, because the term chemicals (like chemical compounds) is mistaken: a compound is a compound, whether it is extracted from a natural or a synthesized product; it becomes a chemical only if it is used or studied by a chemist. The water we drink, for example, is a chemical compound only insofar as it is the object of chemical analysis.
I also suggested that cooks might find it entertaining to experiment with compounds. But entertainment is not really the point, or at least not the only one. A few drops of vanillin (whether synthetic or natural hardly matters because the molecules are the same and have the same effects on sensory receptors) do, in fact, give roundness to unaged alcohols. The reasons it does so are, to the best of my knowledge, unknown. The phenomenon has something to do with the fact that during the barrel aging of brandies the lignin in the wood reacts with the ethanol and over time produces a number of compounds, including syringaldehyde as well as sinapic and vanillic aldehydes. For a lover of brandy who is not rich enough to buy expensive brands that have spent many long years in cask, the appeal of adding vanillin should be obvious.
Finally, I now believe that even if adding benzyl mercaptan to bouillon is a good choice from the culinary point of view (in solutions of low concentration, benzyl mercaptan has notes of onion, garlic, horseradish, mint, and coffee), there is a risk that my fellow chemists, who think of mercaptans as volatile sulfur compounds, will balk. In 1994, however, it seemed to me that the idea of adding compounds to foods did not go nearly far enough. Why not
make foods out of compounds? With this question, note-by-note cooking was born.
Experience nonetheless has proved that people have a hard time imagining what note-by-note dishes might look like. In the pages that follow, I try to show how they can be constructed. Because cooking is an art, however, let me first suggest a comparison with two other arts, music and painting, in the hope that even the most reluctant readers will find the idea of note-by-note cooking less off-putting.
In the past, music was made with instruments—string instruments, wind instruments, and so on. The trumpet, the violin, the piano, and other instruments were used alone or in combination with one another when greater variety was desired. Each instrument had its own timbre. Each century added its own style of composition, its own ways of arranging notes for different instruments, singly or in unison.
With the advent of electronics about a hundred years ago, engineers who saw that the new technology could be used to make music joined forces with musicians who saw in it a new and less cumbersome way to compose and perform music. They were visionaries, not opportunists. Their response expressed a perfectly human desire. As children playing in the park, we feel the urge to go outside it and explore the world beyond. This urge doesn’t leave us once we have grown up.
In the 1940s, musicians such as Pierre Schaeffer, Karlheinz Stockhausen, and Edgar Varèse began to mix sounds produced by electronic devices with sounds produced by classical instruments. Electronic music has made enormous strides since then—to the point that today it is everywhere. Using synthesizers to assemble elementary sound waves, it is now possible to produce any sound whatever, whether one seeks to recreate the sounds of classical instruments (but why bother, since these instruments already exist?) or to invent novel sounds.
When we perceive a sound, we perceive an attack, a timbre, a pitch, an intensity. In cooking, when we perceive a flavor, we perceive a consistency, a taste, an odor, a level of heat. The comparison is obvious. And the culinary equivalent of a sound wave is a compound. In cooking, as in music, the arrangement of well-chosen sensory units allows us unlimited freedom: we can produce any sound, any music or any flavor, any food. And, in either case, everything has to be constructed. One might imagine that cooking from molecular scratch, as it were, on the basis of compounds, is somehow more difficult than traditional cooking; but the same reservation in the case of music (it used to be said that instruments without a fixed, predetermined timbre would be very difficult to play) was proved to be unfounded by the imagination of modern composers, acousticians, sound engineers, and musicians themselves.
The construction of a dish from compounds, note by note, is bound to be a long and involved business. But there is no reason why we should not look for shortcuts. The very same thing, after all, is true of perfumes—which themselves are nothing more (or less) than novel combinations of compounds, and not just ones that have been extracted from plants. The same is true as well for the various compositions and extracts (essential oils, resinoids, supercritical carbon dioxide extracts, and so on) that the food industry uses to make fragrances and other synthetic products (the meaning of the word aroma, as I say, has been abusively enlarged to include this class of artificial compounds).
Next, a visual comparison. We know that when yellow and blue are mixed together the result is green and that by mixing blue with red we get purple. By contrast, mixing purple and brown together will never yield yellow, blue, and red. These three primary, or basic, colors can be combined in various ways to obtain any color, but no mixing of such combinations will give us back their primary colors. The analogy with note-by-note cooking? Meats, fish, fruits, and vegetables are mixtures of a very great many compounds, and mixing them together creates complex flavors. But it will never be possible to obtain a “pure” flavor by combining mixtures. Combine beef and carrots any way you like, you will never succeed in producing the flavor of lemon.
Even people who understand that chemistry will never be a part of cooking are apt to find the idea of note-by-note cooking unsettling. Does it have any nutritional value, they ask? Can we be sure it isn’t harmful? What implications does it have for agricultural production? For traditional cooking? For our traditional notions of conviviality?
Their greatest fear is that traditional cooking—cassoulet, pot-au-feu, choucroute, and so on—will disappear. The virtues of these dishes are often praised with much insincerity, considering how little the dishes themselves are really liked! Chocolate, however, is very much liked. But why should we insist that it contain at least a few milligrams of potassium (have you ever seen a milligram, by the way?) when it consists overwhelmingly of fats and sugar (a particular kind of sugar, sucrose)—compounds that no one recommends for their nutritional value? Why should we praise meats grilled over an open fire when they are packed with two thousand times the amount of benzopyrene (a notoriously carcinogenic organic compound) allowed by European law in industrially processed smoked-meat products?
The neophobia that has protected primates through the ages leads us to place our trust in the foods we eat when we are young and to fear new foods. From an evolutionary perspective, neophobia is a salutary reflex because it prevents us from indiscriminately consuming foods that may be harmful, even toxic. Even so, it is very much an animal behavior that we should want to go beyond, or at least to critically examine, so that we cease to be in thrall to it—and all the more since human beings have progressively become omnivores, which means that we no longer run the risk of starving to death when there is a shortage of the food that our nonhuman primate cousins consume almost exclusively: fruits.
How do we go about transcending our animal nature? By symbolizing, ritualizing the act of eating; by rationalizing, finding reasons for eating one way rather than another. Over time we have come to think differently about how we eat. But it is a gradual process. We no longer reject new foods outright, as our primate ancestors instinctively did; still, we disparage new foods and champion familiar foods, even when their virtues are far from being proven. The least persuasive of the justifications given for this is that because familiar foods are ancient, their dangers, if any, would surely have become apparent by now.
Arguments made in bad faith are obviously bad, for exactly this reason. Smoked products, for example, have been legitimized by long-standing custom. Although it is true that smoking was useful over the course of human history as a way of preserving perishable food stuffs (fish and meat, especially), the large number of cancers of the digestive tract observed in the populations of northern Europe, heavy consumers of smoked products, furnish indisputable epidemiological evidence of the dangers they pose to health. The same thing may be said of salting, which increases the risk of hypertension and stroke, and indeed of every method for preserving foods that yields alarmingly high levels of fats and sugars. The truth of the matter is that no wholly safe method exists and never will exist. Sensible nutritionists recognize this. Their advice recalls Paracelsus’s wise dictum of almost six hundred years ago: “Nothing is poison. Everything is poison. The difference is in the dose.” Long before Paracelsus, of course, the Greeks had stigmatized excess, advocating moderation in all things.
Insincerity founded on food neophobia is therefore not a satisfactory reason for refusing to take note-by-note cooking seriously. But why should we give up traditional cooking and adopt note-by-note cooking in its stead? Or, if this is a false choice, why shouldn’t we follow the example of molecular cooking and practice note-by-note cooking alongside traditional cooking? Or combine the two with a view to inventing hybrid dishes? It is the responsibility of proponents of note-by-note cooking to persuade doubters, not the other way around. Let me therefore proceed to consider the most serious objections—without fear of answering them as frankly as possible, for it is my intention neither to sell products nor to propagate an ideology nor to wield power. Quite to the contrary, my purpose is to advance the cause of enlightened connoisseurship. I begin by briefly introducing six sets of questions that will be treated in fuller detail later on, involving technology, nutrition, toxicology, art, economics, and politics.
A first group of questions concerns the nature of the compounds to be used in note-by-note cooking. Food manufacturers already use very pure compounds, such as water, sodium chloride (salt, in its purest form), sucrose (table sugar), gelatin, and so on. Many people are unaware, perhaps willfully, that these ingredients have long been used in a great many products, whether they are extracted, purified, or modified in various ways. Think of flour and other powdered products, to which anticaking agents are added to prevent them from forming lumps.
A broad range of other compounds might also be used, from saccharides to amino acids, and all the more easily as the food industry already manufactures them. Makers of food additives, for example, market coloring agents, vitamins, and preservatives in addition to polysaccharide gels and other thickening agents. It is true that such additives are not regulated in the same way as food products themselves. But that may change in the not so distant future.
Then there is the question of how pure compounds should be in order to be approved for commercial use. If the standard required by law is not unreasonably stringent, cooks, like the musicians who pioneered the use of synthesizers, will be able to enlarge the palette of ingredients to include simple mix-tures—something the food industry already makes, particularly through the milling of wheat and the processing of milk: milling wheat gives us the husk, starch, gluten, and so on; processing milk gives us fats, powders, and various proteinacious preparations. Gelatin, for example, is not pure, in the technical sense that it does not consist of a single sort of molecule, for the extraction of collagen from animal tissues produces a massive dispersion of polypeptide chains. A sheet of gelatin, in other words, contains more or less large molecules of various kinds. Similarly, the starchy matter of vegetables (also known as native starch) is not pure because it is made of two major compounds, amylose (one really ought to say “amyloses” since here again there is no perfect molecular homogeneity) and amylopectin (or amylopectins, for the same reason). Keep in mind, by the way, that since glutinous rice starch is made exclusively of amylopectins, the modified (or processed) starch that is obtained from it is among the products that can be used in note-by-note cooking right away, without any further legal determination regarding admissible degrees of purity. Pastry chefs have been doing something very similar to note-by-note cooking for quite a while now.
Let us return for a moment to fractionation, the chemical term for the procedure of separating a mixture into its component parts, which I mentioned a moment ago in connection with vegetable and animal products. From wheat, a variety of ingredients are routinely recovered, including polysaccharides, proteins and amino acids, tensioactive agents; from milk, we get amino acids, peptides, proteins, glycerides, and so on. Why shouldn’t the same thing be done using other such products? In principle, laboratory separation processes (filtration, direct or reverse osmosis, cryoconcentration, vacuum distillation, and the like) could be employed to obtain reasonably pure fractions for use in note-by-note cooking.
Government-sponsored research teams in France are hard at work investigating this idea. In Montpellier, for example, experiments are being conducted with specially modified membrane filters to recover water, sugar, acids, and what is known as the “total phenolics fraction” (a brightly colored powder with a powerful flavor) from the juice of grapes. The really interesting thing is that the character of this fraction is strikingly different depending on whether it is extracted from the juice of Syrah or Pinot grapes, for example. The distinctive nature of the initial ingredients, in other words, is not flattened out by the separation process any more than the specific qualities of a piece of meat are masked or diminished by cooking. This means that note-by-note cooking can preserve traces of terroir!
Now that we have some idea of the sort of ingredients that can be used, the question arises of how they are to be combined. We mustn’t lose sight of two things: first, that most of the foods we eat today are made up mostly of water, and, second, that the solubility of many compounds is reduced in an aqueous environment. This is why emulsification—the dispersion of oil droplets in water—is the primary operation in creating note-by-note dishes. But it is not the only one. Indeed, all techniques of dispersion will have their use.
The various biological properties of foods will need to be taken into account when we look to combine compounds. To be sure, nutritional content is important. But it would be a mistake to forget that if we are to find foods agreeable to eat, they must stimulate various kinds of sensory receptor: visual, olfactory, sapictive (the term I suggest we use in speaking of taste), trigeminal (referring to fresh, spicy, and tingling sensations), tactile, and thermal. This raises a whole host of questions to which we do not yet have any answers. Even if it were possible to determine the light-absorption spectrum of a mixture of pigments whose individual spectra are known, for example, we would not be able to predict the exact color of the mixture. Similarly, mixing odorant compounds in proportions close to their perception thresholds will produce unpredictable results. Not even the result of combining only two odorant compounds is known in advance. Will the mixture have a flavor in which the presence of each of the two compounds can be detected or a new flavor, distinct from that of either compound? No one can say.
Matters are still more uncertain with respect to flavor because the biological receptors of sapid compounds in the mouth remain largely unknown. Receptors of long-chain saturated fatty acids in the papillae were identified fewer than twenty years ago. Their discovery has stimulated a great deal of promising research, much of it yet incomplete. In the meantime, however, there should be no hesitation in using citric, tartaric, malic, acetic, and other acids or saccharides such as glucose and fructose in addition to our old standby, sucrose. With regard to trigeminal effects, a number of refreshing or pungent compounds are known, such as eugenol (noted also for its contribution to the fragrance of cloves), menthol (which has two forms, though only one is perceived to be refreshing, as we shall see later in
chapter 3 in connection with flavor), piperine (largely responsible for the pungency of black pepper), capsaicin (a principal source of heat in hot peppers and a variety of spices), ethanol, and sodium bicarbonate.
The consistency of foods is another thriving field of research. Colloidal “soft” materials are poorly understood. Creating multiple emulsions, at first sight a rather straightforward proposition, has turned out to be much more difficult than anyone expected. More generally, anyone who imagines that all the challenges presented by texturing processed foods have been met with the development of surimi and related artificial products is sadly mistaken. Will it be possible one day to fabricate a consistency similar to that of an apple or a pear? So long as it is unclear what making even a prototype artificial fruit would entail, the prospect of large-scale commercial production remains very far off.
In short, there is much to be done. Chefs and food scientists must nevertheless be made to see, as I have already emphasized, that there is little or no reason to reproduce food ingredients that already exist. Just as a synthesizer can copy the sounds of a piano or a violin, note-by-note cooking could copy the flavors and other properties of wines and vegetables and meats—but to what purpose, apart from particular applications, such as dishes famously associated with astronauts working in outer space for long periods of time? We would do better, much better, I believe, to explore that vast continent of dishes that have never yet been created.
A simple calculation will show that the phrase “vast continent,” far from being an overstatement, is much more nearly the opposite. Suppose that the number of classical food ingredients is on the order of a thousand and that a typical traditional recipe uses about ten of them. The number of possible combinations is therefore one thousand raised to the power of ten, or 1030. By contrast, if we suppose that the number of different compounds present in foods is likewise on the order of a thousand, but that the number of compounds that will be used in a typical note-by-note dish is on the order of a thousand, rather than ten, then the number of possibilities is roughly 103000—without taking into account the fact that in note-by-note cooking the concentration of each compound may vary, which means that the new continent is, for all practical purposes, infinitely more immense than the old one. What would be the point, then, of trying to replicate the almost insignificantly small world we already know?
NUTRITION
I need hardly point out that traditional foods are no guarantee of good health. The proof is the pandemic of obesity we presently see in much of the world. No doubt the unbalanced quality of modern diets has not helped matters. But it is probably truer to say that the sheer abundance of food today, unprecedented in the history of our species, has put us in a position for which we were not prepared by biological evolution.
Over the course of many thousands of years humanity has had to confront alternating periods of abundance and scarcity. Nutrigenetics, a branch of nutritional genomics, continues to make progress in identifying the physiological mechanisms that have assured the survival and propagation of the human race. In the past, excessive food intake enabled the body to stockpile fats in anticipation of future shortages, while restricted intake and the gradual loss of appetite it brought about made it easier to adapt to periods of famine as well. The nutritional implications of note-by-note cooking need to be considered in the context of the food industry’s use of sweeteners for several decades now and a growing consumer preference for “lighter” foods and beverages (lighter, that is, owing to the addition of air and water), which raise the question of whether low-calorie products cause compensatory overeating. Research now being carried out in this connection will be a fruitful point of departure for the study of the long-term benefits of note-by-note cooking by comparison with the manifest inadequacies of the present regime.
There is also the question of the effectiveness of dietary supplements—vitamins, oligoelements, and so forth—and, indeed, of nutriments in general. Scientists have diligently investigated the various claims made on behalf of such products. Once again, however, it would be naive to believe that all outstanding issues have been resolved. A planned European study of vitamin E supplements (a term that designates a group of hydrophobic compounds having particular antioxidant properties), for example, was recently cancelled because of the abnormally high number of fatalities due to lung cancer and coronary disease among subjects in the supplemented group (many of them, of course, smokers). So far there is no evidence that vitamin E supplements lower rates of lung cancer and coronary disease, but in the absence of further research no firmer conclusion can be reached.
TOXICOLOGY
It is quite true that the harmful effects of some compounds on the human organism are not yet well understood. Extraordinary discoveries continue to be made with encouraging regularity, however, such as the polymorphism of P450 cytochrome enzymes (it turns out that we are equipped with much more elaborate detoxification systems than was previously thought) and, quite recently, the transfer of bacterial genes that colonize seaweed to the intestinal bacteria of persons who eat it.
Yet uncertainty surrounding the long-term epidemiological consequences of note-by-note cooking does not put it in a fundamentally different position than traditional cooking, which uses vegetable and animal ingredients whose innocuousness has never been satisfactorily established. It is one of the paradoxes of modern diet that new foods are subject to far more severe scrutiny than older foods, the sale of many of which would be prohibited if they did not enjoy the advantage of having long been familiar. But note-by-note cooking will be able to avoid the carcinogenic danger of benzopyrenes in smoked products, for example, simply by not using such compounds. In the same way it will be able to avoid the toxic risks associated with myristicin (found in nutmeg), estragole (in tarragon and basil), glycoalkaloids (in potatoes and tomatoes), certain glycosinolates (in cauliflower), and certain phenolic compounds (in various vegetable tissues).
Restrictions on the sale of compounds are likely to resemble the rules that presently govern the sale of liquid nitrogen and ultrasound probes for culinary purposes as well as of the heating elements used to ensure the even distribution of heat in temperature-controlled water baths required by sous-vide cooking. The ongoing refinement of cooking techniques will make new forms of regulation inescapable, just as the introduction of gas and of electricity in homes and businesses a century ago made it necessary to take special safety precautions.
Accidents are no doubt to be expected—not because note-by-note cooking is more dangerous than using a kitchen knife, but because the culinary world is no less likely than any other to have its share of negligent or reckless behavior. In July 2009, to mention only the most recent sensational example, a young German cook blew off one of his hands and suffered serious injuries to his lower abdomen and legs when liquid nitrogen he had kept hermetically sealed in a thermos bottle exploded.
Art is obviously a complicated subject. For the moment, I shall say simply that culinary art, like painting, music, sculpture, and the other arts, seeks to arouse our emotions. The culinary artist’s fondest hope, after all, is that his guests will look at one another and exclaim, “Oh, that’s good!”
Just as artists in other fields continually introduce novel elements of various kinds in their works, so too chefs constantly seek to create original sensations. In this respect, at least, note-by-note cooking cannot help but meet with their approval, and that of their customers, because the new possibilities it offers are virtually unlimited.
Producing the first works of note-by-note cuisine nevertheless proved to be difficult. Cooks who accepted the challenge had to learn the chemical alphabet of compounds before they were able to form meaningful gustatory words. Note that I use the past tense here because note-by-note cooking is already a reality. In 2006 I prevailed upon my friend Pierre Gagnaire (who has restaurants in Paris, London, Tokyo, Dubai, Hong Kong, Moscow, Courchevel, Berlin, Las Vegas, and Seoul) to become the first chef in history to produce an entirely note-by-note dish. After several months of work, during which I offered advice, assistance, and encouragement, he presented the result as part of a special dinner in Hong Kong on April 24, 2009. This dish was called “Note-by-Note No. 1” (see the color illustrations).
Then, in the summer of 2010, the Alsatian chefs Hubert Maetz and Aline Kuentz created a note-by-note dish of their own on the occasion of a Franco-Japanese scientific conference in Strasbourg. More recently chef-instructors at Le Cordon Bleu, the culinary arts school in Paris, prepared a full menu of note-by-note dishes for a limited number of guests. And on January 26, 2011, to mark the advent of the International Year of Chemistry, sponsored by UNESCO, Jean-Pierre Biffi and his team at the Paris catering company Potel & Chabot prepared a note-by-note meal for almost a hundred persons. Not a day goes by without some new advance being announced. The reason for this could not be more plain: the true culinary artists of our day are fascinated by a new method of cooking that permits them an unprecedented freedom of expression.
A word for those who fear that this new method spells the end for their beloved pot-au-feu, cassoulet, and choucroute: in the domain of art, there is no such thing as replacement, only addition. This has the consequence that the range of choice is perpetually being enlarged. Debussy did not cause Mozart or Bach to disappear, any more than Picasso and Buffet prevented us from continuing to admire Rembrandt and Brueghel. Similarly, molecular cooking has not done away with nouvelle cuisine or with the style of mixing culinary influences from various cultures known as fusion or with traditional cooking in either its classical or regional forms. Note-by-note cooking will not be any different in this regard.
ECONOMICS
Will note-by-note cooking be more expensive than current methods of cooking? Not only must the cost of investment in new equipment be taken into account, but also the increasing cost of energy. The likelihood that the price of conventional fuels will continue to rise may eventually be decisive in assuring the success of note-by-note cooking. Wasting up to 80 percent of the energy used to heat pots and pans on kitchen stoves is considered acceptable today. It will not be considered acceptable tomorrow, when fossil fuel reserves will be nearly exhausted and energy will have become prohibitively expensive.
What advantage does note-by-note cooking present from this point of view? Consider, for example, the reduction of wine in preparing a sauce. This is mainly a matter of evaporating water. Assuming a reduction of the sort performed by professional cooks (roughly by two-thirds), the energy consumption looks to be on the order of 0.417 kilowatt hours, and the cost about ¤0.05 (not quite seven cents on a U.S. dollar) per individual serving. That may not seem like much—but wait a while longer. As the price of natural gas moves ever more sharply upward, energy costs will soon become a rather greater source of concern.
The promise of note-by-note cooking can hardly be understated. Dissolve some phenolic compounds extracted from grape juice or wine, together with a little tartaric acid, a little glucose, and a little salt, then heat the mixture for a few seconds until it is warm enough to serve, and you will have consumed virtually no energy (in addition to saving a great deal of time). Obviously some energy will be needed to prepare the ingredients, but if you use the kind of filtration processes that bottled-water companies have been using for several decades now, energy consumption will be much lower than in traditional cooking. What’s more, restaurants and caterers will be able to exploit economies of scale: the larger the quantity of food being prepared, the greater the savings. As more and more chefs adopt the techniques of note-by-note cooking, the cost of producing meals in this way will begin to fall and profits will rise, slowly at first, but then at a gathering pace.
The making of sauces is only one example, selected at random from among a thousand or more such examples. Energy consumption has never much mattered in traditional cooking. Still today, no chef thinks twice about cooking meats at temperatures of 200°C (almost 400°F) or more in order to produce compounds that could be obtained much more quickly using the techniques of note-by-note cooking, even in the case of large-scale production, and at a far lower cost per serving. In an oven, it costs just as much to roast one chicken as ten!
Note, too, that not all the compounds used in note-by-note cooking will have to be synthesized. Indeed, it will very often be preferable to extract them from vegetable products. Chemists well remember the years of hard work it took to synthesize vitamin B12. In the absence of a proven experimental method, then, we should look to the farm rather than to the laboratory because obtaining compounds from plants will be both less expensive and more expeditious.
POLITICS
From its inception, note-by-note cooking could not help but stimulate fears that it would force people to eat “chemistry.” It revived the dread of an earlier age, when science fiction seemed to herald a world of nutrient pills, soylent steaks, and foods made from petroleum and coal. Here again, as in the case of genetically modified organisms (GMOs), reasoned political debate is contaminated and confused by ideology. The value of note-by-note cooking will become apparent only if uninformed prejudice is combated and persuasive reasons are given for accepting new foods. One of the first to appreciate the importance of the role played by elite tastes in this regard was Antoine-Augustin Parmentier (1737–1813), who persuaded the king of France to eat potatoes, thus setting an example for the people of his kingdom.
Quite apart from the difficulties involved in introducing note-by-note cooking to the widest possible audience, however, ought we not to be worried, as in the case of GMOs, that it will have adverse consequences for traditional forms of social and political organization? What will become of farmers, for example, in the event, however improbable, that all cooking will one day be note-by-note cooking? No one can say with certainty, of course. But just as the owners of vineyards now make more money producing wine rather than grapes, so farmers of all kinds (and not merely large agribusinesses) may be expected to do very well by producing fractions from vegetable and animal products, which today cannot always be sold at prices high enough to be profitable. Note-by-note cooking stands to make farmers better off by encouraging them to manufacture higher-value fractions at the farm for culinary use. Instead of selling fruits and vegetables that are liable to spoil on the way to market, they could sell the extractable parts of these fruits and vegetables. Note, too, that this is where politics links up with the energy question. Transporting fresh fruits and vegetables is mainly a matter of transporting water. Wouldn’t it make more sense to eliminate the water at the point of origin and ship lighter products at lower cost?
There remains, finally, the challenge that note-by-note cooking poses for science itself, which has often developed in response to advances in what used to be called the chemical arts. Yet another such occasion presents itself today.
LEARNING TO COOK NOTE BY NOTE
Note-by-note cooking is something that must be learned. In one sense the present situation is no different than the one thirty years ago, when cooks had to be trained in a new style of cooking. But learning note-by-note cooking will be more difficult than molecular cooking because this time a whole new way of thinking about flavor has to be mastered before dishes can be made. With molecular cooking, the tools were new but the ingredients weren’t. Cooks were still working with veal, crab, and leeks. They knew what these things tasted like, and they prepared them in more or less familiar ways. With note-by-note cooking, the ingredients themselves are new. Many of them will be unknown to begin with, and cooks will have to learn to combine compounds having unfamiliar properties whose effect on the sensory faculties is apt to be quite different than what we are used to. Thus, for example, beta-carotene, a marvelous powder that even in very small amounts imparts a bright orange to certain substances, has neither flavor nor smell, only color. Salt (sodium chloride) has flavor, but neither smell nor color. Many odorant molecules have no flavor, either because they are used in very weak concentrations or because they are not soluble in water, which constitutes the main part of saliva.
Even so, note-by-note cooking will not seem terribly difficult once cooks have become acquainted with the new ingredients and new methods it involves. When you learn a new language, you need to learn words and rules for combining them to form sentences. In the pages that follow, we shall see how to do the same thing in the kitchen—and have fun doing it.
There’s no need to be gloomy in order to be serious.
Kurti, Nicholas, and Hervé This. “Chemistry and Physics in the Kitchen.” Scientific American 270, no. 4 (1994): 44–50.
This, Hervé. Building a Meal: From Molecular Gastronomy to Culinary Constructivism. Translated by M. B. DeBevoise. New York: Columbia University Press, 2009.
——. “Molecular Gastronomy: A Chemical Look to Cooking.” Accounts of Chemical Research 42, no. 5 (2009): 575–583.
——. Molecular Gastronomy: Exploring the Science of Flavor. Translated by M. B. DeBevoise. New York: Columbia University Press, 2007.
——. “De quelles connaissances manquons-nous pour la cuisine note à note?” L’Actualité chimique 350 (March 2011): 5–9.
This, Hervé, and Pierre Gagnaire. Cooking: The Quintessential Art. Translated by M. B. DeBevoise. Berkeley: University of California Press, 2008.






