5 The Parasite Within
Malaria was a mystery. For millenia, the disease appeared to strike without rationale or logic. It affected people in different ways at different times. It often appeared as if by divine intent, then sometimes disappeared from cities, even entire countries. It was as mysterious as the fogs and vapours of the marshes it haunted and its only explanation was supernatural. Some tried supernatural means to prevent it.
In November 1709, a Norfolk vicar – ‘Mr Forbes, a Scotchman’ – died at his living in Rougham, near Bury St Edmunds, and the woman who laid out the body found a small silk bag, tied by a ribbon around his neck. In it was a scrap of paper ‘discolour’d very yellow with sweat. I transcrib’d it in the same charectar &c words as they stood there.’ The unknown writer who copied the peculiar words from the paper onto a manuscript now in the British Museum thought it might be a sign of some coded political conspiracy. It began: ‘Eywm uydlab ase byw udgaa eywdgmw yw esa lbib . . .’ and continued for another 41 incomprehensible words. This odd language had to wait until 1932 for antiquarian Warren R. Dawson to finally translate it.1 It was a simple cipher in which the letters are substituted, so E=W, Y=H, W=E, M=N, and so on. Dawson had discovered a charm against ague:
When Christ saw the cross wherone he was to be crusified the Jews asked him ‘art thou hafraid or hast thou an ague?’ Jesus said ‘I am not afraid nor have not an ague’. Whoesoever w[e]ars these words shall never be troubled with an ague. Amen. Amen. Sweet Jesus.

In a cartoon by Thomas Rowlandson, 1788, fever, represented as a frenzied beast, stands centre stage, while the blue monster of ague ensnares its victim by the fireside. The doctor, right, writes out a prescription.
Today, we know that malaria/ague is caused by a microscopic protozoan blood parasite spread by mosquitoes. The Plasmodium organisms that cause the disease are many orders of magnitude bigger and more complex than simple bacteria and viruses, and their transmission is as convoluted as the disease’s aetiology is mystifying. This complexity masked the precise cause of malaria throughout history, but it also left clues in the symptoms recorded by contemporary observers, in descriptions of historical events detailed by chroniclers, and in the biological material still being examined by archaeologists today.
Written records from ancient Egypt are scant, but recent DNA evidence has suggested that, around 1323 BCE, Tutankhamun died as a result of a broken leg, exacerbated by congenital disorders and severe malaria infection.2 In the canon of Chinese medicine, diseases translated as ‘malaria’ are first mentioned about 800 BCE, and are seasonally linked to the cold, wet airs of autumn. At some point, it jumped to Japan, perhaps linked with the introduction of rice paddy fields around 400 BCE. Likewise, Indian medical scholars identify malaria-like diseases around 1200 BCE. The Hindu text Atharaveda comments that fevers are common after excessive rains. It also appears to distinguish between fevers that recur on the first and third (or fourth) day – typical malaria symptoms.3
Ancient Greece seems to have been virtually free of the disease until about 500 BCE.4 Invading Persians were blamed for bringing it, and in fact three fevers are described accurately enough to be identifiable as three different malarias today. At a remarkably similar time, the Roman Republic, which had been seemingly malaria-free in its early years, began to report more cases. The epidemic drove peasants from the low-lying countryside into the cities (which had at least been drained), and this disruption to the Roman economy was noted.5 The ill winds of the marshlands were well known. Vitruvius, writing his De Architectura in the first century BCE, advises against building farms or settlements near marshes because of the diseases, suggesting that the morning mist becomes infected with the poisonous breath of noxious creatures, especially near stagnant water.6 Varro, writing at about the same time, links marshes to organisms that are too small to be seen by the naked eye, and which are carried on the air.7
For over 11,000 years, the history of the Americas is oral and therefore vague, but both north and south continents were malaria-free when European adventurers first made contact with the native populations.8 Other diseases like smallpox and measles were the first epidemics to ravage here. But, by the middle of the seventeenth century, malaria was well established throughout the New World, and particularly troublesome across the Caribbean.
An incident of striking exactitude happened in Mauritius. This island was uninhabited until the seventeenth century, when it was settled by the Dutch, followed by the French, then the British. It was also populated by immigrations from India, Africa and China. For over 200 years of colonization, malaria was known only from a few isolated cases, always in newly arrived immigrants who had carried the disease with them, mostly from southern India. But it never spread through the general population . . . until 1866, when a huge epidemic erupted, killing 3,700 people.9 Malaria has been present (albeit at low levels) ever since. First, though, as with any disease, it is important to give it a name – a label.
The word ‘malaria’ first entered the English language around 1740 in letters written from Italy by Horace Walpole.10 However, the physician and naturalist John MacCulloch is credited with bringing the term into widespread use, with his essay written in 1827.11 The disease was thought to be caused by ethers, like smokes or plumes, wafting through the air. An excellent description is given in Lloyd’s Encyclopaedic Dictionary (1895):
ma-lär’-i-a, [Ital. mal’ aria, for mala aria = bad air: mala (Lat. malus) = bad, and aria = air.] A morbid poison of unknown character generated in paludal or littoral districts, affecting the system through the blood often as long as twelve months after one has been exposed to it, and exerting its deadly influence in many cases through life. Hydrophobia is the only other form of disease in which the period of incubation may be as long or longer. Malaria emanates from marshy land in a decomposed state under the influence of heat above 60ºF acting on the moisture; when thoroughly drained, flooded or frozen, malaria is not generated. An elevation from 1,000 to 1,200 feet is, generally speaking, a protection against it. Malaria causes ague, intermittent and congestive fevers, and one kind of yellow fever, marked by periodicity. The Roman Campagna and the West Coast of Africa are noted haunts of malaria and malarious fevers; and rice-fields are also well-known sources of it.
 |
Malaria was often portrayed as the malingerer’s disease. |
THE AGUE
In Britain, the endemic malaria was known by that other name – ague. Characterized by recurring fevers (every two or three days), shivers and aches, it was frequently attached to marshes. Written in the late 1300s, Chaucer’s ‘The Nun’s Priest’s Tale’ from his Canterbury Tales talks of ‘some ague that may be your bane’.12 Shakespeare mentions ague in eight of his plays. Sometimes this is by allusion; in The Tempest (1608–13), Caliban describes the ‘unwholesome fen’ and curses: ‘All the infections that the sun sucks up / From bogs, fens, flats, on Prosper fall’. Later, Stephano mistakes Caliban’s trembling for an attack of malaria: ‘This is some monster . . . who hath got, as I take it, an ague . . . He’s in his fit now . . .’. Stephano offers alcohol, a popular cure for ague: ‘open your mouth . . . this will shake your shaking . . . the wine in my bottle will recover him, I will help his ague . . .’. A similar play on popular knowledge is at work in Twelfth Night (c. 1601), where Sir Andrew Aguecheek (also called Agueface behind his back) is a friend of the equally daftly named Sir Toby Belch. Both are drunkards, and Aguecheek in particular is a comic fool, slow-witted and slow in speech. Ague, as well as meaning the malaria of our modern understanding, was also often a very broad term for general fever and illness, shakes and shivering – a kind of Tudor or Elizabethan man-flu. Since it was self-diagnosed and self-treated, it made the ideal affliction for anyone wanting to partake of the medicinal qualities of the bottle.
| Oddly, Dr J. C. Ayer claims his ague cure contains no quinine, emphasizing its vegetable origins; the frogs and crocodiles are not impressed. The tonic probably had a high alcohol content. |  |

| A cartoon from an 1881 issue of Harper’s Weekly poked fun at a Washington health officer called Townsend after he facetiously described malaria as a ‘fashionable disease’; doctors diagnosed it when faced with anything they did not understand. |  |
Daniel Defoe, in his Tour through the Eastern Counties of England (1722), paints a sometimes grim picture of life, where many people have an ‘Essex ague on their backs’. He recounts how the men ‘seasoned to the place’ always went to the uplands for a wife. They ‘took the young lasses out of the wholesome and fresh air’, but when they brought them ‘into the marshes among the fogs and damps, there they presently changed their complexion, got an ague or two, and seldom held it above half a year, or a year at most’. He noted, in particular, that these men got through a remarkable number of wives: ‘5 or 6 to 15 or 16’ was very frequent. One such farmer was on his 25th wife.13
This resignation to the pattern of disease and death is both stoical and grotesque, but shows a local acceptance of ague. Folk names echo this degree of intimacy – the Bailiff of the Marshes, Lord John’s Fever and Old Johnny Axey.14 On the whole, illness from ague may have been chronic and incapacitating, but deaths were generally rare. Nevertheless, Sir Joseph Fayrer, writing in the Transactions of the Epidemiological Society of London in 1881–2, tells us that we have lost two kings, a queen, a cardinal and a lord protector to malaria. Horatio Nelson reputedly suffered from ague as a boy growing up in Norfolk in the 1750s and ’60s, and his strength was reduced by it.
Exact disease records are sparse. There were ague epidemics in Cambridgeshire and Lincolnshire in 1826–9 and 1857–60.15 In 1864 a Privy Council report looked at infant mortality rates across the country and found them higher than average in fenland towns. How much of this is due to ague is not clear; the report rather unsympathetically blamed much of the high mortality rate on a large number of premature births (leading to premature death), high incidence of infant neglect and inbreeding.16
Everywhere, though, the ague was linked to the water. In Great Expectations (1861) Charles Dickens begins with Pip in the local graveyard, when he meets Magwitch, drenched and shivering, escaped from the convict hulks across the mud. ‘I think you have got the ague . . . It’s bad about here’, says Pip, standing in front of his parents’ tombstone, and those of his five infant brothers. The evocation of the malarial mud is laid on thick: ‘Ours was the marsh country . . . this bleak place . . . the dark flat wilderness beyond . . . the low leaden line beyond was the river . . . the distant savage lair from which the wind was rushing was the sea.’ Masterful.
In 1864 a special report on the ‘quantity of ague and other malarious diseases now prevailing in the principal marsh districts of England’ was commissioned by and presented to the Privy Council, but the mechanisms of infection were still tantalizingly clouded. Ague sufferers were sometimes thought to get the disease by drinking ditch water. One correspondent to the leading medical journal, The Lancet, suggested changing the name malaria to mal’aqua.

The listless effects of malaria and the misty miasma-shrouded Pontine Marshes near Rome made Antoine Auguste Ernest Hébert’s name when he painted La Malaria around 1850.
When Jim Hawkins, Long John Silver and the crew of the Hispaniola land on Robert Louis Stevenson’s Treasure Island (1883), there is no mention of mosquitoes, but near the end of the tale Doctor Livesey is concerned to move away from the stockade up to the two-pointed hill. Ostensibly this is to keep guard on the treasure that Ben Gunn has disinterred, but also ‘there to be clear of malaria’.
Seamen have long been wary of malaria, and with good cause. In 1585, Sir Francis Drake left Plymouth with 1,500 seamen and 800 soldiers in 29 ships. They went ashore for a short time in the Cape Verde Islands. Later, Drake wrote in his diary:
We were not many days at sea but there began among our people such mortality, as in a few days there were dead above two and three hundred men. And until some seven or eight days after our coming from Santiago, there had not died one man of sickness in all the fleet; the sickness showed not his infection wherewith so many were stroken, until we departed thence, and there seized our people with extreme hot burning and continual agues.17
The typical incubation period for malaria is ten to twelve days, but seven to eight is not uncommon. Drake lost a further 500 men when he reached the Caribbean, putting this down to ‘first night air’: ‘who so is then abroad in the open air, shall certainly be infected to death’.18 It is easy to imagine mosquitoes biting the newly landed sailors, celebrating their first landfall in a more casual, carefree and perhaps inebriated manner than they ought, paying less attention than they should to swatting the bloodsuckers.
FIRST LINKS TO MOSQUITOES
Strangely, the link between mosquitoes and killer diseases was first mooted centuries ago. There have been suggestions that the Brahmin priest Susruta linked mosquitoes and malaria 2,500 years ago.19 However, he made no precise judgement; he merely differentiated between strongly venomous ‘insects’ like spiders and scorpions (likening them to snake bites) and those with mild poison like mosquitoes. More definite links are implied in 1572, when English writer Richard Hakluyt repeated details from his correspondent Henry Hawks, who had spent five years in Vera Cruz, Mexico:
This towne is inclined to many kinde of diseases, by reason of the great heat, and a certeine gnat or flie which they call a musquito, which biteth both men and women in their sleepe; and as soon as they are bitten, incontinently the flesh swelleth as though they had bene bitten with some venomous worme. And this musquito or gnat doth most follow such as are newly come into the countrey. Many there are that die of this annoyance.20
Into the nineteenth century, there continue to be many tantalizing instances of nearly, but not quite, making that correct link between malaria and mosquitoes. The great German explorer and writer Alexander von Humboldt made lengthy observations on mosquitoes during his journeys through Latin America in 1799–1804. His text is littered with remarks about their links to disease:
at the Orinoco, the banks of which are very insalubrious, the sick blame the mosquitoes for all their sufferings . . . the insects irritate the epidermis, and stimulate its functions by the venom which they deposit in the wounds they make . . . The frequency of gnats and mosquitoes characterises unhealthy climates only so far as the development and multiplication of these insects depend on the same causes that give rise to miasmata . . . May not the mosquitoes themselves increase the insalubrity of the atmosphere?21
He ponders, since there are so many insects floating about in the air: ‘we are led to inquire whether the presence of so many animal substances in the air must not occasion particular miasmata’.22
In what is perhaps his most portentous statement, Humboldt makes the direct suggestion that mosquito bites cause disease: ‘wherever the air is very unhealthy, the sting of the mosquito augments the disposition of the organs to receive the impression of miasmata’.23
He finishes by suggesting that all will be well, health returned and the insects diminished, as soon as the rainforests can be cut down and the land cleared so that the rivers can be ‘bordered with cottages, and the plains covered with pastures and harvests’. This had already started in the high cordilleras:
From these fertile and temperate table-lands, from these islets scattered in the aerial ocean, knowledge and the blessings of social institutions will be spread over those vast forests extending along the foot of the Andes, now inhabited only by savage tribes whom the very wealth of nature has retained in indolence and barbarism.24
This sentiment, that the disease was withholding the advance of civilization, would be used again when the true nature of malaria was finally deciphered.
Just before the final pieces of the puzzle were dropped into place, the notion that biting flies might spread diseases was already very much ‘in the air’. David Livingstone wrote about the tsetse fly in 1850:
it is well known that the bite of this poisonous insect is certain death to the ox, horse, and dog . . . We lost forty-three fine oxen to its bite . . . symptoms seem to indicate what is probably the case, a poison in the blood, the germ of which enters when the proboscis is inserted . . . The poison germ, contained in a bulb at the root of the proboscis, seems capable, although very minute in quantity, of reproducing itself.25
It was later discovered that the bite of the tsetse fly spreads sleeping sickness.
At the same time, French physician Louis Daniel Beauperthuy, working in Venezuela, firmly identified the local mosquitoes, ‘zancudo bobo’ (a species of Aedes), as being the carrier of yellow fever. He knew that ‘the affection known as yellow fever, or black vomit . . . is in no way to be regarded as a contagious disease . . . The disease develops itself under conditions which favour the development of mosquitoes.’ Although he incorrectly believed that the virus originated in the soil or water, he rightly believed that it was the mosquito bite, and the injection of infected saliva, that spread the disease to new victims: ‘the mosquito plunges its proboscis into the skin . . . and introduces a poison akin to that of snake venom. It softens the red blood corpuscles, causes their rupture . . . and facilitates the mixing of the colouring matter with the serum’.26
In the early 1880s Albert Freeman Africanus King, a successful gynaecologist and obstetrician in Washington, DC, suggested that mosquitoes might spread malaria through their bites. At that time, he visited the US Department of Agriculture and spoke to the government entomologists Charles Valentine Riley and Leland Ossian Howard ‘at some length’. 45 years later, Howard was to recall the meeting slightly apologetically: ‘I am sorry to say that we gave him no encouragement. The idea appeared to us to be altogether too farfetched.’27 King went on to publish his thoughts, but perhaps because he was in the wrong medical field, or he wrote in Popular Science Monthly rather than an academic journal, his suggestions were overlooked until examined in hindsight.
THE VECTORS IDENTIFIED
Today, malaria is the disease that everyone associates with mosquitoes, but malaria actually came third. Mosquitoes, it turns out, are responsible for a number of deadly and debilitating illnesses.
In 1877 Patrick Manson, medical officer with the Chinese imperial maritime customs, demonstrated by dissection under the microscope that one of the forms of filariasis (elephantiasis) was incubated in the bodies of mosquitoes. The disease, characterized by gross (elephantine) swelling of legs and scrotum, is caused by minute (0.2–0.3 mm /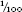 in long) parasitic blood worms accumulating in the lower limbs and blocking the lymphatic drainage vessels, resulting in fluid build-up, bloated flesh and thickening skin. Manson knew that an adult worm, 70–100 mm (3–4 in) long, could be found in the liver, and that the microfilariae, which blocked the blood vessels, were the larvae – but there was no intermediate form, and how did the worms move from one human to another? He settled on mosquitoes, because he knew that the blood worm had to move on to the next stage of its development in some agent ‘capable of piercing the skin of the human body’. He allowed females of Culex fatigans to bite a filariasis sufferer, and made a series of dissections over several tense days. ‘I shall not easily forget the first mosquito I dissected so charged. I tore off its abdomen, and by rolling a pen-holder from the free end of the abdomen to the severed end, I succeeded in expressing the blood the stomach contained.’ Under the microscope: ‘I was gratified to find that far from killing the filaria, the digestive juices of the mosquito seemed to have stimulated it to fresh activity.’28 He went on to show that the worms taken in with the blood migrated into the body of the mosquito, grew and developed – first a digestive tract, then
genitalia. At the time, Manson (later Sir Patrick, Nobel Prize winner) thought that mosquitoes then died transferring these microscopic parasites into the water, and that humans developed the disease by drinking in infectious particles. Manson (and everyone else) was wrong; mosquitoes transfer the worms from person to person during their blood meals, but his initial discovery set the stage for great revelations ahead.
in long) parasitic blood worms accumulating in the lower limbs and blocking the lymphatic drainage vessels, resulting in fluid build-up, bloated flesh and thickening skin. Manson knew that an adult worm, 70–100 mm (3–4 in) long, could be found in the liver, and that the microfilariae, which blocked the blood vessels, were the larvae – but there was no intermediate form, and how did the worms move from one human to another? He settled on mosquitoes, because he knew that the blood worm had to move on to the next stage of its development in some agent ‘capable of piercing the skin of the human body’. He allowed females of Culex fatigans to bite a filariasis sufferer, and made a series of dissections over several tense days. ‘I shall not easily forget the first mosquito I dissected so charged. I tore off its abdomen, and by rolling a pen-holder from the free end of the abdomen to the severed end, I succeeded in expressing the blood the stomach contained.’ Under the microscope: ‘I was gratified to find that far from killing the filaria, the digestive juices of the mosquito seemed to have stimulated it to fresh activity.’28 He went on to show that the worms taken in with the blood migrated into the body of the mosquito, grew and developed – first a digestive tract, then
genitalia. At the time, Manson (later Sir Patrick, Nobel Prize winner) thought that mosquitoes then died transferring these microscopic parasites into the water, and that humans developed the disease by drinking in infectious particles. Manson (and everyone else) was wrong; mosquitoes transfer the worms from person to person during their blood meals, but his initial discovery set the stage for great revelations ahead.
| An engraving from 1614 shows that filariasis (elephantiasis) was long known as a discrete disease. It was the first protozoan disease identified as being spread by mosquitoes. |  |
In 1881 Cuban doctor Carlos Finlay, overlooking the previous work of Beauperthuy, suggested yellow fever was spread by mosquitoes. This disease was intimately linked with the failure of the French effort to build a Panama Canal between 1882 and 1889; 25 years earlier, the Panama railroad had exacted a horrible price, with 40 per cent mortality. Porto Bello on the Caribbean coast was described as ‘an open grave ready to swallow all who resorted there’.29 To dig the proposed canal, the French engineer Ferdinand de Lesseps was brought in after success on the Suez Canal. Much of Suez was cut through comparatively mosquito-and disease-free land; it was completed in 1869, after ten years of construction, and malaria only broke out seriously in 1877 when, by a convoluted irony, Italian workmen were blamed for bringing it. Central America proved different and with heavy disease loads from the start, the Panama project was eventually abandoned.30
 |
Quinine was widely used as a febrifuge long before its specificity for malaria was discovered. |
It took nearly twenty years of back-and-forth mosquito research before, in 1900, American army surgeon Walter Reed finally proved that yellow fever transmission was by mosquito bite. It was, however, a success tinged with failure. At first, Reed did not believe Finlay’s ideas and spent much wasteful time trying to prove some soil- or water-borne pathogen. Yellow fever is a virus, a speck of barely living material so small that, unlike filariasis, it cannot be seen with a light microscope and it passes through the ceramic filters that stop even bacteria. It also has a different modus operandi, and rather than simply multiplying in the body fluids, it disappears into the DNA machinery of the mosquito for up to a month before it returns to an infectious state. In September 1900, at the successful climax of the research, Reed’s friend and assistant in Cuba, Jesse Lazear, died of yellow fever. Reed died in 1902, aged just 51, of a ruptured appendix. Two years later, armed with the knowledge to fight the mosquito, and the disease, the USA began a new assault on the Panama Isthmus, and although sickness continued to afflict the workers, the canal was completed by its better health-protected engineers and labourers in 1914, two years ahead of schedule. US government entomologist L. O. Howard later confirmed that, had he survived, Reed would undoubtedly have been awarded a Nobel Prize for his yellow fever work.31
The unravelling of malaria started in 1880, when French physician Charles Louis Alphonse Laveran, working in Algeria, found strange, moving particles in the blood of malaria sufferers. He deduced that these were a protozoan parasite. By 1884 he suspected that these parasites might also be found in the bodies of mosquitoes.32
The year 1897 was the pivotal year for malarial research. In that year, Ronald Ross, working in Secunderabad, in southeast India, found malaria granules in the stomachs of mosquitoes fed on a patient with the disease. Unfortunately, he was then posted to another part of India, but by Patrick Manson’s intercession he was sent to Calcutta to continue his malarial research. Human malaria was not common in Calcutta, so Ross turned his attention to bird malaria and was able to trace the cycle of the parasite’s development to a local mosquito species. But the mugwumps had not finished with Ross and he was now sent to Assam to study kala-azar (leishmaniasis, another protozoan parasite, this time spread by sandflies).
Meanwhile, still in 1897, with Ross chasing sandflies in Assam, Giovanni Battista Grassi, in Rome, proved that the female Anopheles mosquito was the human vector for malaria by successfully infecting a healthy man living in a non-malarial area by having an experimentally malaria-laden mosquito bite him. Medical ethics committees might baulk at this today, but it was the evidence that clinched the link between malaria and mosquito.
The final malarial breakthrough of 1897 came in dar es Salaam, in modern-day Tanzania, but then in German East Africa, where Prussian physician Robert Koch demonstrated that the chemical quinine destroyed the malarial parasites in human blood. Koch, especially, is credited with helping blow away the medieval humoral miasma idea of disease, and establishing the germ theory we now know to be correct. Earlier, in 1876, he had proved that anthrax was a bacterial infection and he had made similar discoveries with tuberculosis and cholera.

Quinine, as well as a remedy for fevers, was seen as a useful treatment of other disorders, including ‘terrible itching’.
The exact details of who, when, how and why malaria was ‘discovered’ may be lost to the general public’s psyche today, but it happened at a time of blossoming scientific enquiry, often labelled as the golden age of medico-veterinary entomology. The idea of a Victorian gentleman scientist labouring in some outpost of Empire, poring over his microscope, rattling Petri dishes and test tubes, and scratching endless letters to his contemporaries back in England, is a familiar image and one used regularly in literature, theatre and film. In Holmes of the Raj (2006), one of many modern sequel spin-offs from Sir Arthur Conan Doyle’s popular hero, the famous London detective’s sidekick, Dr John Watson, is portrayed as discovering the malaria–mosquito link, but in his usual gallant way he remains in the background and passes his observations to one Ronald Ross, medical Resident of Bangalore, and of course the rest is history.
Unfortunately, no such gentlemanly good sportsmanship really existed between the rival national scientific groups, and they all sought to claim as much of the discovery limelight as possible. In his later years, there was some unease when Ross took to ‘much controversial writing’33 as he vigorously fought the rival claims of the Italians. Writing in 1902, for example, Ross wrote to the same L. O. Howard who had not been very encouraging towards A.F.A. King’s early malaria suggestions:
I think that the Italian school requires a little medicine in the shape of plain speaking. I suppose that you have seen the last effort of Grassi and Noe, who pretend that they have found out about Filaria bancrofti [the elephantiasis parasite]. As a matter of fact they have hardly ever seen one, much less found out anything about them. Believe me.34
When Howard visited the retired Ross in Putney Heath in 1927, the great man was no less vociferous. Despite having suffered a serious stroke that paralysed the left side of his body, Ross was as belligerent and inflammatory as ever:
He showed us a big cabinet in which he had systematically filed and indexed all of the papers relating to his malaria work. He swore about the Italians, spoke of Grassi as a damned liar . . . He spoke of De Kruiff’s book The Microbe Hunters [which he believed gave too much credit to Grassi] with profanity . . . He gave us each a copy of his latest paper on the Grassi claims . . . Grassi was a damned pirate.35
Despite Ross’s and Grassi’s rival competing claims, the understanding of malaria cannot be attributed to one single discovery. Ross continued to work on malaria for many years, often in difficult circumstances. Back in Secunderabad in 1907, he worked daily in the intolerable heat, without a punkah for fear of disturbing the delicate mosquito specimens he was dissecting under his worn and broken microscope, ‘the screws being rusted with sweat from my hands and forehead, and my only remaining eye-piece being cracked . . . Fortunately my invaluable oil-immersion object glass remained good.’36 He was rewarded by the further discovery of the next stages of the malaria parasite’s life cycle within the body of the mosquito. In the end, Sir Ronald Ross, Nobel laureate, Knight Commander of the Order of the Bath, after whom roads, buildings, medical institutes, lectures and medals are named, received more than enough awards and accolades during and after his life, and he is certainly worthy to be nominated as the major determiner of malaria’s cause.
THE MECHANISM OF MALARIA
Malarial transmission is not a simple case of infected blood being picked up from one person and arbitrarily transported to another by the passive dart of a mosquito’s mouthparts. The entire complex life cycle of the parasite is intricately tied into the completely different physiologies of both human and mosquito hosts.
Malaria is caused by a tiny microbe called Plasmodium. An Anopheles mosquito infected with malaria injects anticoagulant saliva when it bites; it also injects spindle-shaped Plasmodium ‘spore’ cells, technically called sporozoites, which enter the victim’s bloodstream. They end up in the liver, where they divide to produce new Plasmodium cells called merozoites. Some of these reinvade the liver while others pass into the bloodstream, where they enter the red blood cells. Inside these red corpuscles, they increase in size before again splitting into more merozoites. The red cells burst, ejecting haemoglobin (the ‘mixing of the colouring matter with the serum’ seen by Beauperthuy) and releasing the multiplied merozoites back into the blood to attack the liver again and yet more red blood cells. This repeating and increasing cycle of production within the red blood cells creates huge numbers of circulating merozoites and huge numbers of damaged red cells, often causing anaemia. When they rupture, the red cells also unleash toxins into the blood, and it is the cyclical release of these toxins, every two or three days, that produces the regularly recurring fevers for which malaria is well known.
While all this is going on, some merozoites take a different developmental pathway and form pre-sexual cells called gametocytes. These red cells do not rupture, but circulate in the blood waiting to be drunk down by another Anopheles mosquito. Only when they are ingested into the mosquito gut do the gametocytes undergo further development and form the male and female cells (gametes), which combine to form fertilized cells called zygotes. The Plasmodium zygotes pass into the mosquito’s intestinal wall, where they eventually develop into progenitor ‘egg’ cells called ovocysts, which divide and multiply to form large numbers of the sporozoites that started the whole process. The sporozoites migrate to the mosquito salivary glands and wait to be injected with the mosquito’s next human bite. The parasite’s development within the mosquito takes about fourteen days, and it was this hidden part of the cycle that had previously so confused the medical profession. It was too great a time lag to link individual malaria sufferers and threw any calculations about contagion times and transmission modes into complex disarray. It would, however, later prove to be an important fourteen-day window during which malaria outbreaks might be contained and controlled.
All the transforming cell types, from sporozoites to merozoites to gametocytes and zygotes, may seem ludicrously complex, but malaria’s astonishing cycle of mosquito/human parasitism has a simple biological process at its heart – amplification. A small number of Plasmodium particles injected into a human victim reproduce vast numbers of themselves in the blood; all the better to be picked up by the next mosquito. A small number of these Plasmodium particles ingested by a mosquito again reproduce large numbers of themselves; all the better to be injected back into the next human victim to keep the cycle going. It works very well, and it has been working for at least eight million years when, according to one study, human malaria started to evolve away from the proto-malaria that infected our hominid ancestors.37
One of the most complicated things about malaria is that it is not one disease, but four (at the last count).38 Four species of Plasmodium infect humans (and plenty of others infect birds or mammals). Two of these, P. malariae and P. ovale, are rare; P. ovale is hardly known outside tropical West Africa. It is the ‘big two’ that have had the greatest effect on mankind. Plasmodium vivax is the most widespread, and the one reckoned to be behind ague in temperate climes. This is because it can develop in mosquitoes in much cooler regions (15ºC/59ºF for at least one month; most of North America and much of Eurasia). The death toll from vivax is about 1–2 per cent of severe untreated infections during epidemics; higher than many other infectious diseases, but nothing compared to the last and most deadly malarial parasite. Plasmodium falciparum is the danger malaria of the tropics, and with a death rate of 25–50 per cent of severe untreated infections, is the disease that has been responsible for most of the malaria deaths in the world. This is a dark shadow of a disease that has probably killed more people on the planet than any other single cause, and which is still killing about one million people every year, mostly infants in sub-Saharan Africa.
Differences between at least three of the four parasites have been known, even if not fully appreciated, from time immemorial. They each have slightly different symptoms. The cyclical mass release of the blood-inhabiting merozoites, together with the fever-inducing toxins when the red blood cells burst, have a fairly fixed periodicity. With vivax, falciparum and ovale, there is a crippling sledgehammer attack of fever every 48 hours, traditionally called ‘tertian’ fevers, because they appeared every first and third day. Chaucer, in ‘The Nun’s Priest’s Tale’, warns not to ‘get yourself with sudden humours hot; / For if you do, I dare well lay a groat / That you shall have the tertian fever’s pain’. For P. malariae victims, the attacks come every 72 hours, so the term ‘quartan’ fevers is used, because they appeared every first and fourth day. In his Inferno (c. 1308–21), Dante speaks of ‘one who has the shivering of the quartan so near, / that he has his nails already pale, / and trembles all’.
 |
Fritz Schaudinn, blood cells being invaded by Plasmodium vivax parasites, 1903. |
Seasonality was also well known, especially away from the perennially warm tropics where the mosquitoes and disease were present all year round. On his travels through Arabia, noted traveller, linguist, soldier and spy Captain Sir Richard Burton relayed: ‘In the summer, quotidian and tertian fevers (Hummah Salis) are not uncommon, and . . . are frequently fatal. The attack generally begins with the naffazah, or cold fit, and is followed by the al-hummah, the hot stage.’39
Physicians also noted the relatively low mortality rate of vivax, calling it ‘benign tertian’ fever, and the more deadly falciparum, which was ‘malignant tertian’. Other differences are seen in the incubation period after being bitten by an infected mosquito – falciparum hit fast and deadly (as it did for Drake’s crew) in 7–12 days, ovale after about 17 days, malariae 18–40 days and vivax (as suffered by Alfred Russel Wallace) anything from 15 days to a year. Vivax also had a dormant stage, which could remain, asymptomatic, in the liver for many months or years, only to recur spontaneously, without warning, after a long period of what seemed like cure. (Ovale, likewise, can have a ‘resting’ stage.) Differences between the various Plasmodium species also help to explain why it is that some people can survive inside the malaria zone, while others – usually newcomers – quickly fall victim to its fevers, febrile convulsions, delirium and, all too often, its rapid, fatal end.
When he received his Nobel Prize, on 12 December 1902, Ronald Ross stirringly summed up this effect on newcomers in his acceptance speech ‘Researches on Malaria’. It’s worth publishing part of it here, capturing as it does the imperial frustration that the disease engendered.
Malarial fever is important, not only because of the misery which it inflicts upon mankind, but because of the serious opposition which it has always given to the march of civilization in the tropics. Unlike many diseases, it is essentially endemic, a local malady, and one which unfortunately haunts more especially the fertile well-watered, and luxuriant tracts – precisely those which are of the greatest value to man. There it strikes down not only the indigenous barbaric population, but with still greater certainty, the pioneers of civilization – the planter, the trader, the missionary, and the soldier. It is therefore the principal and gigantic ally of Barbarism. No wild deserts, no savage races, no geographical difficulties have proved so inimical to civilization as this disease. We may also say that it has withheld an entire continent from humanity – the immense and fertile tracts of Africa. What we call the Dark Continent should be called the Malarious Continent; and for centuries the successive waves of civilization which have flooded and fertilised Europe and America have broken themselves in vain upon its deadly shores.
Powerful stuff. It was not unravelled until the last half of the twentieth century, but there is an underlying biological reason why Ross’s European march of civilizing expansion (for which also read commercial exploitation) into Africa was much slower than in southern Asia and the Americas – resistance within the bodies of the indigenous population to attack from Plasmodium parasites.
It had long been known that newcomers, usually Europeans, were especially susceptible to malaria, and virtually every tropical African city has probably been described as a white man’s grave at some point in its history. When researchers knew which microbes to look for in the blood, they often found that many of the indigenous population were infected, yet showed no disease signs. Knowing that they were surrounded by a walking reservoir of symptom-free carriers was justification for segregating the community – keeping the diseased natives away from the vulnerable expatriate colonists.
Today, two mechanisms of resistance are known. Across tropical Africa, 97 per cent of contemporary populations carry a gene in their DNA called ‘Duffy antigen negativity’, named after the haemophiliac in which the positive antigen was first found, in 1950. The antigen, on the surface of the red blood cell, is the door through which the malaria merozoites invade. The ‘negative’ gene – which means that the antigen is missing – is thought to have appeared, as a result of a random genetic mutation, 100,000 years ago. The negative gene does not disadvantage the carriers, but since the antigen is not there, Plasmodium vivax cannot invade the blood cells, and this prevents the disease from taking hold in the body. In effect, 97 per cent of the tropical African population – those carrying the Duffy negativity gene – are immune to vivax malaria.
Resistance to the more dangerous falciparum malaria is not so benign. Another series of random genetic mutations also took place in Africa (maybe also Arabia or southern India) 70,000–150,000 years ago, in the DNA that codes for haemoglobin (the oxygen-transporting red pigment in the blood cells). The mutation causes sickle-cell disease, so named because the altered haemoglobin changes the shape of the normally round red blood cells into long, thin sickles. The falciparum parasite cannot get into the sickle blood cells to multiply. Unfortunately, the sickle cells carry less oxygen, are shorter lived than normal red cells, and do not flow smoothly through the capillaries. These problems combine to create a parade of life-threatening complications from anaemia and kidney disease to stroke. An individual receiving a mutant sickle cell gene from one parent and a normal gene from the other, can also produce the normal haemoglobin, and normal round red cells. In this case, the sickle cells do not cause as great a problem, but do give some immunity to malaria. However, individuals receiving sickle cell genes from both parents have greatly reduced life expectancy, usually death before adulthood. In some West African communities, 25–30 per cent of the population carry a single copy of the sickle cell gene. This means around one in ten of the children born there will have the dangerous, ultimately fatal, double mutant gene combination. There is a very high price to be paid for falciparum immunity.
UNRAVELLING HISTORY
These discoveries, of blood parasites, mosquito vectors and partial immunities, start to make clear some of the historical conundrums about the origin of malaria and the spread of epidemics. Unlike smallpox, measles and the other dreadful contagious pandemic diseases, malaria needed two fuses to light the keg of disease gunpowder – an immigration of malaria-carrying humans, and the presence of suitable malaria vector mosquitoes.
Back in Tolkien’s Midgewater Marshes, stout country hobbit Sam Gamgee is being eaten alive by midges and asks: ‘What do they live on when they can’t get a hobbit?’ Little does he realize, he’s just asked a very pertinent biological question. If humans (and maybe hobbits) are thin on the ground, mosquitoes will bite other animals, including wild birds and mammals and, in agricultural country, farming stock. For the relatively short-lived mosquito, this in effect dilutes the probability of malaria being passed on to other humans. If stock animals are bitten, they will not harbour the disease for long (or at all if their blood physiology is significantly different to that of humans), because they are almost inevitably slaughtered for their meat. Of course, eating, even raw, meat carries no risk of malaria infection. Biting of wild creatures can, very rarely, create a natural reservoir for the disease when no humans are about, but if mosquitoes evolve, say, bird-biting behaviour, they are unlikely to transfer their attacks back to humans; once learned, they stick to birds. In order for malaria to become the dreadful killer it is today, human population density had to achieve a critical mass. In Neolithic Africa, hunter-gather populations were just not dense enough to sustain the heavy malarial loads we see today. The disease was a ‘minor’ problem, allowing the immunities of Duffy negativity and sickle-cell anaemia to evolve.
In most of Europe, the chronically debilitating, but usually less deadly, vivax malaria was the cause of ague. Seasonal flooding affected the yearly patterns of mosquito abundance and human activities. Fenland drainage for agriculture started removing the mosquito breeding grounds long before any medical significance could be appreciated.
When Europeans arrived in the New World, they found sparsely scattered nomads in the north, and their Old World poxes devastated the metropolitan civilizations of the centre and south. Malaria was not a problem for many decades, perhaps even a couple of centuries, but increasing population in concentrated settlements eventually allowed the disease to run out of control. When the colonial powers decided on sugar cane economics around the Caribbean, the local indentured labour, mostly brought in from South America, succumbed quickly to the malaria which had become established in the relatively densely populated lowland settlements, so hardier, disease-resistant slaves, bringing their long-acquired immunities, were imported from Africa.
The fall of Rome, and the eclipse of the ancient Greek civilization, has recently been linked (through archaeological DNA studies) to the arrival of the much more deadly falciparum malaria into southern Europe around 450 CE. Until then, Europe had only to deal with the ‘mild’ vivax ague form of the disease.40 High mortality, leading to economic collapse and civil chaos, is suggested by some to have resulted partly from the likely combined arrival of suitable falciparum vectors and falciparum carriers.
Was the arrival of rice, 2,500 years ago, with its mosquito-friendly aquatic cultivation, the spark that made malaria flare up in Japan? Increased trade, and the exchange of paddy engineering know-how, would also have brought infected individuals from China into the changing and increasing Japanese agricultural settlements.
The mysterious Mauritian malarial outbreak of 1866 is now explained with the crystal-clear vision of hindsight. Although malaria-infected immigrants arrived in a constant stream for two centuries, and their individual fits and tremors may have stayed with them all their lives, it was not until the accidental introduction (and subsequent breeding) of the key mosquito, Anopheles gambiae, from Africa, in 1866, that the disease could become established in the wider population. Until that year, there was no suitable mosquito vector on the island. Mean while, back on Stevenson’s fictional Treasure Island, malaria should not have been a threat. Although it was in the malarial Caribbean, the secret island on Billy Bones’s map was deserted, apart from Ben Gunn. He may have been a ‘half-idiot maroon’, but he was not ill with the fever. Even if malaria vector mosquitoes had been present, there would have been no pool of malaria-carrying humans to infect the newly landed adventurers.
At the end of the nineteenth century, the revelations of Manson, Ross, Grassi and all the other great medical men heralded the start of a new war against disease. The weapons were still in the making, but the enemy had been clearly identified. All across the world, mosquitoes were in the cross-hairs of the gun sights and the hunt was on.