Major Features of Tetrapod Evolution
Farish A. Jenkins Jr.
OUTLINE
1. Tetrapod ancestry
2. The fish-tetrapod transition
3. Amniote origins
4. Synapsids
5. Diapsids: Lepidosaurs and their relatives
6. Diapsids: Archosaurs
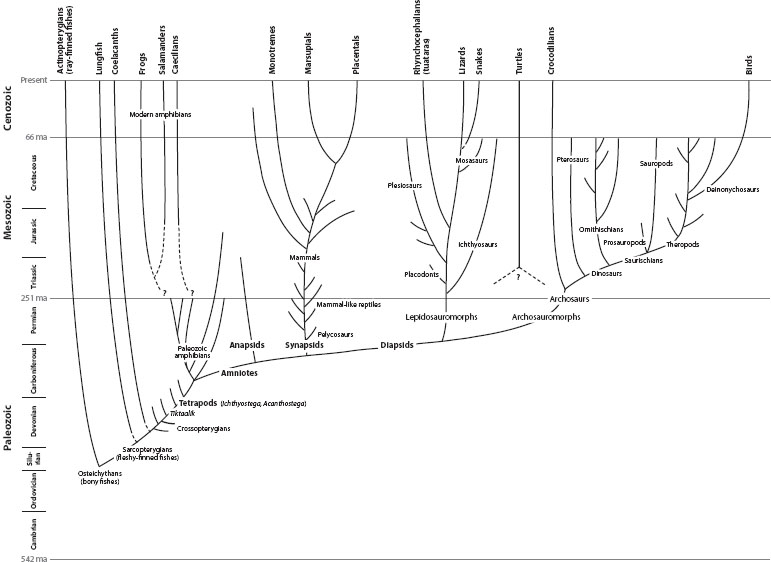
Tetrapod evolution spans the last 375 million years, beginning with the origin of limbed terrestrial vertebrates from finned fishes. The earliest tetrapods were amphibians that radiated during the Late Paleozoic; only three groups of highly specialized amphibians survive today. The development of an amniotic egg provided novel mechanisms to enhance embryonic growth and allowed eggs to be laid on land. Amniotes came to dominate Mesozoic and Cenozoic terrestrial faunas worldwide, generated powered aerial flight three times, reverted to aquatic habitats and the marine realm repeatedly, created endothermic temperature controls, evolved limbs for running, digging, and climbing, flew in water with fins converted from limbs, many times lost limbs altogether, forged armor, concocted venoms and other weapons, adapted to eat almost everything macroscopic of biological origin, and attained body sizes across four orders of magnitude. A skeletal outline of tetrapod evolution and diversity is presented here.
GLOSSARY
Amniote. Tetrapods that reproduce by means of an amniotic egg, comprising an eggshell membrane and extraembryonic structures (including an amnion and chorioallantoic membrane) that provide a protective environment, nutrition, waste storage, and respiratory exchange.
Anapsid. The earliest recognized of three clades of amniotes that arose in the Late Paleozoic. Anapsids are characterized by skulls in which the chamber housing major jaw-closing muscles is completely enclosed. The term anapsid signifies lack of any external openings (fenestrae) that otherwise occur in diapsids and synapsids.
Diapsid. Amniotes in which the chamber housing jaw-closing muscles possesses two fenestrae. Diapsids include numerous extinct Mesozoic taxa such as dinosaurs, pterosaurs, and ichthyosaurs. Lizards, snakes, crocodilians, and birds are extant diapsids.
Synapsid. Amniotes that possess a single fenestra in the chamber housing jaw-closing muscles. Synapsids include extinct Late Paleozoic pelycosaurs and Permo-Triassic mammal-like reptiles (therapsids). Mammals are the only extant synapsids.
Tetrapod. Literally, four feet. Tetrapods are vertebrates with limbs instead of fins. The fish-tetrapod transition and the emergence of vertebrates onto land was initiated during the Late Devonian, some 375 million years ago.
1. TETRAPOD ANCESTRY
The ancestry of tetrapods may be traced to bony fishes (Osteichthyes) that first appear in the fossil record during the Silurian (439–408 million years ago, Ma). By Devonian times (408–362 Ma), bony fishes had diverged into two distinct clades, ray-finned fishes (Actinopterygii) and fleshy-finned fishes (Sarcopterygii). In their pectoral and pelvic fins, both possess elongate, slender, rodlike scales (lepidotrichia) and a skeletal base for fins within the trunk. In actinopterygians, lepidotrichia radiating from the skeletal base constitute the fin’s sole skeletal framework, with fin movements controlled by musculature within the body wall. Sarcopterygians are distinctive for the muscles and robust skeleton present within the fin; in this group, lepidotrichia are largely confined to the fin margins.
Sarcopterygians diversified into three distinctive assemblages that were major components of Devonian fish faunas: lungfish (Dipnoi), coelacanths (Actinistia), and a variety of so-called crossopterygians (figure 1). Lungfishes and coelacanths have few living representatives, and neither is generally considered closely related to tetrapods. Rather, tetrapods arose from a particular crossopterygian group (the elpistostegalids) that did not survive the Paleozoic in their piscine form. Lungs were probably present in Devonian sarcopterygians as accessory respiratory organs, for they are present in extant lungfish and, in modified form, in living coelacanths. Lungs were thus not a novel development among emergent tetrapods. Although the respiratory function of gills was eventually abandoned, tetrapods retained and adapted the skeletal elements of the gill system for feeding and other functions.
Figure 1. Overview of tetrapod evolution in the context of geologic time.
Tetrapods carried into a terrestrial sphere many crossopterygian features, including the pattern of skull bones persisting in modified shapes throughout tetrapod evolution. A distinctive tooth structure in which the enamel and dentine are deeply infolded into the tooth’s interior (labyrinthodonty) was also retained. The tetrapod vertebral pattern likewise originated among crossopterygians; vertebrae comprised a single midline element (neural arch) with three crescentic, supporting ossicles (paired pleurocentra, and an intercentrum) surrounding a large notochord (Benton 2005). Although the proximal homologues of the bones in the tetrapod forelimb (humerus, radius, and ulna) and hindlimb (femur, tibia, and fibula) can be confidently identified in crossopterygian fins, the distal elements as precursors to tetrapod feet have long remained problematic. Nonetheless, extant lungfishes and the coelacanths both paddle with alternating strokes of contralateral fins, much as tetrapod limbs are used in walking.
2. THE FISH-TETRAPOD TRANSITION
Elpistostegalid sarcopterygians, initially recognized from incomplete fossil remains of two taxa, Elpistostege and Panderichthys, exhibit features suggesting a prototetrapod condition. The discovery by Daeschler and colleagues (2006) of Tiktaalik roseae, an elpistostegalian from the Late Devonian Fram Formation (375 Ma) in the Canadian Arctic, documents many anatomical details of an amphibious transitional stage in the emergence of tetrapods (figure 1).
Tiktaalik possesses eyes elevated above the skull roof, a feature shared with various living vertebrates that gaze just above the air-water interface (notably the mudskipper Periophthalmus, frogs, crocodilians, and hippopotamus). The broadly triangular skull and rather flat body bear greater resemblance to those of a Paleozoic amphibian than to a fish. Most surprisingly, Tiktaalik possesses a neck. Typically, a fish skull is connected to the shoulder girdle through a series of intermediate bones (operculo-gular and extrascapular series), but in Tiktaalik these are lost, allowing the head independent mobility. Fish feeding in water can readily position the entire body to direct the mouth toward prey. Such maneuvers are more difficult on land, where a mobile head is distinctly advantageous for a predator.
Shubin and colleagues (2006) described the forefin skeleton, which is homologous to the vertebrate forelimb and includes precursors to the tetrapod wrist and possibly digits. In addition to a humerus, radius, and ulna, there are wrist bones (an ulnare and intermedium) and a robust distal skeleton of small, short bones (radials). The eight rows of radials making up the bony margin of the Tiktaalik fin are possible precursors to the polydactylous feet of early tetrapods. The geometry of the shoulder, elbow, and distal joints provides evidence that the pectoral fins could assume both finlike and limb-like postures.
The interpretation that the fins of Tiktaalik supported the body on land is corroborated by other aspects of skeletal architecture. The expanded, overlapping ribs recall those in the earliest-known tetrapods, which augmented rigidity for the trunk and axial support for the body. Such support was critical as elpistostegalid fishes left the buoyancy of water and encountered gravitational forces on land. This specialization for terrestrial existence, once believed to have evolved as an adaptation in the earliest-known tetrapods, in fact developed in amphibious fishes that were behaving like tetrapods. Although Tiktaalik is clearly piscine in retaining a well-developed branchial skeleton to support gills, a scaly body covering, and lepidotrichia, this amphibious sarcopterygian could move from shallow water margins onto shore.
What selective forces may have promoted a land invasion? The open seas of the Devonian were populated by large, predaceous fish—such as sharks and placoderms. Elpistostegalids, with flattened head and body and no dorsal fins, were adapted for nearshore shallow waters; Tiktaalik was recovered from deposits created by freshwater rivers overrunning their banks. In the shallowest habitats, elpistostegalids entered a natural refugium free of large predators and provisioned with abundant, unexploited invertebrate faunas on which to feed.
The earliest and most primitive tetrapods known, Ichthyostega and Acanthostega of Late Devonian age, retain many crossopterygian features, including labyrinthodonty, the pattern of skull bones, the location of lateral line sensory canals, the configuration of vertebral components, a finned tail, and gills. Relative to Tiktaalik, major alterations appear in the vertebral column and limbs. In Ichthyostega, the vertebral column is robustly constructed to support gravitational loading of the trunk. The closely abutted neural spines of the vertebrae are arranged to reinforce the back against sagging. Zygapophyses, processes that interconnect adjacent neural arches, are developed to reinforce spinal stiffness and regulate intervertebral movements. A specialized vertebra, the sacrum, anchors the hindlimb to the spine. The limbs are robust, with numerous small bones that form flexible wrists and ankles; fore- and hind feet are polydactylous. Tetrapod anatomy underwent relatively modest changes during the initial radiation of amphibians through the remainder of the Paleozoic and early Mesozoic. By comparison, extant amphibians (frogs, salamanders, and caecilians) are morphologically quite specialized, yet most remain dependent on an aqueous environment for the development of externally laid eggs (Clack 2002; Carroll 2009).
Vertebrate development from a fertilized ovum requires a protected environment, and supplies of oxygen and nutrients. Extant amphibians (anamniotes) and amniotes exhibit divergent solutions to providing these necessities. An amphibian ovum is surrounded by a vitelline membrane and successive “jelly” (mucopolysaccharide) capsules. Water absorbed through the membrane and capsules enlarges the space for embryonic growth and provides oxygen for the embryo. Although the capsular layers have diverse physiological and protective functions, they limit oxygen diffusion, which in turn limits the size of the hatchling. No longer bound to aqueous or moist environments, the amniotic egg overcame these constraints.
3. AMNIOTE ORIGINS
Turtles, snakes, lizards, tuataras, crocodiles, birds, and mammals present a rich array of living tetrapod diversity. Each group has evolved such distinctive anatomical, physiological, and behavioral characteristics that they cannot be confused. Yet each lineage converges on a singular event in its common ancestry: the development of an amniotic egg. The eggshell membrane and extraembryonic structures form an elaborate support system, including a protective casing (shell membrane, shell), a watery environment (amnion-enclosing amniotic fluid), a nutritional source (yolk sac), waste storage (allantois), and a respiratory exchanger (chorioallantoic membrane). The system’s origin remains obscure. The only feature of egg development shared by both amphibians and amniotes is the derivation of a maternally derived envelope in the oviduct. The Paleozoic fossil record has yet to reveal any evidence of amniotic eggs (Sumida and Martin 1997).
Amniote evolution can be traced only by a suite of skeletal features inherited and further transformed by amniote lineages that survive to the present day. The earliest-known amniotes, entrapped within hollow, upright stumps of giant lycopods (see chapter II.3), exhibit distinctive features shared with later amniotes. Some represent losses from the common amphibian pattern, such as loss of palatal fangs and labyrinthodonty. Others are novel, including additional skull components. On the palate, enlarged fang-like teeth are developed, and a prominent transverse flange of the pterygoid is tooth bearing. In the postcranial skeleton, amniotes fuse three separate bones in the amphibian ankle—the intermedium, tibiale, and proximal centrale—into a single bone, the astragalus.
By the Late Carboniferous, three types of amniotes are known on the basis of skull roof patterns (figure 2). In anapsids, the chamber behind the eye from which jaw-closing muscles originate is completely enclosed by external skull bones. Numerous extinct Late Paleozoic taxa exhibit an anapsid lack of fenestration. Synapsids are defined by a single lateral opening (temporal fenestra) exposing the muscle chamber, whereas diapsids have two fenestrae, one above the other. In contrast to anapsids, synapsids and diapsids underwent extensive Mesozoic and Cenozoic radiations, ultimately yielding the vast variety of amniote life (Benton 2005). An anapsid ancestry for turtles has been suggested, but not unequivocally demonstrated. Most recently, molecular and morphological analyses have suggested that turtles are diapsids that have secondarily achieved an anapsid condition, but it is unclear to which diapsid group (lepidosauromorphs or archosaurs) they are most closely related (figure 1). Turtle origins remain an unresolved problem in tetrapod evolution.
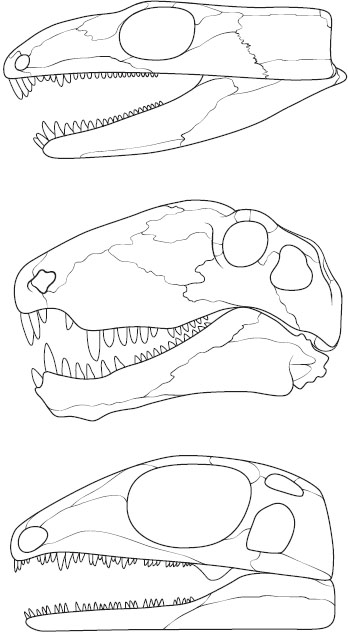
Figure 2. Representative skulls of the three major groups of amniotes. Top: The anapsid condition, in which there is no opening (fenestra) behind the orbit. Middle: The synapsid condition, with a single fenestra to the chamber from which jaw-closing muscles arise. Bottom: The diapsid condition, exhibiting two fenestrae of the chamber. (Top: Eocaptorhinus laticeps modified from M. J. Heaton. 1979. Cranial Anatomy of Primitive Captorhinid Reptiles from the Late Pennsylvanian and Early Permian, Oklahoma and Texas. Oklahoma Geological Survey, Bulletin 127, fig. 1; middle: Pelycosaur Dimetrodon limbatus modified from A. S. Romer and L. W. Price. 1940. Review of the Pelycosauria. Geological Society of America Special Papers, 28, fig. 62; bottom: Petrolacosaurus kansensis modified from R. R. Reisz. 1981. A Diapsid Reptile from the Pennsylvanian of Kansas. Special Publication of the Museum of Natural History, University of Kansas, 7, fig. 1.)
4. SYNAPSIDS
Living mammals are synapsids possessing such distinctive skeletal characteristics as a jaw joint between the dentary and squamosal bones, a secondary (bony) palate separating the nasal cavity from the mouth, and three middle ear bones (malleus, incus, and stapes). Although none of these skeletal configurations was present when synapsid evolution began, the development of these and other mammalian features is now traceable through 300 million years of the geological record with considerable precision.
Synapsids evolved through three successive radiations: pelycosaurs, therapsids (so-called mammal-like reptiles), and mammals. The earliest-known undoubted synapsid, the pelycosaur Archaeothyris florensis, occurs in Middle Pennsylvanian deposits in Nova Scotia. Pelycosaurs, the largest tetrapods in Late Pennsylvanian faunas, diversified in the Early Permian to occupy both carnivorous and herbivorous niches, with some forms developing greatly elongated neural spines supporting a dorsal “sail.”
Therapsids supplanted pelycosaurs during the Late Permian. Substantially more varied than pelycosaurs, therapsids ranged from large, heavy-bodied, robust-limbed Dinocephalia to relatively gracile Theriodontia. During the Permian, therapsids underwent considerable cranial diversification; dental specializations were accompanied by substantial increases in the size of temporal fenestrae and the mass of jaw-closing muscles.
Only two groups of therapsids survived the widespread extinctions during the Permo-Triassic transition. The most abundant Triassic therapsids were the anomodonts, robust herbivores that replaced teeth with a keratinous, slicing mechanism. Most important in terms of mammalian origins were cynodonts. Members of certain families of cynodonts (Thrinaxodontidae, Probainognathidae) exhibited dental, cranial, and postcranial features that represent the most advanced level of therapsid organization from which mammals were derived.
Approximately 5000 diverse species of living mammals testify to the huge scope of synapsid evolution since the Early Jurassic. Throughout this radiation, the defining mammalian characters in the skull and postcranial skeleton have largely persisted. These characters did not arise as an integrated complex, but rather as a mosaic initiated at different times in synapsid history. Whereas brain enlargement and diphyodonty (deciduous, or “milk,” teeth followed by the adult dentition) are novel attributes of early mammals, the development of other features can be traced to earlier stages. Carnivorous pelycosaurs possessed elongate, paired caniniform teeth set in an otherwise homogeneous dentition. Various therapsids subsequently developed prominent single canine teeth, together with modest differentiation of incisors and postcanines. Distinctive cusps on anterior and posterior postcanine teeth are a cynodont feature, presaging the differentiation of mammalian premolars and molars as well as tooth-on-tooth occlusion.
In early cynodonts the size of the temporal muscle substantially increased, and an incipient coronoid process on the jaw (a lever for the temporal muscle) and a masseter muscle first appeared. In pelycosaurs, a dentary (the tooth-bearing bone) and eight postdentary bones composed the lower jaw. Throughout synapsid evolution, postdentary bones diminished as the dentary enlarged. In the most advanced cynodonts, a mammalian jaw joint between the dentary and squamosal evolved alongside the primitive quadrate-articular joint. Although most postdentary bones were ultimately lost, several persisted to serve novel functions. The angular (with a distinctive flange, the reflected lamina) persisted as the tympanic bone supporting an eardrum. The articular and quadrate were retained as the malleus (adherent to the inner surface of the tympanic membrane), and incus (linking the malleus and stapes). In therapsids the close association of the stapes, the original bone of the middle ear, with the quadrate set the stage for the quadrate (incus) and articular (malleus) to associate as a miniaturized, sound-conducting bony chain between the tympanic membrane and inner ear.
Major advances toward a mammalian grade of postcranial organization occurred among cynodonts. The joints between the skull and first cervical vertebra, and the first and second cervicals, became specialized for flexion-extension and rotation of the head, respectively, which are movements important in feeding, defense, and other behaviors. Cervical, thoracic, and lumbar regions became distinctly differentiated, contributing to the wide range of movements and postures utilized by mammals. A small tuberosity on a major ankle bone, the calcaneum, was the forerunner of an elongate lever, the calcaneal tuberosity, into which flexor muscles insert to power mammalian walking, running, and jumping. But not all mammalian characteristics had their beginnings among cynodonts; numerous features emerged later among Jurassic and Cretaceous mammals.
Despite the rich synapsid fossil record, the origins of such quintessential mammalian attributes as hair, endothermy, and mammary glands remain obscure. A furry coat in Early Cretaceous mammals is the earliest persuasive evidence of endothermy. Given the plausible thermoregulatory function of Permian pelycosaurs’ extensive dorsal sails, which might have served to gather radiant heat, synapsids conceivably could have had a long history of temperature regulation.
5. DIAPSIDS: LEPIDOSAURS AND THEIR RELATIVES
Extant diapsids are represented by lepidosaurs (the squamates: tuataras, lizards, and snakes) and certain archosaurs (crocodilians and birds). As diverse as this assemblage may seem, diapsid evolution during the Mesozoic yielded an even more extraordinary array of tetrapods. Some invaded the oceans (placodonts, plesiosaurs, ichthyosaurs, mosasaurs), others became the first powered fliers (pterosaurs), and still others dominated terrestrial herbivorous and carnivorous niches (ornithischian and sauropodomorph dinosaurs, and theropod dinosaurs, respectively). A Late Pennsylvanian precursor to this diversity, Petrolacosaurus kansensis, is clearly diapsid by virtue of the double fenestration of the skull (figure 2), but it lacked many of the specialized features found in lepidosaurs and archosaurs.
Lizards (>4,000 species), snakes (~3,000 species), and tuataras (1–2 species; technically, rhynchocephalians) share numerous derived features indicative of common ancestry. The epidermis is shed entirely at discrete intervals. The cloaca, an opening for the digestive, urinary, and reproductive tracts, is a transverse slit. A fracture plane within each caudal vertebra allows the tail to break away (autotomy), facilitating escape.
Although rhynchocephalians were moderately diverse during the Mesozoic, and included herbivorous, possibly venomous, and even aquatic forms, today they are represented only by two relict species of the genus Sphenodon that survive on coastal islands off New Zealand. Tuataras differ from true lizards in a number of features, including the presence of a premaxillary process, or “beak,” in place of premaxillary teeth. The upper dentition is a double row of teeth between which the lower teeth precisely occlude. Tuataras lack an external ear.
The Early Jurassic Gephyrosaurus bridensis is the earliest-known well-documented lizard with such defining features as fusion of the primitively paired frontals and parietals, fracture planes for caudal autotomy, and an astragalocalcaneum. Lizards that are possibly related to modern groups appear during the Middle and Late Jurassic. Limb reduction or loss is widespread. Iguania (including iguanids, agamids, and chamaeleonids) is generally considered the most generalized assemblage of extant lizards. Most are territorial ambush predators; the fleshy tongues of iguanids and agamids snatch prey with sticky saliva. Scleroglossa is a diverse assemblage of mostly active, foraging predators without established home territories. The highly protrusible tongues of many of these lizards serve both prehensive and chemosensory functions (Pianka and Vitt 1989).
Snakes were derived from lizards, and are likely most closely related to monitors (Varanidae) and alligator lizards (Anguidae). Fossil snakes are rare and not known before the Late Cretaceous. Najash rionegrina, a Late Cretaceous terrestrial snake, possessed a pelvis with diminutive hindlimbs. The positioning of the pelvis and definitive sacral vertebrae suggest external hindlimbs, in contrast to hindlimb rudiments in some modern snakes that lie within the body wall. Some interpret this as evidence that snakes derived from a terrestrial, burrowing ancestor, but for lack of adequate evidence there is no consensus on snake origins. In modern snakes, as in other elongate squamates, only the right lung is functional. In some taxa, the respiratory surface is limited to the anterior part of the very elongate lung; posteriorly, the surface is nonrespiratory, but the bag serves as an air reservoir during the prolonged period of prey ingestion when the glottis is blocked. In one feature, however, snakes are unique among tetrapods: the right aortic arch predominates, rather than the left. Also, in the most specialized taxa, almost all bones save for those encasing the brain (frontal, parietal) are involved in highly mobile articulations and capable of displacement. Unfettered from the constraints of the primitive amniote cranial plan, serpents suspend from the protective braincase a startlingly flexible and expansive feeding apparatus (Greene 1997).
During the Mesozoic, numerous lepidosaurs and their relatives turned to the sea. Aquatic varanoid lizards gave rise to the large, open-water, predatory Late Cretaceous mosasaurs, some exceeding 10 m in length, with paddling limbs and sculling tails. Triassic placodonts, armored and superficially turtle-like, developed broad, flat teeth for crushing shelled invertebrates in coastal waters. Pachypleurosaurs, likely propelled by undulation, were small, fish-eating forms (ca. 0.5 m in body length) with long necks. Nothosaurs were similar to pachypleurosaurs, but larger (1–4 m body length); these anguilliform (eellike) swimmers were likely ancestral to plesiosaurs.
Plesiosaurs, appearing in the Jurassic and persisting almost to the end of the Cretaceous, diverged into two distinctive morphotypes. Pliosauroids, with huge heads, relatively short necks, and robust teeth, were adapted for large prey. Plesiosauroids, with slender teeth indicative of smaller prey, bore undersized heads on enormously elongated necks. The expansion of ventral elements in the shoulder and pelvic girdles, together with limb shape, are evidence that plesiosaurs engaged in subaqueous flight, much as do extant sea turtles, seals, and penguins.
Ichthyosaurs are the epitome of aquatic diapsids. Early Triassic ichthyosaurs were anguilliform swimmers; their backbone of cylindrical centra permitted a wide range of undulations. Jurassic and Cretaceous ichthyosaurs developed a thunniform (tuna-like) body shape, a pronounced dorsal fin and crescentic tail, and deep discoidal centra that contributed to body rigidity—all features of powerful swimmers. The disproportionately large eyes of later ichthyosaurs, with diameters greater than 200 mm in Ophthalmosaurus and 260 mm in Stenopterygiius, were evidently an adaptation for visual foraging at great depths in near darkness.
6. DIAPSIDS: ARCHOSAURS
Archosaurs, a diverse assemblage of diapsids that dominated terrestrial faunas throughout much of the Mesozoic, are represented today only by crocodiles and birds (figure 1). Their origins are to be found among Early Triassic archosauromorphs; distinctive features, such as socket-implanted (thecodont) teeth were largely retained in later archosaurs. True archosaurs also appear in the Early Triassic. They are distinguished from other diapsids by openings in front of the orbit (antorbital fenestra) and in the lateral side of the jaw (mandibular fenestra). The morphological diversity among the ankle joints of early archosauromorphs and later archosaurs reflects shifts in stance and gait, and defines major archosaur groups. In Ornithodira, which includes pterosaurs and dinosaurs, the astragalus and calcaneum are immobile, fixed to the distal ends of the tibia and fibula, respectively. Ankle flexion and extension occurs at a mesotarsal joint between these fixed elements and the next row of anklebones, the distal tarsals. In Crurotarsi, which includes crocodilians and various extinct Triassic taxa, the ankle joint is developed between the astragalus and calcaneum. The calcaneum (and the remainder of the distal elements of the foot) flexes and extends on the immobile astragalus (Benton 2005).
The relatively flattened skulls, fused parietals, and small temporal fenestrae of primitive Late Triassic crocodilians are features that persist in modern crocodiles. Whether bipedal or quadrupedal, the gracility and length of their limbs indicate that early crocodilians were agile, perhaps even cursorial, predators. Early Jurassic crocodilians, initiating a trend toward separating the nasal and oropharyngeal passages, formed a complete secondary bony palate with internal nasal openings (internal choanae, linking the mouth and nasal cavities) halfway along its length. There is no evidence that these early forms were particularly aquatic, but later in the Jurassic mesosuchian crocodiles occupied aquatic habitats. Mesosuchian skulls elongated and flattened; the internal choanae displaced posteriorly, invading the pterygoids at the back of the palate. In some mesosuchians the limbs were relatively small and the tail was prolonged for sculling. Eusuchians appeared in the Cretaceous and have changed relatively little since. A major eusuchian specialization was the completion of the posterior migration of the internal choanae, now completely surrounded by the pterygoids. Crocodilians thus achieved a complete separation of the nasal and oropharyngeal passages, an adaptation uniquely useful to semiaquatic ambush predators (Ross and Garnett 1989).
Pterosaurs, the first vertebrates to have evolved powered flight, present one of the most spectacular radiations in Mesozoic history, encompassing a great range of size, diverse skull and tooth shapes, and an array of diets. The principal wing membrane (brachiopatagium) arose from the trunk and forelimb and extended to the distal tip of a hyperelongated fourth digit. Exceptionally well-preserved pterosaurs provide unequivocal evidence of a furry pelage on the body and wing membranes, a strong indicator that pterosaurs were partly, if not fully, endothermic. Braincase endocasts reveal that pterosaurs possessed relatively large brains, although not as large as those of birds of comparable body mass. The immense size of the flocculus, where reflexes are coordinated that maintain a fixed gaze under dynamic conditions, together with very long semicircular canals in the inner ear, indicate that pterosaurs possessed a highly refined sense of equilibrium.
Beaks, cranial crests, body size, and dental specializations are the sources of pterosaur diversity. Beaks varied from short and deep to long and attenuated, and from downturned and prehensive to upturned and probing. Crests occurred as bony ridges on beaks, or as soft tissue or bony adornments on the head. Extreme dental specializations ranged from bristlelike teeth in filter feeders to batteries of blunt teeth fused to the jaws of shellfish crushers. Sizes ranged from the robin-sized Pterodactylus elegans (wingspan 25 cm) to Quetzalcoatlus northropi, the largest-known flying vertebrate, with a wingspan of 11–12 m. Found in Late Cretaceous Texas floodplain deposits 400 km from the coastline, Quetzalcoatlus possessed an unusually long neck and a robust (but toothless) beak, evidence that these huge pterodactyls might have been either predatory or carrion feeders (Wellnhofer 1991).
Relatively few features unite dinosaurs as a group. Among them are loss of the postfrontal in the skull and the tendency to reduce manual digits V and IV. Structures associated with the insertions of locomotor muscles are accentuated; the deltopectoral crest of the humerus is elongate, and a cnemial crest (for insertion of the quadriceps femoris) appears on the tibia. More than two vertebrae contribute to the sacrum.
By Late Triassic time, two major lineages of dinosaurs are distinguishable on the basis of pelvic structure. Ornithischians, ultimately comprising a great diversity of herbivorous forms, had a pubis with two processes: a prepubic process projecting anteriorly, and a postpubic process lying posteriorly near the ischium. The postpubic process superficially resembles the pubis of birds, and thus originated the term Ornithischia (“bird hip”), although pubic configurations in birds and ornithischians were derived independently. The Saurischia, comprising herbivorous sauropodomorphs and carnivorous theropods, had pubes that retained the primitive anteroventral orientation and were widely separated from the ischium. In both groups the acetabulum (hip socket) was widely fenestrated (Weishampel et al. 2004).
Ornithischian dinosaurs developed several masticatory specializations to process plant material. A toothless premaxilla and a neomorphic predentary bone in the lower jaw supported a cropping, presumably keratinous beak. Lanceolate (leaf-shaped) tooth crowns bore multicusped edges; wear facets developed through tooth-on-tooth occlusion. In many forms the enamel was asymmetrically distributed to promote self-sharpening. A robust, elevated coronoid process was a mechanical lever for powerful jaw-closing muscles. But unlike many mammalian herbivores, few major radiations of ornithischians seem to have employed rapid escape as an antipredatory tactic. Rather, as defenses against contemporary carnivores, they variously developed large body size (iguanodontids, hadrosaurids), armor and tail clubs (ankylosaurids, nodosaurids), spikes and plates (stegosaurids), shields and horns (ceratopsians), and thickened skulls for head ramming (pachycephalosaurs).
Saurischian dinosaurs comprise two divergent radiations: herbivorous sauropodomorphs (prosauropods and gigantic sauropods), and predaceous theropods. By the Late Triassic, prosauropods were well established as the dominant herbivores in practically all known terrestrial faunas. Typically, prosauropods had small skulls, robust bodies, and long tails. They range in size from the diminutive Thecodontosaurus (0.5 m) to the bulky melanorosaurid Riojasaurus (8–9 m). Prosauropods persisted into the Jurassic, but their niche as large-bodied browsers was subsequently occupied by sauropods, huge animals with very small heads mounted on elongate necks, shortened trunks, and massive, straight (graviportal) limbs. The huge size attained by sauropods represented, in part, a defensive strategy, but for some, long necks and legs also permitted foraging at canopy levels, a resource unavailable to any contemporary ornithischian.
Theropods are far less well represented in the fossil record; the biomass of carnivores invariably is far smaller than that of their prey. The earliest group, the coelophysians, was widely distributed and present from the Late Triassic through the Early Jurassic. Coelophysis (Late Triassic), bipedal and gracile, is a representative early form with features of a generalized theropod. The limb bones have extensive marrow cavities, giving them a hollow appearance when fossilized. The forefoot retains four digits, although the fourth is commonly lost in later, more derived forms. The fourth digit on the hind foot is sufficiently reduced in size that the foot is functionally tridactyl. Tridactyl trackways are a common trace fossil signature of theropods.
Later theropods developed larger heads and deeper mandibles than those of coelophysians. Allosaurids, ranging up to 12 m in length, were dominant predators from the Late Jurassic through the Early Cretaceous. Tyrannosaurids were the largest predators of Late Mesozoic ecosystems. Relative to an allosaurid skull, the tyrannosaurid skull was longer, deeper and more massively built, with a very wide snout. In cross-section, the extremely robust teeth were as wide as long, and thus thicker than allosaurid teeth. Bearing such a heavy head, the neck and trunk are shortened. The very slender, small shoulder girdle bore tiny, short forelimbs with only two divergent digits. But not all theropod groups depended on a powerful dental battery. Both the ornithomimosaurs and the oviraptosaurs were toothless, the latter bearing a cutting beak.
The Deinonychosauria, comprising two families (Dromaeosauridae, Troodontidae), share a few skull characters (such as loss of the prefrontal bone bordering the orbit) that unite the group and are shared with birds. Postcranial evidence for a deinonychosaurian-avian relationship is stronger. Both modern birds and the dromaeosaurid Deinonychus possess tubercles (epipophyses) for muscular attachment on the second and third cervical vertebrae; these are well developed in the primitive bird Archaeopteryx. The length of the forefoot in deinonychosaurs and Archaeopteryx equals or exceeds the length of the hind foot. The pubic orientation in Deinonychus is almost vertical, approaching the backwardly reflected avian position, in contrast to the primitive theropod anteroventral orientation.
Representatives of several Early Cretaceous theropod families possessed a covering of protofeathers; another theropod (Caudipteryx) was fully feathered but flightless nonetheless. Together this evidence indicates that feathers likely evolved initially for thermoregulation rather than for flight. Their development was a critical stage in the evolution of powered flight. Two theories have offered seemingly contrasting interpretations of the origin of powered flight. In the “cursorial” or “from the ground up” scenario, incipiently developed feathers on the forelimbs of cursorial theropods provided sufficient lift to launch a runner into the air. In the “arboreal” or “from the trees down” scenario, primitive feathers provided sufficient lift to support gliding. In both cases, the development of an aerofoil generating even the most incipient aerodynamic lift (even if insufficient for sustained, powered flight) represents the structural innovation from which avian flight evolved. Although long represented as competing theories, until recently there was little evidence to support either theory, but two studies now provide evidence that both gliding and running with protowings are realistic precursors to powered flight and are not mutually exclusive. From their initial radiations in the Mesozoic (Chiappe and Witmer 2002), birds now constitute more than 10,000 living species, a major component of tetrapod diversity, and testimony to the vast scope of evolutionary change within archosaurs.
Benton, M. J. 2005. Vertebrate Palaeontology. 3rd ed. New Jersey: Blackwell. An updated edition of a synoptic textbook encompassing the relationships, morphology, and evolution of major vertebrate clades.
Carroll, R. A. 2009. The Rise of Amphibians: 365 Million Years of Evolution. Baltimore: Johns Hopkins University Press. A leading paleontologist’s summary of discoveries and interpretations of the evolution of Paleozoic and modern amphibians.
Chiappe, L. M., and L. M. Witmer, eds. 2002. Mesozoic Birds: Above the Heads of Dinosaurs. Berkeley: University of California Press. Well-illustrated research reports and subject summaries on avian origins, avian-theropod relationships, the anatomy and systematics of Mesozoic birds, and locomotor evolution.
Clack, J. A. 2002. Gaining Ground: The Origin and Evolution of Tetrapods. Bloomington: Indiana University Press. A compendium of geological, paleoecological, anatomical, paleobiological, and taxonomic data bearing on the origin and early evolutionary history of tetrapods.
Daeschler, E. B., N. H. Shubin, and F. A. Jenkins Jr. 2006. A Devonian tetrapod-like fish and the evolution of the tetrapod body plan. Nature 440: 757–763.
Greene, H. W. 1997. Snakes: The Evolution of Mystery in Nature. Berkeley: University of California Press. An extravagantly illustrated survey of the diversity and biology of snakes, with perspectives on evolution and biogeography.
Pianka, E. R., and L. J. Vitt. 2003. Lizards: Windows on the Evolution of Diversity. Berkeley: University of California Press. An authoritative and well-illustrated summary of the adaptations, life history, and diversity of lizards.
Ross, C. A., and S. Garnett, eds. 1989. Crocodiles and Alligators. New York: Facts on File. A superbly illustrated account of the evolution, diversity, life histories, and conservation of crocodilians.
Shubin, N. H., E. B. Daeschler, and F. A. Jenkins Jr. 2006. The pectoral fin of Tiktaalik roseae and the origin of the tetrapod limb. Nature 440: 764–771.
Sumida, S. S., and K.L.M. Martin, eds. 1997. Amniote Origins: Completing the Transition to Land. New York: Academic. Research summaries of the phylogeny, biogeography, feeding, reproductive biology, functional anatomy, and physiology relating to amniote origins.
Weishampel, D. B., P. Dodson, and H. Osmólska, eds. 2004. The Dinosauria. 2nd ed. Berkeley: University of California Press. The sourcebook for current interpretations of the evolution and major features of all dinosaurian taxa, including biogeography, taphonomy, paleoecology, extinction, and the early history of birds.
Wellnhofer, P. 1991. The Illustrated Encyclopedia of Pterosaurs. London: Salamander Books. A compendium on the origin, evolution, and diversity of pterosaurs, including detailed illustrations of diverse taxa as well as life reconstructions.