Speciation Patterns
Timothy G. Barraclough
OUTLINE
1. Testing the nature of species
2. Speciation patterns in sexual eukaryotes
3. Speciation patterns in asexuals
4. Speciation patterns in prokaryotes
5. Speciation and global diversity patterns
6. Linking patterns with process
Speciation refers to the splitting of a single ancestral species into two or more descendant species. The way in which speciation occurs can affect many aspects of biodiversity, such as phenotypic variation among organisms, the geographic distributions of related species, and the number of species in a geographic region or clade. All these aspects of biodiversity, if studied in relation to speciation, are referred to as speciation patterns. These patterns provide vital clues into the causes of speciation and their variation among different organisms. By studying patterns we can understand processes occurring over many human life spans and over large geographic scales that are difficult to study experimentally. Fundamental questions such as the importance of reproductive isolation and sex in speciation can be addressed by comparing speciation patterns among organisms with different lifestyles. Documentation of speciation patterns is also a major goal for understanding the distribution of biodiversity across the planet and among major groups of organisms.
GLOSSARY
Allopatric. Describing species with nonoverlapping geographic ranges.
Clade. A group of organisms descended from a single common ancestor. The pattern of diversity within a clade is determined in part by the speciation events that generated the separate species.
Crossing Experiment. An experiment that tests species boundaries in sexual organisms by attempting to interbreed individuals believed to belong to different species.
Eukaryote. An organism that has a cell nucleus and other organelles enclosed by membranes, such as mitochondria. Eukaryotes include single-celled protists and multicellular animals, plants, and fungi.
Prokaryote. An organism that lacks a cell nucleus or other organelles surrounded by membranes. The two major groups are the true bacteria and the Archaea.
Recombination. The bringing together of genes from different individuals into a single genome. In sexual eukaryotes, it occurs by crossing-over events during gamete formation. In bacteria, it occurs through a variety of mechanisms.
Reproductive Isolation. The result of prevention of interbreeding between two populations or species of sexual organisms, either by prezygotic or postzygotic isolation.
Sexual Reproduction. The formation of an offspring by the combination of genetic material from two parents. There are two steps: the formation of gametes such as sperm and eggs that contain half the genetic material of the parent followed by fusion of two gametes from different parents (called fertilization).
Speciation. The splitting of a single ancestral species into two or more descendant species.
Sympatric. Describing species with overlapping geographic ranges.
Taxonomy. The science of classifying organisms and giving them names.
1. TESTING THE NATURE OF SPECIES
Most biologists agree that species represent a real and fundamental unit of biodiversity. In sexually reproducing organisms, species arise because of reproductive isolation caused by barriers to gene exchange. Evolutionary processes of gene flow, selection, and drift maintain coherence within species but cause divergence between isolated species over time. Repeated speciation events should therefore lead to a discrete pattern of phenotypic and genetic variation within clades and communities.
Perhaps the fundamental question about speciation patterns is, Does diversity really fall into discrete species units, rather than fitting some alternative model? For example, there might instead be a continuum of variation among individuals without the phenotypic gaps predicted by the concept of species. This question is hard to answer using traditional methods for describing biodiversity. Taxonomists have long cataloged the diversity of life and applied names to species taxa. However, although informed by biological understanding, traditional alpha-taxonomy is often subjective and based on expert opinion rather than formal analyses of measured variation. Also, in most cases, it is assumed a priori that organisms fall into distinct species. Consequently, taxonomic species may not reflect real evolutionary entities but instead simply provide convenient labels for categorizing variation.
The solution to this problem is to collect genetic and phenotypic data and to analyze the pattern of variation against alternative hypotheses for the occurrence of diversification. A classic example was the work of G. Evelyn Hutchinson, who measured the morphology of planktonic rotifer species in an attempt to understand the importance of sexual reproduction for generating a pattern of distinct species. By taking repeated measurements for large samples of individuals from a pond, he was able to show that individuals’ morphology fell into distinct clusters, indicative of species. The hypothesis that diversity fell into a continuum of variation was rejected. Similar studies have been applied in problematic groups such as asexuals and prokaryotes, as described in later sections.
In a large-scale study to test the reality of species in sexual eukaryotes, Loren Rieseberg and colleagues investigated the correspondence between phenotypic clusters (based on morphological measurements) and the results of crossing experiments across 400 plant and animal genera. Phenotypic clusters were found in more than 80 percent of genera, and 75 percent of those were confirmed to be reproductively isolated from crossing experiments. Although the possibility that an alternative pattern of diversity might better explain the observed variation in these genera was not ruled out, the results did establish the presence of phenotypically distinct groups of individuals that tend to be reproductively isolated. The study also refuted the widely held belief that plant species are less distinct than in animals, because of their assumed greater propensity for hybridization, asexuality, and other evolutionary modes that are believed to blur species boundaries: in fact, distinct phenotypic clusters were just as frequent in plant genera as in animal genera, and plant species were more likely to correspond to reproductively isolated groups than were animal species.
Surprisingly few studies have adopted a quantitative approach to delimiting species, and most species in multicellular eukaryotes are still defined by taxonomic opinion. As an illustration, there are 13,000 genera of flowering plants alone, compared with the 400 genera of plants and animals for which quantitative data were available for the Rieseberg study. In some groups, such as fungi, in which useful morphological characters are either lacking or often deceptive, it has been routine to use formal crossing experiments to identify reproductively isolated species. However, in most organisms this is impractical—imagine the effort required to delimit more than 40,000 species of weevils by pairwise crossing experiments. A recent solution is to use DNA sequence data to test species boundaries. Using a single genetic marker, such as the mitochondrial gene cytochrome oxidase I now widely used for DNA identification, called DNA bar coding, it is possible to identify genetic clusters of individuals and check for correspondence between morphological characters and genetic divergence. With multiple genetic markers (i.e., several nuclear genes), it is possible to estimate the level of gene flow between putative species. Finally, in the age of genomics, it is becoming possible to identify genes underlying key ecological differences between species and to test detailed scenarios of divergence of species’ genomes.
2. SPECIATION PATTERNS IN SEXUAL EUKARYOTES
Theories of speciation were largely first developed with animals and plants in mind, and speciation patterns played an important role in shaping ideas about which mechanisms of speciation were most prevalent in the real world. Classic work by Ernst Mayr and others used taxonomic information of animal and plant groups to infer likely causes of speciation based on the characteristics of closely related species. Widely held views such as that most speciation involves geographic isolation, and that hybrid and polyploid speciation is common in plants, derived largely from observations: closely related species tend to occupy different geographic areas, and closely related plant species often differ in chromosome number.
Perhaps the biggest question concerning speciation in sexual animals and plants is, How do reproductive isolating mechanisms evolve? (See chapter VI.1.) Given that most reproductive and ecological traits important for the coexistence of species are polygenic, how can these traits evolve from a starting point of gene flow in the common ancestor? Also, what types of barriers to gene flow are most important in initiating speciation? Prezygotic isolation refers to barriers to gene flow caused by mating preferences or incompatibility of gametes prior to the formation of a zygote. Postzygotic isolation refers to genetic incompatibilities occurring within the zygote, such as poor growth or survival of hybrid offspring. In broad terms, the former emphasizes divergence in mating preferences and sexual characters between populations, whereas the latter emphasizes genetic incompatibility in hybrid offspring.
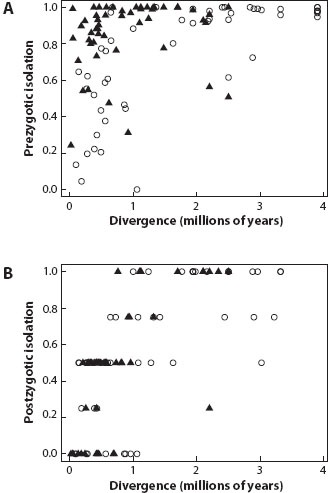
Important evidence for testing alternative theories has come from analyses of patterns of isolation across pairs of recently diverged species. By focusing on recently diverged species, it is hoped that patterns reflect early stages of speciation rather than subsequent evolutionary changes accumulating in the species. The classic study, which stimulated much of the comparative work on speciation patterns, was by Jerry Coyne and H. Allen Orr in the 1980s. They compiled data on levels of reproductive isolation from published crossing experiments between Drosophila species, and asked two questions. First, does prezygotic or postzygotic isolation evolve faster between species pairs? Second, does the rate at which isolation evolves differ in crosses between sympatric pairs (which might have experienced a history of gene flow) versus between allopatric pairs (which are assumed to have diverged without experiencing gene flow)? The finding was striking: prezygotic isolation evolves faster than postzygotic isolation (figure 1A versus figure 1B), but only between sympatric species pairs. The conclusions were far-reaching: first, natural selection is acting to increase reproductive isolation (which is also known as reinforcement; see chapter VI.4), as only when species were in contact did strong prezygotic isolation evolve; second, although postzygotic isolation remains a phenomenon of considerable interest for understanding genetic systems, it appears to play a minor role in solving the conundrum of how sexual species are able to coexist. In sympatric Drosophila at least, most species are already completely isolated by prezygotic mechanisms by the time postzygotic isolation evolves to a sufficient level to reduce interbreeding.

Figure 1. The pattern of (A) prezygotic and (B) postzygotic reproductive isolation between pairs of Drosophila species in relation to the divergence time between them. Divergence was calculated from genetic distances calibrated using a rough molecular clock. Black triangles are sympatric species pairs, and white circles are allopatric species pairs. Prezygotic isolation evolves faster than postzygotic isolation between recently diverged species when sympatric but not when allopatric. (Redrawn using data from Coyne and Orr 1997.)
Since then, other studies have looked for correlates of reproductive isolation in a wide range of groups. For example, Mikael Le Gac and Tatiana Giraud found the same pattern of greater premating isolation between sympatric than allopatric species of mushroom-forming fungi as had been found in Drosophila. Subsequent studies have sought to identify the correlates driving the evolution of reproductive isolation. Comparison of patterns of reproductive isolation and ecological differences among sister species pairs have shown that ecological shifts play an important role in driving the evolution of reproductive isolation. For example, in closely related stickleback species diverging between different habitats, such as between freshwater and marine populations, reproductive isolation is caused by changes in breeding habitat and body size associated with adaptation to the local habitat, and by poor survival of hybrid offspring in either habitat, rather than by genetic incompatibilities.
3. SPECIATION PATTERNS IN ASEXUALS
Our knowledge of speciation has been strongly shaped by a few taxa: birds, Drosophila, fish, a few plant taxa. It has long been realized that some ideas formulated for these taxa become problematic when applied to the charming variety of eukaryotes that are less well understood. Because of the importance of reproductive isolation for understanding speciation in sexual eukaryotes, perhaps the biggest challenge is posed by speciation in asexuals. A discreet but abundant set of microscopic eukaryotes reproduce, as far as we currently know, purely by asexual reproduction, in which offspring derive all their genetic material from a single parent individual. Clearly, the biological species concept based on reproductive isolation does not usefully apply, as none of their individuals interbreed: reproductively isolated units coincide, redundantly, with the individual level. This has led some authors to argue that species are a property of sexual organisms: asexuals do not “do” species. While this conclusion is true if we subscribe to a strict biological species concept, it is at odds with population genetic theories of speciation. If interbreeding is the very process that makes speciation difficult in sexuals, because it tends to erode genetic differences arising between populations (see chapter VI.3), then removing the ability to interbreed should make divergence easier.
Population genetic theories show that the same conditions promoting divergence and the evolution of reproductive isolation in sexuals, namely, geographic isolation or adaptation to distinct ecological niches, should also cause divergence in asexuals. Distinct genetic and phenotypic clusters are expected to emerge that evolve independently from one another and are maintained coherent by ongoing drift and selection within each cluster (figure 2). The pattern of variation within clusters should differ between sexuals and asexuals. Sexuals inherit genes from many different ancestors, and variation in traits such as body size should display normal distributions owing to averaging of multiple genes. Asexuals inherit all their genes from a single individual (i.e., their mother) in each preceding generation, and traits such as body size should exhibit a hierarchical pattern of variation reflecting maternal ancestry in the population. The pattern of variation between clusters, however, should be qualitatively similar in sexual and asexual clades if they encounter the same conditions favoring divergence. Whether sexuals or asexuals show a stronger pattern of genotype clustering will depend on the balance of two contrasting effects: asexuals might show stronger patterns of genotypic clustering because they do not have the limiting factor of interbreeding that limits speciation in sexuals, but they might show weaker patterns if they adapt more slowly to new niches than do sexuals (see chapter IV.4).
Figure 2. Hypothetical example of variation within an asexual and a sexual clade, both of which have diversified into six independently evolving species. The axes represent two independent measures of trait variation: they could represent morphological traits such as body length and wing length, or a summary measure of genetic variation among individuals. In both cases, the six independently evolving groups that form distinct clusters are indicated by solid ellipses. Processes of selection and drift maintain low variation among individuals within a cluster (a feature known as coherence or cohesion), but independent evolution allows divergence between clusters. The asexual clade also displays discrete variation within clusters, indicated by the dashed ellipses: each dashed ellipse represents a group of individuals sharing a relatively recent maternal common ancestor. In contrast, variation within sexual clusters should be homogeneous, as long as there is not further population subdivision within clusters, because the traits are determined by many genes, each with a different ancestry in the population. In the example shown, clusters are more divergent and distinct in the sexual clade, which might be expected if faster rates of adaptation in sexuals allow their species to adapt more efficiently to different niches. (Redrawn using data from Barraclough, Birky, and Burt 2003.)
The main reason that this question has been hard to answer is the difficulty of interpreting observed patterns. Traditional taxonomic approaches for delimiting species cannot help, because species tend to be defined differently in sexual and asexual taxa and so are not comparable. Even with quantitative measurements of phenotypic or genetic clustering, comparisons are limited by complications in how asexuals originate. Most asexual organisms tend to be recently derived from sexual ancestors, which means that variation in part reflects variation in their sexual ancestor and also that there has likely been insufficient time for conditions favoring speciation to have arisen and to have produced their effects on the population. A robust comparison of speciation in sexuals and asexuals requires lineages of asexuals that have survived over long enough timescales (many millions of years) without sexual reproduction.
Diego Fontaneto and colleagues evaluated the pattern of divergence in a celebrated clade of long-lived asexuals, the bdelloid rotifers. Bdelloids are microscopic animals living in permanent or temporary freshwater habitats. By comparing patterns of genetic and morphological variation with null models of variation in a single asexual population, these researchers showed that bdelloids have diversified into distinct and independently evolving clusters broadly equivalent to sexual species. The extent of morphological variation relative to genetic variation revealed that divergent selection on feeding morphology was one cause of their diversification. Whether bdelloids display a stronger or weaker pattern of clustering than equivalent sexual organisms, such as the facultatively sexual monogonont rotifers, remains to be answered. Also, although there has been no evidence for sexual reproduction, Gladyshev and colleagues showed that bdelloids can take up bacterial, plant, fungal, and protist genes from their environment. In principle, bdelloids could use this mechanism to acquire genes that help them to adapt to new environments and in part compensate for the reduced adaptive abilities expected of strict asexuals. Whether this unusual (for eukaryotes) means of acquiring genetic variation has affected their diversification is an interesting question for future work.
Many other groups of eukaryotes display features departing from those of the classical sexual eukaryote “models” for speciation. As well as asexuality, many microscopic eukaryotes have very high dispersal potential, which could affect the relative importance of geographic isolation in driving speciation. Genetic mechanisms might also differ between animals and plants in ways affecting the nature or magnitude of diversification. For example, many mushroom-forming fungi have complex life cycles in which true mating is preceded by a period of “cohabitation” of nuclei belonging to each mating partner within the same cells (these cohabiting fungi are referred to as heterokaryons). Cohabitation sometimes ends with each partner going its separate way rather than in the formation of sexual spores. In this way, genetic compatibility of the mating partners can be “tried out” before taking the irreversible step of combining their genes in the same nuclei. For example, poor growth in the heterokaryon resulting from the combination of genes from the partners might be used as a signal to end the relationship. DNA sequencing technology has opened up the opportunity to characterize diversity in the many eukaryotes not amenable to classical biological investigation (i.e., difficult to culture and with few morphological characters). Our view of how speciation occurs is likely to broaden substantially as these organisms are studied in increasing detail.
4. SPECIATION PATTERNS IN PROKARYOTES
Prokaryotes present a big challenge to theories of species and speciation developed for eukaryotes. The dominant concept of species as reproductively isolated groups assumes that individuals engage in sexual reproduction, but prokaryotes do not. Many prokaryotes are predominantly clonal and reproduce by cell division, but they also exchange genes by a variety of mechanisms. So-called homologous recombination occurs when naked DNA is taken up from the environment and incorporated into the genome. Such recombination mostly involves DNA from closely related cells: the probability of homologous recombination declines exponentially with increasing sequence divergence between the recipient and the DNA being incorporated. Alternatively, genes can also be transferred by independently replicating viruses or DNA molecules called plasmids. Plasmids often contain a set of functionally related genes, such as those conferring antibiotic resistance, and sometimes cross between distantly related taxa. The frequency of these different mechanisms varies widely among taxa and in all cases departs from the dominant mechanism of recombination in sexual eukaryotes. Speciation patterns therefore might differ between prokaryotes and eukaryotes and among prokaryote taxa.
The complexity of mechanisms of recombination in prokaryotes, and their failure to conform to the assumptions of the biological species concept, has led many biologists to an agnostic approach to prokaryote species: they use the term “species” for convenience but often follow statements on bacterial species with “whatever they are” or claim that bacteria do not have species. DNA sequence data provide the framework for prokaryote taxonomy, but species are usually classified using simple thresholds in pairwise genetic divergence (originally calibrated against “known” species units from traditional culture-based methods), without reference to an evolutionary definition for what those species units represent. For example, current efforts to catalog bacterial diversity in seawater, soil, and other habitats typically amplify and sequence the 16S ribosomal RNA gene and calculate species richness by counting the numbers of groups differing by more than 1 percent in their 16S sequences.
A more satisfactory view of prokaryote species comes from applying population genetic theories to predict patterns of diversity. Irrespective of how genetic inheritance and transfer occurs, the same forces that cause speciation in sexual eukaryotes can also cause diversification in bacterial populations. Frederick Cohan and colleagues pioneered evolutionary definitions of bacterial species by showing how specialization to different ecological niches causes the emergence of independently evolving genetic clusters of individuals. Mutations that are useful for survival and reproduction in one niche spread within the genetic cluster adapted to that niche but not into genetic clusters adapted to different niches. Simulation models of these processes have been used to demonstrate multiple distinct genetic clusters, which they call ecotypes, in Bacillus simplex and B. subtilis–B. licheniformis isolated from dry canyons in Israel. These models apply especially to bacteria with rare recombination, because the spread of new beneficial genes during adaptation to the distinct niches creates a pattern of genetic clustering at neutral DNA markers only if recombination is rare, unless a barrier to recombination evolves in association with ecological divergence.
Christophe Fraser, William Hanage, and Brian Spratt showed how speciation occurs in bacteria with high rates of recombination. When the recombination rate is lower than the mutation rate per gene, bacterial populations behave equivalently to populations without recombination, and their expected speciation patterns are similar to those described for asexual eukaryotes, and by Cohan and colleagues for bacteria with rare recombination. When recombination equals or exceeds the mutation rate, bacterial populations behave effectively as “sexual” organisms with the result that recombination prevents individuals within a population from becoming too genetically divergent from one another. Distinct species can evolve because of barriers to gene exchange caused by the decline in homologous recombination with increasing genetic distance between them, similar to the process of reproductive isolation in sexual eukaryotes. For example, in the mitis group of Streptococcus, there are four distinct genotype clusters based on variation in six genes involved in basic metabolic functions within the cell. These clusters conform to four traditional taxonomic species named on the basis of phenotypic traits.
Prokaryote diversity can therefore be understood in the same framework as developed for eukaryotes. A survey by Michiel Vos and Xavier Didelot found that 27 of 48 bacterial species had recombination rates above the threshold identified by Fraser and colleagues, and therefore are expected to evolve more like “sexuals” than “asexuals”; 13 fell in a gray area between clear-cut “sexual” or “asexual” evolution, and 8 had effectively “asexual” evolution. There remain important differences, however, from eukaryotes even in bacteria with high recombination rates: in the Streptococcus example described, a seventh gene linked to a locus involved in penicillin resistance is exchanged between these otherwise-distinct species. The question therefore remains whether prokaryote diversity is best described by simple units that could be called “species” or whether this model of diversity should be replaced with something more complex.
Our current knowledge of evolutionary processes in bacteria derives from species that can be cultured in the lab or that cause disease. Most prokaryotes cannot be cultured, and their diversity is only just being uncovered through large-scale DNA sequencing. Surveys of marker genes such as the 16S ribosomal RNA gene show that genetic variation falls into discrete clusters, as it does in surveys of single gene markers in both sexual and asexual eukaryotes. At least at neutral loci, it seems that most bacteria display the same pattern of discrete, independently evolving sets of genotypes that eukaryotes do. Metagenome sequencing—in which all DNA in a sample of soil or seawater is sequenced, and not just marker genes—is beginning to uncover ecological variation in bacterial communities, by identifying sets of genes required by the bacterial community at large to function in particular environments. However, piecing together the whole genomes needed for evolutionary and population genetic analyses from the “soup” of genetic information resulting from metagenome sequencing remains difficult.
5. SPECIATION AND GLOBAL DIVERSITY PATTERNS
The number of species in a group of organisms depends on the balance between the origin of new species by speciation and the loss of species by extinction, on the age of the group, and on the availability of space and resources to sustain species within the region occupied by the group (as space or resources diminish, the speciation rate will either decrease, or the extinction rate will increase). Although it is not the only process affecting diversity, speciation is likely to play an important role in shaping many different aspects of diversity (see chapter VI.16). For example, whether closely related species tend to share similar ecological characteristics will depend on the role of ecological divergence in speciation. If speciation tends to involve ecological shifts, then close relatives will tend to differ in ecological traits, whereas if it tends to occur through geographic isolation in identical habitats, then close relatives will tend to have similar ecological traits. These alternatives will also affect whether local communities are made up of distantly related species or of closely related species.
Perhaps the biggest question is whether speciation patterns can explain patterns of species richness among different organisms and in different geographic regions. Key parameters important in speciation models, such as dispersal rate and the strength of mating preferences, have been shown to correlate with species richness among clades. It is difficult, however, to separate effects of speciation from those of extinction, because current diversity is the net product of both processes. By including information on species relationships or species age distributions, it can be possible to tease them apart using statistical models. For example, Jason Weir and Dolph Schluter showed that recent speciation rates in northern latitudes exceeded those in more tropical latitudes, perhaps because of exposure of new ecological opportunities following the retreat of glaciers. Extinction can be detected directly from the fossil record, but few taxa display sufficient taxonomic and temporal resolution to document speciation, extinction, and morphological evolution along lineages. Analyses of the fossil record of single-celled marine eukaryotes called planktonic foraminifera by Thomas Ezard and colleagues showed that speciation rates depend more on the number of species present at the time (and decline as the number of species increases) than by changing physical environments, whereas extinction was driven largely by climate. Growing evidence from these and other studies indicates that speciation rates are strongly influenced by feedback from geographic and ecological limits on available resources within a region. As the number of species within a region and using a particular set of ecological resources increases, then the opportunity for new species to originate and persist declines.
The main frontier for understanding global diversity patterns is in hyperdiverse or previously poorly studied organisms such as tropical insects, tiny marine animals, and especially prokaryotes. To date, our knowledge of global diversity is heavily skewed toward macroscopic eukaryotes, especially vertebrates. Efforts to quantify how many species there are on earth are strongly dependent on being able to define comparable species units among disparate taxa and then to identify those units operationally. In the past, much attention has focused on tropical insects because of their known high diversity from museum collections, but generating a species list for the planet has seemed a distant goal based on the rate at which new species can be described. Extrapolations to estimate the likely total number of species from smaller samples have used assumptions about the turnover of diversity between different regions, about the level of specialization to tree species and the number of tree species in different regions, and the rates of description of new species over time. High-throughput DNA sequencing techniques could speed up the rate of discovery of new species, by reducing the need for the slow process of matching specimens morphologically to museum collections. Even with these methods, the turnover of hyperdiverse groups between regions means there will always be gaps in our sampling. However, by quantifying turnover and habitat specificity at different spatial scales for key groups, it will be possible to generate robust models for extrapolation to estimate global totals.
Prokaryotes present the biggest challenge. Even with the latest high-throughput sequencing techniques and using single marker genes, only a tiny fraction of the true diversity in the environment can be quantified in a given sample. Estimates of true diversity rely on estimating the total abundance of bacteria and the abundance of common species, and then extrapolating to estimate the total number of species likely to be present. This extrapolation requires an assumption of how species tend to vary in their abundances. One approach is to use neutral theory, which is an ecological theory that predicts the abundance of different species from mathematical equations based on the simplifying assumption that species do not differ in their ecological characteristics. Graham Bell used this approach to estimate that the estimated 1029 soil bacterial cells worldwide belong to perhaps 1014 species. Documenting and understanding diversity patterns of this many bacteria in the detail achieved for, say, vertebrates will keep researchers busy for decades, but the work has begun. For example, several studies have shown that pH is a major factor determining species richness and community similarity in soil bacteria.
Strangely, as noted by Graham Bell, the estimated diversity of single-celled eukaryotes is low (only around 50,000 species of protists have been described) relative to the astronomical numbers for bacteria and even compared with multicellular eukaryotes. DNA sequence data are revealing considerable cryptic diversity within protist species defined based on traditional taxonomy, which might increase their estimated diversity by one or two orders of magnitude, but this still leaves them depauperate compared with expectations from their global abundance. The reason for lower diversity in single-celled compared with larger eukaryotes could be that their higher dispersal rates (by wind, rain, or fowl) reduce speciation rates by limiting the opportunities for geographic isolation that arise abundantly in larger organisms. Another reason for lower diversity than bacteria could be the constraining influence of sex described earlier: if the average recombination rate were much higher for protists than for bacteria, and if the main effect of recombination were to limit genetic divergence, then this might explain the lower diversity of protists. Final resolution of this paradox awaits species units to be defined using comparable methods and estimation of rates of recombination in both bacteria and protists, which are both answerable with multilocus genetic techniques.
6. LINKING PATTERNS WITH PROCESS
Together with mathematical models and laboratory crossing experiments, studies of speciation patterns in nature have been at the heart of developing ideas about speciation (see chapter VI.3). Future work will entail establishing even stronger links between patterns and process. Population genetic theories of speciation are well developed, and the mechanisms of genetic divergence are increasingly well known from case studies of pairs of recently diverged species. It has been hard, however, to collect detailed genetic data for broader scales necessary to document speciation patterns across clades and regions. Traditional work focused on easily measurable characteristics such as geographic range and secondary sexual trait differences, and relied on taxonomic lists of species. The next generation of studies will use high-throughput genetic techniques to perform systematic evaluation of population genetic process such as gene flow and selection across broad samples of species. This will allow the nature of species units to be compared across organisms and the causes behind their formation to be revealed. One sticky issue will be where to draw the line for species versus higher levels in the hierarchy of nature, such as genera, or lower levels, such as populations. All biologists know that diversity is often more complex than can be encapsulated in simple units—species are not like chemical elements—and as quantitative approaches are increasingly used, we will be able to investigate the processes promoting diversification without needing to make simplifying assumptions a priori. At the broader scale of global diversity patterns, dynamic models are needed to connect the population genetic processes occurring in populations with the broader environmental context of how conditions favoring speciation and coexistence come and go (see chapter VI.16). This remains a big challenge, as it depends on complex interactions between organisms and their environment over elusive medium timescales: too long for direct observation but too short for most fossil records (with the notable exception of planktonic foraminifera).
FURTHER READING
Acinas, S. G., V. Klepac-Ceraj, D. E. Hunt, C. Pharino, I. Ceraj, D. L. Distel, and M. F. Polz. 2004. Fine-scale phylogenetic architecture of a complex bacterial community. Nature 430: 551–554. Study that used genetic data to demonstrate the existence of clusters indicative of species in prokaryotes.
Barraclough, T. G., C. W. Birky Jr., and A. Burt. 2003. Diversification in sexual and asexual organisms. Evolution 57: 2166–2172. Verbal theory comparing expected patterns of speciation in sexuals and asexuals.
Bell, G. 2009. The poverty of the protists. In R. Butlin, J. Bridle, and D. Schluter, eds., Speciation and Patterns of Diversity. Cambridge: Cambridge University Press. A thought-provoking account of differences in species numbers among the major forms of life.
Coyne, J. A., and H. A. Orr. 1997. Patterns of speciation in Drosophila revisited. Evolution 51: 295–303. Revisit of the classic paper on speciation patterns with some new data added.
Coyne, J. A., and H. A. Orr. 2006. Speciation. Sunderland, MA: Sinauer. The best single source of information and insight on all forms of speciation research.
Fontaneto, D., E. A. Herniou, C. Boschetti, M. Caprioli, G. Melone, C. Ricci, and T. G. Barraclough. 2007. Independently evolving species in asexual bdelloid rotifers. PLoS Biology 5: e87. A study using quantitative methods to test whether asexuals diversify into species.
Fraser C., W. P. Hanage, and B. G. Spratt. 2007. Recombination and the nature of bacterial speciation. Science 315: 476–480. Theory demonstrating how recombination shapes diversity patterns in bacteria.
McKinnon, J. S., and H. D. Rundle. 2002. Speciation in nature: The threespine stickleback model systems. Trends in Ecology & Evolution 17: 480–488. A good overview of evidence for speciation patterns reviewed in a well-studied group of fish.
Rieseberg, L. H., Wood, T. E., and E. J. Baack. 2006. The nature of plant species. Nature 440: 524–527. A critical investigation of the reality of species—more work like this is greatly needed to answer questions raised in this chapter.
Weir, J. T., and D. Schluter. 2007. The latitudinal gradient in recent speciation and extinction rates of birds and mammals. Science 315: 1574–1576. An example of how speciation patterns affect diversity patterns, and how models can be used to separate the effects of speciation and extinction.