Coevolution and Speciation
John N. Thompson
OUTLINE
1. Coevolution and the divergence of species interactions
2. Speciation with character displacement
3. Predators, parasites, and diversification
4. Mutualistic networks and speciation
5. Coevolved symbionts and speciation
6. Escape-and-radiate coevolution
7. Cospeciation
8. Conclusions
The web of life constantly changes as species impose strong natural selection on one another. During the past century alone, there have been dozens of examples of rapidly evolving interactions between parasites and their hosts, predators and their prey, competitors, and mutualists. This process sometimes involves reciprocal evolutionary change in interacting species driven by natural selection, which is called coevolution. We know that the coevolutionary process is responsible for many of the adaptations found in species, and it may also be responsible for many instances of speciation and adaptive radiation. This chapter explores the current hypotheses and results regarding coevolution as a driver of speciation, and the possible contributions of the coevolutionary process to the adaptive radiation of species.
GLOSSARY
Character Displacement. Evolutionary shifts of the ecological traits of two of more competing species in environments in which they co-occur.
Coevolution. Reciprocal evolutionary change among interacting species driven by natural selection.
Coevolutionary Hot Spots. Environments in which selection on interactions between species results in reciprocal evolutionary change; in contrast with cold spots, where selection does not favor reciprocal change.
Cospeciation. A macroevolutionary pattern of speciation in which two or more interacting lineages undergo matched speciation events during their phylogenetic history.
Ecological Speciation. Divergence of populations driven by divergent natural selection among environments.
Geographic Mosaic Of Coevolution. Variation among environments in the structure and strength of selection on interspecific interactions and in the genes and gene combinations under selection
Selection Mosaic. Divergent natural selection on interspecific interactions driven by differences in the expression of genes or the ecological outcomes of interactions among environments.
Symbiont-Induced Speciation. Speciation in host species driven by divergence in interactions between host and symbiont populations.
1. COEVOLUTION AND THE DIVERGENCE OF SPECIES INTERACTIONS
Much of the diversity of life is a result of the diversity of interactions among species. Many of the morphological, physiological, behavioral, and life history traits that we use to distinguish species from one another are traits involved in their interactions: the various forms of flowers, the beaks of birds, the running abilities of mammals, and the warning colors of many toxic invertebrates. It is therefore likely that much of the diversification of life may be the result of speciation driven by interactions among species (see also chapters VI.4, VI.10, and VI.16). Nevertheless, it has turned out to be much simpler to demonstrate that species adapt to one another than to demonstrate that interactions among species cause speciation. Part of the problem is time. Although adaptive change can occur in some interactions over a decade or even less, speciation is a much longer process.
The crux of the problem is to understand the extent to which speciation is ecological speciation—that is, a direct result of divergent natural selection acting on populations living in different environments. If speciation is a continuation of the process of adaptation, then studies of divergent adaptation of populations are studies of the process of speciation. The evidence for that view has been increasing steadily. In recent years, multiple studies have provided direct and indirect evidence that natural selection commonly plays a central role in speciation (see chapter VI.4). Many examples involve divergent selection imposed by interactions with other species, such as plant-feeding insect populations adapting to different host plants, or fish populations adapting to environments that differ in competition and predation.
Where speciation is driven by interactions with other species, geographic differences in the structure and strength of coevolution may contribute to the process. There is no reason to assume that all interspecific interactions coevolve, but when coevolution does occur, it may differ among populations living in different environments. Moreover, divergent coevolution among populations may increase the overall rate of adaptive divergence among populations over what would occur if populations were adapting only to their physical environments. When a population adapts to a physical environment, selection can often act to improve adaptation of the population to that particular range of physical conditions. A population is able, over time, to climb toward an adaptive peak. But as a population of one species adapts to a population of another species, the environment (i.e., the other species) often becomes a moving target; the adaptive peaks continue to change. Each adaptation in the first species can produce a counteradaptation in the other species. If populations coevolve in different ways in different places, then the coevolutionary process can rapidly lead to multiple highly divergent populations.
More broadly, coevolution can fuel the divergence of populations through three sources of variation in interspecific interactions: geographic selection mosaics, coevolutionary hot spots, and trait remixing. Any interaction between two or more species can be characterized as a genotype by genotype by environment interaction (G×G×E). The fitness of any genotype in one species will often depend not only on the distribution of genotypes found in the local population of the other species but also on the particular environment in which the interaction takes place. The expression of genes often differs among environments, making some traits more effective in some environments than in others. In addition, the ecological and evolutionary outcomes of interactions among species are bound to differ among environments, because the surrounding web of life will affect how any two species interact with each other.
The result is a selection mosaic across landscapes as natural selection favors different traits in different environments. An interaction may be antagonistic in all environments, or mutualistic in all environments, but natural selection can favor different defenses, counterdefenses, or mutualistic traits in different environments. Ecologically important selection mosaics have been demonstrated for multiple interactions. These selection mosaics sometimes include coevolutionary hot spots, where reciprocal selection on interacting species is strong, and coevolutionary cold spots, where selection is nonreciprocal. For example, interactions between some woodland star (Lithophragma spp.) species and their pollinating Greya moths are mutualistic in environments where copollinating bees and bee flies are rare, but commensalistic or even antagonistic in some environments where copollinators are common. In addition, the geographic mosaic of coevolution is further fueled by new beneficial mutations that appear only in some populations, move through gene flow to some populations but not to others, become lost in some populations because of random genetic drift, and continually shift in occurrence and frequency among populations owing to metapopulation dynamics. The combination of traits available for coevolution therefore becomes continually remixed, providing further fuel to coevolutionary change.
The geographic mosaic of coevolution that results from selection mosaics, coevolutionary hot spots, and trait remixing sets the stage for ecological speciation. At the extreme, divergent natural selection may pull mutualistic interactions in one or more directions; antagonistic interactions in multiple other directions; and commensalistic interactions in yet other directions. Multiple interactions have been shown to coevolve as a geographic mosaic. Examples include toxic newts and garter snakes that differ geographically in the levels of tetradotoxin in the newts, and detoxification abilities in the snakes; wild parsnips that differ geographically in the levels of multiple defensive furanocoumarins, and parsnip webworms that differ geographically in the combinations of P450 gut enzymes that detoxify these compounds; and Australian wild flax and its Melampsora rusts that differ geographically in the genes involved in defense and counterdefense in these gene-for-gene interactions. Collectively, divergent selection among populations found in multiple studies suggests that the geographic mosaic of coevolution can, in fact, fuel the early stages of ecological speciation.
2. SPECIATION WITH CHARACTER DISPLACEMENT
Competition between populations adapting to different environments has been suggested as a major form of coevolutionary interaction driving ecological speciation. Evolutionary theory predicts that characters with the greatest effect on competition between populations will be displaced more than other characters when populations come into contact. Either one or both populations undergo this displacement in ecological characters in regions where they co-occur, and only the latter is considered coevolutionary displacement.
Character displacement can take multiple spatial and temporal forms, and it not yet known which conditions most often result in coevolutionary displacement rather than evolutionary displacement of just one of the co-occurring populations. At one extreme, populations could undergo some adaptive divergence in allopatry, then meet again in a hybrid zone during a time of range expansion of both populations. Character displacement would occur within the hybrid zone. Alternatively, two or more populations of the same species could colonize a habitat at different points in time and, hence, have different lengths of time to become adapted to the new environment. Sometime after the first colonist population becomes adapted to the habitat, the other colonist population arrives and imposes selection on the resident population while itself being subject to selection by the resident population. A wide range of intermediate situations is possible in the ways in which allopatric populations become sympatric, sometimes forming complex mosaic hybrid zones. Hybridization within the contact zone could itself contribute to the speciation process as genes introgress from one species into the other and come under selection within their new genomes (see chapter VI.6). A major current problem to be solved in coevolutionary biology is which of these ecological situations involving competition and character displacement is most likely to result in speciation.
Character displacement during sequential colonization of a habitat has been shown in detailed studies of threespine sticklebacks in British Columbia. These coastal fish are commonly found in nearshore saltwater environments, but during the Pleistocene some populations colonized nearby freshwater habitats and became trapped. In some lakes, a secondary colonization occurred, and the two populations diverged into a benthic form and a limnetic form. Sympatric populations of benthic and limnetic forms have now been found in five lakes, and each sympatric pair appears to have resulted from a separate sequential set of colonizations followed by character displacement. The ancestral populations are thought to have been pelagic marine forms, which resemble the limnetic form found in the lakes. Limnetic fish have a slender body, extensive body armor, and many gill rakers. In contrast, the benthic form is less slender, has reduced body armor, and fewer gill rakers. The two forms show a strong tendency to mate with others of the same morphological type. Multiple additional studies have shown that these two forms are morphologically, genetically, and ecologically distinct in ways consistent with divergence from a common ancestor, followed by character displacement in the lakes where they have come into contact.
Repeated character displacement can result in an adaptive radiation of species, as has been shown in Anolis lizards in the Lesser Antilles. These lizards use a wide range of habitats, and they have diverged in size and shape as they have evolved to live mostly in one of three habitats: on the ground, the lower trunks of trees, or the upper tree canopies, creating what are often called ecomorphs. On Cuba, Hispaniola, Jamaica, and Puerto Rico, these lizards have diverged repeatedly into predictable ecomorphs. Each island has between four and six morphs, and phylogenetic analyses have shown that most morphs originated after colonization of each island. The exact sequence of morph divergence on each island is not known, and it is therefore not yet possible to determine the fraction of morphs that originated through reciprocal evolutionary change rather than through evolutionary change in later colonizers adapting to the range of morphs already in place. It is clear, though, that divergence among populations of these species has been driven by selection repeatedly favoring character displacement among coexisting populations on these islands.
3. PREDATORS, PARASITES, AND DIVERSIFICATION
Interactions between trophic levels may be as important a driver of speciation as competition within trophic levels. If divergence in ecologically important traits represents the first stage of most speciation events, then many species show evidence of incipient speciation driven by antagonistic interactions between tropic levels. There are now multiple studies showing that interactions between predators and prey, or parasites and hosts, have resulted in divergent adaptation among populations. Some of the best-studied examples are of plant-feeding insects in which different populations have adapted to different plant species, forming what are commonly called host races. In some insect groups, speciation has been attributed directly to shifts of these populations onto new plant species. In other taxa, there is clear evidence that defenses and counterdefenses have escalated more in some populations than in others.
Speciation appears to be driven both by antagonistic trophic interactions and by competition, as has been shown in the interactions among conifers, squirrels, and crossbills on multiple continents. Squirrels selectively harvest conifer cones that are easy for them to handle, and they cache large quantities of these cones. Where squirrels are abundant, multiple conifer species have evolved cone sizes and shape that are difficult for the squirrels to handle and extract seeds. In some regions where squirrels are absent, crossbills are the major seed predators of conifers, and in these regions conifers have evolved cone structures that are difficult for the crossbills to handle. The result has been a geographic mosaic of coevolution in which some populations of some conifer species have coevolved only with squirrels, others only with crossbills, and yet others with squirrels and crossbills together.
Geographic divergence in the crossbills has been even more extreme. The bill of a crossbill is a precision tool that aids the bird in prying apart the scales of closed cones to reach the seeds. Different populations of crossbills have evolved differ bill sizes and shapes to specialize on the cones of different conifer species. Some crossbill populations show evidence of extreme adaptation to the local population of particular conifer species. In some outlying regions of the North American Rocky Mountains where squirrels are absent, local populations of crossbills and conifers have coevolved to such an extent that the crossbills are regarded as separate species. These birds differ from other crossbills in their bill morphology and their songs.
These studies of crossbill speciation have shown that population divergence can be driven by geographic differences in the unique defenses and counterdefenses found in different populations, sometimes mediated by competition imposed by yet other species. Much work remains to be done to determine how antagonistic trophic interactions and competition work together to drive divergence and speciation. The current studies, though, show that geographic differences in the complex web of antagonistic differences may be a powerful force in speciation.
4. MUTUALISTIC NETWORKS AND SPECIATION
Ecologically important mutualisms among free-living species occur in all major ecosystems. Among the most evident are the interactions between plants and their pollinators and seed dispersers in terrestrial environments, and cleaner fish and host fish in the oceans. In many of these interactions, individuals interact with multiple other individuals of the other species during the course of a lifetime. These interactions therefore differ from symbiotic mutualisms, in which two mutualistic individuals live in intimate contact for an extended period of time. The distinction between mutualisms involving free-living species and symbiotic mutualisms is important for our understanding of speciation, because symbiotic mutualisms often involve symbionts that become adapted to a single host species, whereas nonsymbiotic mutualisms favor the evolution of multispecies webs. Reciprocal specialization between pairs of species is therefore uncommon in coevolved mutualisms among free-living species.
As mutualisms among free-living species continue to coevolve, they often draw additional species into the interaction. Species converge over time in traits that allow them to exploit the interaction. The process often favors the formation of groups of unrelated species that have all converged to exploit a group of relatively closely related species. Examples include plants from multiple families that have converged in their floral traits to attract hawkmoths or hummingbirds as pollinators. At the same time, these insects and birds continue to evolve to exploit these groups of plants with similar floral traits.
Because these interactions tend to form multispecies networks rather than pairwise interactions, it is often more difficult to study exactly how coevolution has shaped speciation. At the broadest evolutionary scale, however, it seems clear that pollinators are a driving force in the divergence of plant lineages, because these animals are directly responsible for plant reproduction through their movement of pollen among plants. It is equally evident that plants have shaped the diversification of pollinators, as can be seen in the diversification of insect mouthparts and bird bills as tools for extracting nectar.
When reciprocally specialized interactions have evolved between pairs of pollinators and plants, other aspects of the interaction often favor specialization. The most extreme example is pollination by floral parasites: adult pollinators lay their eggs in the flowers of host plants while pollinating the flowers, and the larvae feed on a subset of the developing seeds. Specific examples include the interactions between figs and fig wasps, yuccas and yucca moths, Lithophragma plants and Greya moths, Glochidion plants and their Epicephala moths, and globeflower plants and their pollinating flies. Some plant lineages involved in these interactions include hundreds of plant species, each of which is pollinated by one or a few highly specialized pollinating seed parasites. In these cases, specialization in the insects appears to be driven by the parasitic phase of the interaction. The plant has taken advantage of the egg-laying behaviors of these insects and remolded the parasitic interaction into a mutualistic interaction. Because these highly specialized insects are the sole pollinators of their host plants, they completely control the pattern of gene movement among plants and therefore have the potential to control the pattern of divergence among populations of their host plant.
Some other forms of mutualism also can favor population divergence and possibly speciation by favoring local matching of traits among interacting species. The clearest examples include Müllerian mimicry, in which co-occurring distasteful species converge on a color pattern that warns predators of their toxicity. Müllerian mimicry complexes have evolved in many taxa, including butterflies in terrestrial environments and sea slugs in the oceans. Within each mimicry complex, all species at a locality evolve to use the same visual cues to warn predators, but different populations of these species sometimes converge on different warning patterns in different regions. Hybridization between populations that are members of different mimicry complexes could therefore foster speciation, because selection could disfavor individuals with a warning pattern that falls outside the usually warning cues used by the local predators. The role of hybridization in the formation of multispecific mimicry complexes remains an active area of research.
5. COEVOLVED SYMBIONTS AND SPECIATION
All major ecosystems are built on a web of coevolved mutualistic symbioses, and we are still at the early stages of understanding how these symbioses affect diversification in the web of life. In terrestrial environments, major mutualistic symbioses include lichens, which are coevolved interactions between fungi and algae; mycorrhizae, which are interactions between fungi and the roots of most terrestrial plants; and rhizobia, which are interactions between nitrogen-fixing bacteria and the roots of some plants. Tropical communities are dominated by termites, which rely on their coevolved gut symbionts to digest cellulose in their diet, and larger plant-feeding animals rely on the symbionts in their gut for many aspects of digestion. In Central and South American forests, leaf-cutter ants cultivate particular species of coevolved fungi for their food. The richest marine environments are those dominated by corals and the symbiotic zooanthellae that provide nutrients to these corals. And deep-sea vents are built on coevolved interactions between invertebrates and the symbiotic microbes that take over the role of photosynthetic organisms in these lightless environments. Even more broadly, the worldwide proliferation of complex organisms has relied on mitochondria and chloroplasts, which are the products of ancient coevolved interactions. Much of the web of life is therefore a result of the diversification of coevolved interactions that transform inorganic compounds or poorly digestible organic compounds into food and energy.
How these symbiotic interactions affect speciation either in the microbial symbionts or their hosts is one of the questions at the frontier of speciation theory, because it was not possible even to begin exploring genetic divergence in these interactions until the advent of advanced molecular methods in recent decades. So far these methods have shown that the diversity and specificity of microbial symbionts is much greater than previously imagined. All eukaryotic organisms harbor a wide range of mutualistic and potentially pathogenic microbial species. It is easy to understand that hosts offer such different environments that these are likely to be important drivers of speciation in symbionts. It is also likely that symbionts are at least sometimes involved in host speciation, but studies of symbiont-induced speciation are still in their early stages.
One way in which coevolved symbionts might affect speciation is through distortion of sex ratios in their hosts. Bacteria in the genus Wolbachia were discovered just several decades ago. Since then, surveys have suggested that they occur in the cells of the majority of insect species and in a wide range of nematodes, interacting with their hosts as antagonists in some cases and as mutualists in others. Wolbachia, and some similar symbionts, often cause partial reproductive isolation between Wolbachia-harboring host populations and those that either lack these bacteria or have different strains of the bacteria. This partial reproductive isolation could serve as the initial stage of symbiont-induced speciation. It has long been suggested that subsequent coevolution between these symbionts and their hosts could drive speciation among host populations, because the host populations would rapidly diverge in genes favored by natural selection to mitigate the negative effects of the bacteria on host reproduction. Only recently, however, have studies of these interactions begun to analyze how partial reproductive isolation caused by these symbionts could drive speciation among populations during the early stages of population divergence.
At an even broader level, the maintenance of sexual reproduction itself has been attributed in part to natural selection favoring sexual organisms, which are better at keeping pace with coevolving parasites than asexual organisms. An asexual female produces offspring that are genetically identical, except when rare mutations occur. In contrast, a sexual female produces offspring that are each genetically unique. Sexual females are therefore more likely to produce offspring with novel genotypes to which local parasites are not adapted. Much of the process of speciation in eukaryotes is about the development of reproductive isolation among sexual populations, since most eukaryotic species are sexual. Hence, coevolution could lead to speciation not only by driving divergent adaptation among populations but also by favoring the process of sexual reproduction itself. Sexual reproduction then opens opportunities for assortative mating that, in turn, make speciation possible in sexual species. Hence, whether directly or indirectly, coevolution, sexual reproduction, and speciation are linked as driving forces in the diversification of life.
6. ESCAPE-AND-RADIATE COEVOLUTION
Coevolution has the potential to drive even large adaptive radiations by favoring speciation into new adaptive zones, thereby creating macroevolutionary patterns from microevolutionary processes. The leading hypothesis was first proposed by Paul Ehrlich and Peter Raven and is often called escape-and-radiate coevolution. It is a three-step process that was initially described for the adaptive radiation of plants and butterflies, but it could readily apply to any two or more lineages of interacting species. The process begins with one or more mutations in a host or prey population that allows individuals to escape attack from enemies. That mutant population then expands its geographic range and undergoes a starburst of speciation in the absence of the interaction as it colonizes a wider range of environments. Eventually, a mutant parasite population overcomes the new host defenses and radiates in species, with each new parasite species specializing on one or more of the many hosts now available to it. The process then repeats itself.
The result of escape-and-radiate coevolution is a temporal series of alternating starbursts of speciation on both sides of the interaction, forming entire clades with new defenses and counterdefenses. This view of coevolution makes clear predictions. First, novel defenses will occur among, rather than within, clades, because defenses accumulate starburst by starburst rather than species by species. Second, parasites will not colonize hosts within each host clade in any systematic fashion, because there is no ancestral-descendant pattern of accumulation of defenses within each starburst of host speciation. Rather, the pattern of escape-and-radiate coevolution appears at higher taxonomic levels, where each starburst of species on one side of the interaction is matched later with a starburst of speciation on the other side. In fact, different taxa could be involved at different points in the radiation of defenses and counterdefenses in this form of coevolution-mediated speciation.
Escape-and-radiate coevolution was the first hypothesis to explain how coevolution could affect not only speciation but also major patterns in the adaptive radiation of entire lineages. It has inspired a great deal of research on how interspecific interactions might drive speciation, and it remains a major framework for thinking about how the process of reciprocal selection might shape the web of life at multiple levels. It is, however, a difficult hypothesis to test for any particular group of interacting lineages, and it is only one of many ways in which reciprocal evolutionary change can drive the diversification of interacting lineages. Perhaps its greatest impact on evolutionary research has been to show that the coevolutionary process will often not result in a macroevolutionary pattern of matched speciation events during the phylogenetic history of interacting species.
7. COSPECIATION
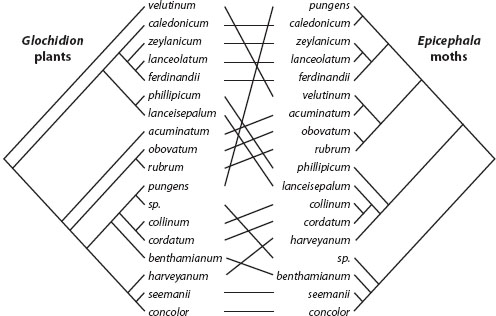
The extreme alternative to escape-and-radiate coevolution is cospeciation, or parallel cladogenesis, in which each speciation event on one side of an interspecific interaction is matched with a speciation event on the other side of the interaction (figure 1). This result is impossible with escape-and-radiate coevolution, because parallel cladogenesis at the species level cannot occur during reciprocal starbursts of speciation.
Figure 1. Cospeciation in Glochidion plants and their coevolving pollinating floral parasitic moths in the genus Epicephala. In perfect cospeciation, each speciation event in either the plants or the moths would be matched with a speciation event in the other lineage. In this case, the plants and moths show an overall pattern of cospeciation with occasional shifts of the moths onto more distantly related plants. (Redrawn from A. Kawakita, A. Takimura, T. Terachi, T. Sota, and M. Kato. 2004. Cospeciation analysis of an obligate pollination mutualism: Have glochidion trees [Euphorbiaceae] and pollinating Epicephala moths [Gracillariidae] diversified in parallel? Evolution 58: 2201–2214.)
The growing number of studies of the geographic mosaic of coevolution suggests that cospeciation should be uncommon in truly coevolving species. Species coevolve as sets of genetically distinct populations that have their own patterns of adaptation, coadaptation, and rates of divergence. As species coevolve, they can undergo speciation at different rates, and they can differ in the tendency of some populations to shift their interactions to yet other taxa. One local population could coevolve strongly with a particular species, while other populations could coevolve with yet different species. Some populations could coevolve equally with two or more closely related species, and occasionally, a population could even shift its interactions to a taxon phylogenetically far removed from the usual coevolutionary species partners. The long-term pattern of coevolution results from the complex mix of successes and failures of these many different coevolutionary experiments spread among all the populations within those species.
There are, however, three situations that favor cospeciation. One is coevolution of maternally inherited mutualistic symbionts and their hosts. Among the clearest examples are those involving obligate symbionts that provide nutrients required by their host. In these interactions, the mutualistic symbionts are often transmitted directly from mothers to their offspring during host reproduction. As a result, population divergence in the host results directly in population divergence in the symbiont. In some cases, bacterial symbionts have diverged in parallel with their insect hosts over millions or tens of millions of years.
The second situation that favors cospeciation is the tracking of hosts by maternally inherited commensalistic, rather than mutualistic, species. In these interactions, the species have not truly coevolved through reciprocal selection. Instead, the species have simply codiverged as their shared environment has fragmented over time. Each local commensal population diverges with its local host from other geographically separated populations. It is an important process contributing continuity in the structure of species assemblages.
The third situation that favors some degree of cospeciation is the special case of pollinating seed parasites. This specialized form of pollination favors highly host specific insect pollinators. Since these pollinators completely control the pattern of movement of pollen among plants, speciation in the plants follows from the pattern of specialization and speciation in the pollinators. Even so, host shifts sometimes occur, leading sometimes to overall cospeciation but with occasional shifts of pollinators to distantly related host plants (figure 1).
In general, few lineages show sustained cospeciation with other lineages. Over the history of a lineage, species often have repeated opportunities to shift their habitats and also their preferences in their interactions with other species. For example, feather lice of birds have switched among avian orders at least twice during their evolutionary history, and the obligate marine worms that live within echinoderms have undergone occasional shifts onto new host lineages during the several hundred million years of their association.
Most codivergence of interacting lineages probably involves a complicated mix of ecological and evolutionary processes. Some species sharing the same habitats will codiverge in some regions and not in others. Some coevolving populations will cospeciate, while other populations of one of the partners will switch their interactions to other species. The result is constantly varying degrees of codivergence of interacting species at different geographic scales and timescales. A well-studied example is the divergence of coevolving leaf-cutter (attine) ants and the fungi they cultivate as food in their fungus gardens. These fungi are directly transmitted by the ants to new colonies generation after generation, creating the opportunity for codiversification of these ants and their symbiotic fungi. Although attine ant and fungal lineages show overall patterns of codiversification, over millions of years there have been multiple instances of acquisition of new fungi by some attine species and occasional shifts of fungal cultivars among attine lineages.
8. CONCLUSIONS
Coevolution is one of the major processes driving the adaptive divergence of populations, shaping interactions between species in different ways in different environments. Coevolution also has the potential to drive population divergence faster than does adaptation only to different physical environments. There is, however, much we still do not know about the genetic and ecological mechanisms by which different forms of coevolutionary selection contribute to speciation and adaptive radiation.
FURTHER READING
Benkman, C. W. 2010. Diversifying coevolution between crossbills and conifers. Evolution: Education and Outreach 3: 47–53. Summarizes research into one of the best-studied examples of how coevolution beween predators and prey may drive speciation.
Futuyma, D. J., and A. A. Agrawal. 2009. Macroevolution and the biological diversity of plants and herbivores. Proceedings of the National Academy of Sciences USA 106: 18054–18061. Reviews the variety of ways in which interactions between plants and herbivores shape large scale patterns in diversification.
Kay, K. M., and R. D. Sargent. 2009. The role of animal pollination in plant speciation: Integrating ecology, geography, and genetics. Annual Review of Ecology and Systematics 40: 637–656. Discusses the mechanisms by which pollinators can shape patterns of speciation in plants.
Losos, J. B. 2010. Adaptive radiation, ecological opportunity, and evolutionary determinism. American Naturalist 175: 623–639. Provides an overview of how environmental heterogeneity and species interactions can shape adaptive radiation.
Moran, N. A., J. P. McCutcheon, and A. Nakabachi. 2008. Genomics and evolution of heritable bacterial symbionts. Annual Review of Genetics 42: 165–190. Describes the pervasiveness of bacterial symbionts in eukaryotes and their potential roles in speciation.
Pfennig, D. W., and K. S. Pfennig. 2010. Character displacement and the origins of diversity. American Naturalist 176: S26–S44. Considers how competition between species can drive the process of speciation and the diversification of lineages.
Schluter, D. 2009. Evidence for ecological speciation and its alternative. Science 323: 737–741. Summarizes current views on how divergent natural selection among environments, especially in species interactions, can lead to speciation.
Schluter, D. 2010. Resource competition and coevolution in sticklebacks. Evolution: Education and Outreach 3: 54–61. Summarizes research into one of the best-studied examples of how character displacement driven by competition and predation may lead to speciation.
Segraves, K. A. 2010. Branching out with coevolutionary trees. Evolution: Education and Outreach 3: 62–70. Provides an overview of the processes shaping phylogenetic diversification, including the process of escape-and-radiate coevolution.
Thompson, J. N. 2005. The Geographic Mosaic of Coevolution. Chicago: University of Chicago Press. Synthesizes research showing how coevolving interactions diverge among environments, leading to local adaptation and ecological diversification.