Sexual Selection and Its Impact on Mating Systems
Rhonda R. Snook
OUTLINE
1. What are mating systems and why are they important?
2. Measures of mating systems
3. Plastic, continuous mating systems and the evolution of behavior
4. Mating systems and evolutionary potential
5. Applied relevance of the study of the evolution of mating systems
In sexually reproducing animals, males and females interact for mating, and the way in which they interact defines an organism’s mating system. Mating systems reflect the action of sexual selection, including sexual conflict, and can be quantified. Mating systems also determine a population’s evolutionary potential and perhaps its ability to respond to anthropogenic changes.
GLOSSARY
Effective Population Size (Ne). The number of individuals that can contribute genes equally to the next generation. This may not equal the census population size.
Environmental Intensity of Sexual Selection. A measure of the degree of competition for mates, which is dependent on the potential reproductive rate (PRR) of each sex and which influences the operational sex ratio (OSR).
Operational Sex Ratio (OSR). Average ratio of fertilizable females (or more generally, the limiting sex) to sexually active males (or more generally, the competing sex) at any given time.
Opportunity for Sexual Selection. Frequently used as a synonym for the intensity of sexual selection but more correctly defined by an equation in which it is equal to the total variance in reproductive success divided by the square of the mean reproductive success, that is, Imates.
Potential for Polygamy (EPP). The degree to which multiple mates, or resources critical to gaining multiple mates, are economically defendable.
Potential Reproductive Rate (PRR). Has multiple definitions; the maximum number of independent offspring that parents can produce per unit time when mating partners are freely available but all other constraints in terms of environmental factors (such as food, number and sizes of nest sites, temperature) remain. The sexual difference in PRR in the population then influences the OSR and has to be estimated experimentally for a sample of the population.
Strength of Sexual Selection. Frequently used as a synonym for intensity of sexual selection.
1. WHAT ARE MATING SYSTEMS AND WHY ARE THEY IMPORTANT?
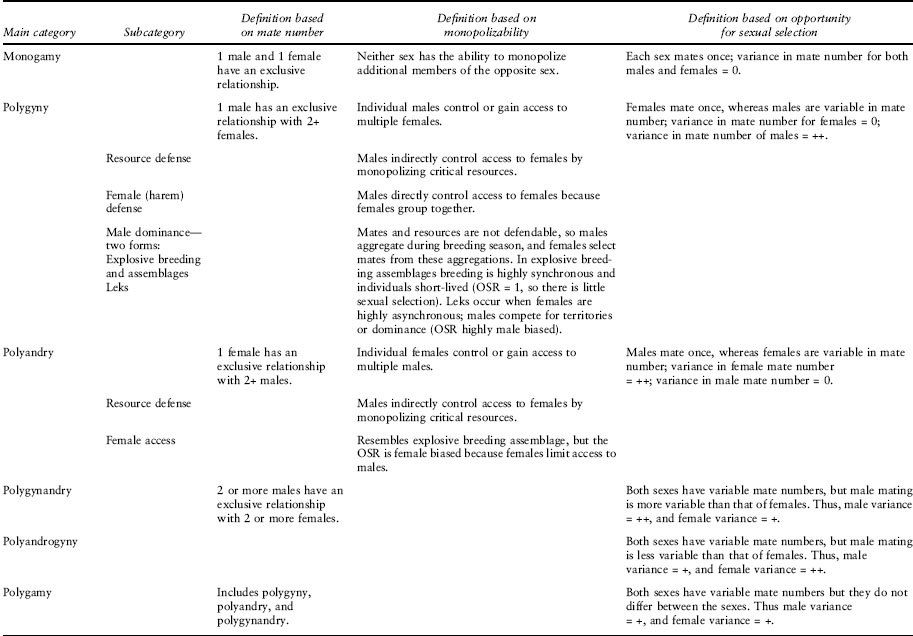
The simplest definition of a mating system for either plants or animals is a description of who mates with whom and when. Beyond this general description, however, discussions of animal and plant mating systems diverge. In plants, genetic relationships define the mating system and capture the degree of outcrossing (see chapter IV.8). In animals, mating system descriptions include the general behavioral strategy employed in obtaining mates, encompassing not only the number of mates acquired and when but other features such as the manner of mate acquisition and the patterns of parental care provided by each sex. Despite such a simple general definition, understanding the evolution of mating systems is complex. This complexity arises because mating system descriptions (e.g., monandry, polygyny; table 1) are idealized, and their definitions are often inaccurate; social and genetic mating systems are not necessarily exclusively the same, and the same species can exhibit plasticity of mating systems across or even within populations. Moreover, methods for quantifying the nature of selection that contributes to the evolution of mating systems are contentious.
The study of mating systems, for both animals and plants, began—like much of the study of evolution—with Charles Darwin. In this context, Darwin contributed his second idea about how evolution works, that of sexual selection. Darwin recognized that the marked sexual dimorphism—one of the most striking and easily observed of natural phenomena—seen in many animals could be contrary to the action of natural selection and thus would undermine his theory of evolutionary change. To resolve this conundrum, he suggested that individuals struggle not only to exist but also to acquire mates, and this selection results in sex-limited traits that are used to either compete for or choose mates. Developments in the 1970s and 1990s extended this arena of competition to sperm-egg interactions, so the modern definition of sexual selection includes that arising owing to competition not only for access to mates but also for fertilization opportunities (see chapters VII.5 and VII.6). The inherent differences between the sexes can lead to conflicts of interest between males and females in evolutionary outcomes; this sexual conflict is now embedded in sexual selection and the evolution of mating systems.
Darwin described animal mating systems using the now-familiar descriptions of monogamy and polygamy. He also intuitively realized that polygamous species, relative to monogamous species, should experience stronger sexual selection because there is a greater opportunity for increased variation in mating success among males in polygamous species. Yet he also believed that most animals exhibited monogamy. Darwin’s prejudiced notion leads to an illogical conclusion using his ideas. If the intensity of sexual selection is positively related to the extent of sexual dimorphism, and sexual dimorphism is so conspicuous, then why would most taxa be monogamous? Biologists now recognize that Darwin was wrong about the extent of monogamy; through better observation and molecular parentage tools—and perhaps a more accepting society—most animal systems are now known to be polygamous to some degree. However, this fact raises another question. Since we now generally have a catalog of the mating systems of taxa, what is the point of modern studies of the evolution of mating systems? What is left to understand?
There are several research questions that are fundamental, but unresolved, regarding the evolution of mating systems; we focus on three. First, while it is generally agreed that the intensity of sexual selection influences the mating system, how to measure sexual selection and therefore how to quantify and predict which mating system will be observed is controversial. Second, mating systems can affect the evolutionary potential of a population through a variety of mechanisms. However, these effects are predominantly theoretical and tested across a limited set of taxa. Third, anthropogenic changes via spatiotemporal changes in resource availability may alter mating systems. Such changes in the mating system can alter genetic variability, potentially affecting the ability of a population to respond to environmental change, but few studies have assessed the relationship among these factors.
2. MEASURES OF MATING SYSTEMS
The modern focus on mating systems is couched in understanding how sexual selection acts on a population. Sexual selection arises from differential access to reproductive opportunities. Such selection occurs in two ways: among individuals of one sex for access to the other sex (intrasexual selection; competition; see chapter VII.5) and between the sexes for choice of mate (intersexual selection; mate choice; see chapter VII.6). The strength of sexual selection can be measured using different, highly debated methods, but in essence the larger the variance in mating success experienced by one sex, the stronger the sexual selection on that sex. Thus, theory predicts that sexual selection should be a much stronger evolutionary force in taxa in which some individuals of one sex are successful at both mating and preventing other individuals of the same sex from reproducing. Such a pattern occurs in polygamous compared with monogamous mating systems.
In 1977 the first attempt at a unified evolutionary hypothesis to explain variation in animal mating systems was put forward by Stephen Emlen and Lewis Oring. Their model, herein called the E and O model, is descriptive, based on the spatiotemporal distribution of receptive mates and/or the resources used to monopolize mates. The model arises from Darwin, and its subsequent development uses economic cost-benefit analyses, applied to ecology. Darwin wrote that competition for access to mates occurs because one sex is a limiting factor for the other; one sex competes among its members for a limiting resource, leading to variance in mating success for the nonlimiting sex. Thus, understanding why different taxa exhibit different mating systems is, in essence, a process of determining why in certain species one sex is less of a limiting resource than in other species. Robert Triver’s parental investment theory, based on A. J. Bateman’s work in Drosophila, suggested that because females invest more in parental care (including the initial cost of larger eggs—anisogamy), females are the limiting resource, and therefore males compete for them.
The E and O model suggests that male variation in mating success, the extent of which influences the intensity of sexual selection, reflects the ability of males to control (or not) access of others to potential mates. This monopolization may be over females themselves or over resources that females may require. Resources vary in space and time, and thus ecological constraints on the sexes determine the intensity of sexual selection and the mating system. The original intention of the E and O model was to allow for predictions regarding how the environment, through ecological constraints, results in the evolution of animal mating systems. To make the verbal model predictive, they introduced two terms that allowed mating systems to be identified: operational sex ratio (OSR) and environmental potential for polygamy (EPP). Emlen and Oring argued that the OSR represents an empirical measure of the potential for monopolization. If the OSR is close to unity, then monopolization is unlikely, whereas if the OSR is skewed toward one sex, then that sex has the opportunity to monopolize the other sex. The OSR may be predicted by the potential reproductive rate (PRR) of each sex. However, EPP has no quantitative definition and therefore may have limited use in the ability to predict mating systems.
Using the concepts of OSR and EPP, the E and O model classifies mating systems into four simple categories: monogamy, polygyny, rapid multiple clutch polygamy, and polyandry (table 1). Both polygyny and polyandry contain subcategories of mating systems (table 1). These categories represent a description of the degree to which a sex can monopolize resources. Thus in monogamy, neither sex has the opportunity for monopolization, whereas in resource defense polygyny, males have the opportunity to control access to critical resources required by females and thereby indirectly control access to females. In the former category, there will be little sexual selection because the OSR is not skewed toward one sex, but in the latter the intensity of sexual selection will reflect the extent to which the OSR is skewed—the greater the skew, the greater the variation in mating success, so the stronger the sexual selection. The influence of the OSR on variance in mating success depends on the economics of the resource defense. If a resource becomes too expensive to defend, then the mating system may move away from polygyny and toward monogamy.
Table 1. Major categories of mating systems using three different classification schemes

Much of the E and O model is intuitive, and it enjoyed considerable success, especially early on. However, despite the model’s intuitive appeal, there are concerns with it that have been extensively discussed elsewhere. Generally, there are two main problems: the OSR is an incomplete predictor of mating systems, and it takes into account only a subset of the population. Use of only the OSR is insufficient to predict the mating system because EPP is not quantitative; in other words, the relationship between OSR and whatever environmental factors affect monopolization of females is not always positive. For example, in the European bitterling fish, larger males are territorial, and most spawning involves breeding pairs. However, if male density is high, the EPP is reduced because the economical defendability of large territories is untenable. Thus, the mating system changes from one of resource defense polygyny to that of scramble (or explosive breeding assemblage) competition, in which large males abandon territoriality, and group spawning occurs. Counterintuitively, the relatively less male-biased OSR of the population during resource defense polygyny has a larger variance in male mating success than when the population has a more male-biased OSR, when a scramble-based mating system is used and there is less variance in male mating success. While the E and O model predicts this pattern, the dual issues are that the verbal model provides no way to quantify when this switch will happen because EPP is not an empirical measure, and the relationship between OSR and variance in male mating success is not always positive. Therefore, the OSR itself cannot necessarily accurately predict the strength of sexual selection and therefore cannot predict the mating system.
The second problem with using OSR as a predictor of mating systems is that it takes into account only breeding individuals at particular times. Nonreproductive males are not included, which is fundamental to the theory, but this omission has two consequences for understanding a population’s response to sexual selection. First, this exclusion causes errors in the calculation of the strength of sexual selection by overestimating population fitness and underestimating variance in fitness. Under strong sexual selection, more individuals are left out of the equation, and these errors become larger. Thus, the predicted mating system, which is based on variance in mating success of one sex, will be underestimated. Second, OSR is an instantaneous measure, and it can change across the breeding season; the effect of this change depends on its magnitude and whether such changes occur relatively early or late in the breeding season.
In an effort to make sexual selection quantifiable, and therefore mating system evolution more predictable, several attempts have been made to move away from the proxy measurement of the OSR to “direct” measures of selection explicitly linked to evolutionary theory (that is, to fitness). Of the several indexes suggested, two have proven most popular: Bateman’s gradient (βss) and the opportunity for sexual selection (Is). Both these measures are consistent with quantitative genetic theory and measurement of selection, and are independent of phenotypic traits. Moreover, they are more general measures of the strength of sexual selection than indexes that require knowledge of the phenotypic trait putatively under selection (e.g., selection gradients, β).
Bateman’s gradient is a selection differential, describing the covariance between values of a particular trait and fitness. In the case of understanding sexual selection and the evolution of mating systems, the particular trait of interest is the number of mates, assuming that higher mating success results in more offspring (i.e., greater reproductive success). If the regression of fitness on mating success gives a gradient of zero, then sexual selection is typically nonexistent. In contrast, if the gradient is steep, then sexual selection will act strongly on some trait correlated with mating success. Thus, it has been suggested that βss provides an estimate of the strength of selection acting on mating success. However, subsequently understanding why either populations or species have different Bateman gradients requires uncovering the cause both of differences among individuals in their ability to acquire mates and of nonzero Bateman gradients.
The opportunity for sexual selection (Is) measures the standardized variance in mating success and is an upper limit of the strength of sexual selection. One proposed advantage to this measure is that it may predict the level of sexual dimorphism as an outcome of sexual selection. While described by some as being a direct measure of selection, it is actually still a proxy, because although Is represents the upper limit of selection, there is little knowledge regarding the relationship between it and the actual strength of selection. Thus, the extent to which Is can predict the mating system is unclear.
Both these measures require knowing mating success. In cases where this parameter is not known, Stephen Shuster and Michael Wade have suggested that calculating how critical resources, particularly females, are clumped in space and time may serve as a measure of the strength of sexual selection, and they use the concept of mean crowding to quantify these variables as m* and t*, respectively. Essentially, these measures enumerate EPP, making the E and O model quantitative. High values of m* reflect spatially clumped resources, whereas low values indicate overdispersion; high values of t* reflect temporal invariability (e.g., synchronous breeding), whereas low values indicate temporal variation. Thus high values of m* and low values of t* represent conditions in which one sex would have the potential to monopolize resources, the opportunity for sexual selection would be great, and the mating system would reflect one based on resource or defense. The contrasting values would represent little ability for one sex to monopolize resources, a low opportunity for sexual selection, and a mating system tending toward monogamy.
These different measures of sexual selection, along with other less used suggestions not discussed here, are contentious. Some measures may be more suitable than others under different conditions. However, studies directly comparing these indexes have reached different conclusions about the congruency between them. Seemingly, the only idea with which everyone agrees is that no current specific measure or combination of measures used to quantify sexual selection, and thus mating systems, satisfies everyone.
3. PLASTIC, CONTINUOUS MATING SYSTEMS AND THE EVOLUTION OF BEHAVIOR
There are a number of classification frameworks for mating systems, although the predominant focus is on the number of mates of each sex for each sex (table 1). These frameworks often lack a common terminology, but mating system descriptions also can overlap, be redundant, represent inadequate descriptions, or lend themselves to incorrect usage relative to formal definitions. For example, monogamy is frequently defined as the condition in which each sex has only one partner. However, this is an inadequate description because this could be the situation for an organism’s entire life or for one season, or an individual might raise offspring with only one mate regardless of whether the offspring are sired solely by that mate (as seen in many passerine birds).
Another problem is multiple definitions of the same word. For example, under the general E and O framework (table 1), polyandry is a mating system in which females have variable numbers of mates, while males mate with a single female. In trying to understand female multiple mating, many researchers have asked, What is the evolutionary benefit of polyandry? But in this context, most researchers are following the definition of polyandry provided by Thornhill and Alcock in which polyandry simply means female multiple mating with no conditions regarding the number of mates for males. In most systems, both males and females mate multiply. Perhaps such a system may be described as promiscuous, in which both males and females mate with multiple partners. Yet the term is loaded with connotations about indiscriminate mating, which is the direct opposite of what is being asked by researchers studying the functional significance of polyandry, which is primarily focused on the evolutionary rationale for female choice.
In an attempt to solve these descriptive problems, Shuster and Wade use their framework to further delineate mating systems. While quantitative (although the variables necessary to determine mating system [i.e., m*, t*, female distribution in brood number, sperm competition level] are largely unavailable), their system is rather unwieldy, resulting in 41 different mating systems under 12 different general categories.
So, how many mating system categories are necessary? Categorizing phenomena is something humans do, but doing so implies rigidness to those phenomena. If the E and O model is mostly correct, then spatiotemporal resource distribution is the driving factor in the evolution of mating systems. Given that resources are frequently ephemeral across space and time, mating systems are likely dynamic rather than static. Intraspecific mating system variability has been associated with changes in such factors as population density, as discussed earlier regarding the European bitterling; predation; food availability; and climate change. Thus, a species “mating system” is likely much more flexible than mating system terminology suggests. Problems associated with correct use of mating system terminology may be more than mere nuisances, as these terms give the impression that mating systems are discrete rather than continuous. The Shuster and Wade model most approximates a continuous distribution of mating systems, but whether it is feasible to implement remains debated.
Sexual selection results in the evolution of behavioral, morphological, and physiological traits. Many of these traits can be plastic and exhibit continuous variation. The ability to predict how these traits change in response to underlying environmental variables that control mating systems is critical. The OSR (and sometimes associated changes in density) has frequently been shown to predictably influence male behavior, such as aggression, territoriality and alternative reproductive tactics, mate guarding, copulation duration, and sperm release. Changes in the OSR can also vary female mate choice. These changes in behavior are in response to the ability of males to monopolize females and to the costs and benefits of mating for females.
The ability to monopolize mates at both pre- and postcopulatory stages is one of the driving forces of sexual conflict and sperm competition. A recent meta-analysis asked whether changes in OSR could predict the outcome on different types of intrasexual selection behavior associated with mating systems: direct mechanisms of monopolization via contest and sperm competition, and indirect mechanisms via courtship behavior, copulation duration, and mate guarding. Overall the study found that behaviors change in response to increasing OSR. In particular, contest competition exhibited a humped distribution, initially increasing but then decreasing as the OSR became more male biased. Sperm competition follows this trend. In mating systems that are predominantly determined through direct competition, males may conserve energy when competition gets extreme and the economic defendability of mates is unsustainable—as predicted by the E and O model. In contrast, behaviors that function indirectly to monopolize mates did not exhibit the same response, either to one another or to direct competition. As the OSR became more male biased, courtship rate decreased, whereas mate guarding and copulation duration/attempts increased. Mate guarding and copulation duration (if it serves as mate guarding) may increase, as it benefits a male to ensure his fertilization success when there are many competitors around rather than to seek additional mates that may already have a partner. If males do not have partners, then increasing copulation attempts is the only option for securing a mate. Additionally, per capita courtship rate decreased because males were competing for the same female. One long-term outcome of increasing male OSR is the evolution of alternative mating tactics.
Female mate choice is also predicted to change when the OSR changes. Females should be more selective when the OSR becomes more male biased because there are more males from which to choose. However, as (receptive) males become more prevalent in the population, sexual coercion by males may increase, and sexual conflict can result. Sexual conflict occurs when the reproductive interests of males and females are incongruent; because of sex differences in PRR, the benefit to males of mating with more females is generally linear, whereas the benefit of multiple mating for females is asymptotic. Sexual conflict is thought to be particularly strong over mating decisions because of this sex disparity in optimal mating rate. Thus, under high male density, when males may be persistent for mating opportunities, the cost to females of resisting these attempts may outweigh the benefits of not mating. In this case, females may become more receptive and acquiesce to superfluous matings (i.e., “convenience polyandry”).
These two hypotheses provide contrasting predictions regarding female behavior when the OSR becomes more male biased. If females become more selective, via affecting the proportion of male displays that a female accepts, then female sexual responsiveness should decrease under more male-biased OSRs. However, if female mating rate is determined by male harassment, then female mating rate should increase under greater male-biased OSR. Female water strider mating activity is positively related to male harassment rate, which supports the convenience polyandry hypothesis. The positive association between male harassment rate and female mating was not found in other taxa, however, which suggests that in these taxa the costs of unnecessary matings is greater than the cost of avoiding harassment.
As emphasized earlier, male density and resources frequently change across the breeding season, so a population’s mating system can be variable rather than rigid. This flexibility has potential consequences for such behaviors as male harassment and female mating decisions. This flexibility also may affect the evolutionary trajectory of populations, as discussed in the next section.
4. MATING SYSTEMS AND EVOLUTIONARY POTENTIAL
The two hypotheses about the relationship between female mating rate and male coercion have different effects on the opportunity for sexual selection because this value is partly determined by female choosiness, which in turn is dependent on the costs of choice. Convenience polyandry has been predicted to decrease the opportunity for and strength of sexual selection because females mate indiscriminately to avoid harassment. If male harassment changes across a breeding season, then the strength of sexual selection also changes temporally. In fact, the strength of and opportunity for sexual selection may frequently vary temporally if the mating system is as dynamic as studies suggest. For example, mating systems are known to oscillate between contest and scramble competition. This oscillation will have an impact on the rate at which traits evolve owing to changes in the intensity of sexual selection and perhaps also in the direction of selection. Access to mates via contests usually predicts the evolution of costly exaggerated traits, such as male body size and armaments. Thus in populations experiencing contest competitions, these traits will be directionally selected. However, if male density changes across a breeding season, subsequently altering the mating system to one of scramble polygyny, then these costly traits may not be beneficial and subsequently may be selected against.
Two types of constraints may complicate the ability to predict evolutionary trajectories of selected traits in different mating systems: indirect genetic effects and between-sex genetic correlations. Moreover, if sexual conflict is operating in a mating system, then the nature of that conflict may have different effects on its evolution.
Indirect genetic effects (IGEs) occur when the phenotype of one individual is affected by genes expressed in interacting partners. In the case of sexual selection, the evolution of traits influencing male mating success is a consequence of the male’s own selective history and the environmental constraint posed by the genes of the interacting female. Thus, males and females have interacting phenotypes, and the evolutionary trajectories of these traits are different from those of traits expressed regardless of social context. The effect is twofold: the evolutionary response may not be constrained by depleted genetic variation in one sex, and the strength of the interaction translates into the relative rate of evolutionary change. Both of these relate to the mating system, since more intense sexual selection is predicted to deplete genetic variation (although condition dependence of such traits may mitigate loss of genetic diversity) but also to result in greater phenotypic change owing to stronger interactions between the sexes. While theory on IGEs is well developed, the empirical application to mating system evolution is nascent.
A separate constraint arises because males and females share a common genome. If the homologous trait under selection is controlled by genes expressed in both sexes, then a between-sex genetic correlation occurs in which the evolutionary change in a trait of one sex is dependent on the magnitude of the genetic correlation with, and patterns of selection acting on, the trait in the other sex. Thus, between-sex genetic correlations are expected to influence the evolution of sex differences given the shared genetic basis and evolutionary history of the trait. In particular, if there is sexual conflict, then these correlations can act as a constraint.
However, these potential evolutionary limitations can be mitigated by, for example, the evolution of sex-specific alleles, which is predicted to occur relatively rapidly under intralocus sexual conflict. This conflict arises when each sex has a different fitness optimum for the expression of a shared trait, which can generate sexual antagonistic evolution. Such antagonism can be resolved through the evolution of sex-specific alleles that putatively can engender the evolution of sexual dimorphism, the pattern that Darwin explained through his ideas on sexual selection. Intralocus sexual selection may also alter the evolution of gene expression to resolve sexual antagonism. Many organisms have been shown to have sex-biased gene expression (that is, genes that are sexually dimorphic in their expression), and these genes are rapidly evolving and nonrandomly distributed across the genome.
Interlocus sexual conflict can also affect the evolution of populations with different mating systems. Such conflict occurs when the sexes differ in the fitness outcome of male-female interactions, and traits associated with this interaction are genetically encoded by independent genetic effects. Because males and females can evolve independently, each sex is predicted to evolve traits that enhance its own fitness at the cost of its mating partner’s fitness, which can generate a coevolutionary arms race. Conflict can occur over sex-specific life history traits related to the mating system, such as mating frequency, relative parental effort, reproductive rate, and clutch size. Moreover, costs and benefits of particular responses are environmentally dependent. Thus, sexual conflict can affect the mating system and vice versa. Interlocus sexual conflict is predicted to accelerate adaptive evolution owing to sexually antagonistic coevolution.
Theoretical and empirical results indicate that the sex chromosomes play a strong role in the evolution of sex-specific traits that have been putatively linked to sexual selection. The X chromosome has a smaller effective population size (Ne) than autosomes because males carry only one copy (that is, they are hemizygous) but two autosomes. Under conditions of random mating and equal fitness, the X/A ratio is 0.75. However, this ratio can increase if variance in male reproductive success is skewed, such as in strong sexual selection. This increase occurs because the fewer males that contribute to the gene pool have a greater influence on autosomes than on X chromosomes, since males account for half of all autosomes but only one-third of X chromosomes. A dominant allele that benefits females but is unfavorable to males can be maintained on the X chromosome more easily than on autosomes. In contrast, a recessive X-linked allele that benefits males will immediately be exposed to selection because it is hemizygous, and such alleles should therefore colonize the X chromosome more readily than the autosomes. The nature of X chromosome transmission and the differential effects of different types of alleles in the sexes will facilitate the evolution of X-linked genes related to sexual antagonism and sexual selection. Such patterns have been found in Drosophila.
This section outlined the influence of mating systems on the evolutionary potential of a population through a variety of mechanisms. However, much of the current understanding is theoretical, with relatively few empirical tests in a limited set of taxa, and some of it is controversial. Additionally, different mechanisms for impeding or accelerating evolution within a mating system, let alone across mating systems, are generally not considered simultaneously.
5. APPLIED RELEVANCE OF THE STUDY OF THE EVOLUTION OF MATING SYSTEMS
Mating systems may affect population viability, because the sex ratio influences pair number, which can alter population dynamics. Male-biased sex ratios can result in harm to females and decreased reproductive output. Female-biased sex ratios, either naturally occurring or due to selective harvesting, can lead to mate limitation and extinction. Additionally, as a population becomes small, Ne will diminish, and this result will be compounded in species with intense sexual selection in which only a proportion of the population reproduces. Both theory and experiments have shown that because male armaments and ornaments are expensive, sexual selection carries a genetic load that negatively affects mean population fitness. However, sexual selection could prevent extinction for a variety of reasons, one being that it accelerates the rate of adaptive evolutionary change. Yet experiments designed to test whether this acceleration occurs in the face of a changing environment have found little support. Overall, whether polygamous or monogamous systems are more at risk depends on the parameters of the models and the analyses performed. For example, comparative analyses addressing whether monogamous or polygamous taxa may experience a greater threat of extinction have been equivocal. In one study of birds, greater extinction probability was associated with taxa having greater post- but not precopulatory sexual selection. Other studies have failed to find a similar relationship.
Mating systems may change as a consequence of anthropogenic environmental modifications and affect population viability. Anthropogenic influences that result in climate change, habitat fragmentation, or pollution—and even selective harvesting—may be potent mediators of mating system evolution by altering predictors of mating system variation (i.e., m*, t*; Lane et al. 2011) and the genetic variation underlying sexually selected traits. For example, one of the first biological alterations in response to climate change is phenological; that is, the temporal distribution of key life history traits is altered. This change may affect mating systems given that it is variation in the life histories of males and females that determines PRR and subsequently the OSR. Habitat fragmentation shifts spatial resource distributions, including females, which may alter mating systems. Pollution can decrease the effectiveness of sexual signals, even to the point of losing species distinctions. Selective harvesting (e.g., trophy hunting) typically removes the largest individuals in a population, which can deplete phenotypic, and the underlying genetic, variation in size and sexually selected traits associated with condition.
The benefits of using a quantitative approach to studying the evolution of mating systems, such as the one Shuster and Wade advocate, is that m* and t* can be used to estimate how anthropological effects may shift these variables and therefore shift the mating system. For example, habitat fragmentation through processes of urbanization and resource extraction leads to clumping of resources and an adjustment in m*. The effect of changes in m* on the mating system depends on the scale of female clumping relative to the organism’s range size. For example, assume a bird species’ mating system is polygynous. If female range size is historically large, but fragmentation results in the loss of nesting sites, then these small areas will support fewer females. This situation will decrease a male’s ability to monopolize many females, thereby reducing the intensity of sexual selection and potentially converting the mating system in that fragmented area to monogamy. Whether this fragmentation influences population viability depends on several factors, including the extent to which genetic factors are also altered as a consequence of a change in mating system and a possible difference in the mean reproductive rate due to limited ability to find mates. If enough is known about a species’ mating system, then proposed changes in habitat use (or pollution or climate change) could be modeled, and the effect on the mating system could be predicted.
Such predictions may be too simplistic, however, as the intensity of sexual selection on a particular trait associated with mating success may depend on an environmental cue not included in current mating system approaches. For example, in the seed beetle (Stator limbatus), larger males are preferred by females, but under conditions mimicking scramble competition smaller males have a searching advantage over larger males. This advantage, however, occurs only in cooler temperatures, because smaller males can heat up more rapidly and thus begin searching earlier. The effect of climate change on this system will depend on whether populations experience either cooler or warmer temperatures and the extent to which scramble competition occurs naturally. Quantitative mating system measures might be able to predict environmental conditions that foster scramble competition as a consequence of changing resource distribution, but the current mating system framework would not be able to predict the evolution of a particular trait, such as body size, in relation to changes in both the mating system and the abiotic environment.
In conclusion, there have been tantalizing, but frequently opposing, findings on the interaction of mating systems, environmental change, and population viability. Understanding the effect of the mating system on population viability and the feedback loops between mating systems and environmental change should be a priority for future research on mating system evolution given its applied relevance. Integrating genetic studies that address the influence of mating systems on the evolutionary potential of populations is vital to this endeavor. Appreciating that mating systems, however quantified, are a dynamic continuum, rather than static and fixed, will help in this understanding and will facilitate progress in predicting the evolutionary trajectories of behavioral traits under selection.
FURTHER READING
Arnold, S. J., and D. Duvall. 1994. Animal mating systems: A synthesis based on selection theory. American Naturalist 143: 317–348. Develops the theory of sexual selection gradients to provide a conceptual framework to predict mating systems. Quantifies Bateman’s principles and provides a direct link to theory for selection on quantitative traits.
Arnqvist, G., and L. Rowe. 2005. Sexual Conflict. Princeton, NJ: Princeton University Press. The first source accumulating the evidence for the role of sexual conflict in influencing evolutionary trajectories of populations.
Bateman, A. J. 1948. Intra-sexual selection in Drosophila. Heredity 2: 349–368. The famous paper that empirically demonstrated that males benefited more from multiple mating than females. While aspects of Bateman’s experimental design and interpretation have been justly criticized, subsequent studies across a variety of species have demonstrated sex-specific variation in the benefits of multiple mating.
Darwin, C. 1871. The Descent of Man and Selection in Relation to Sex. London: John Murray. Darwin’s answer to some of the logical problems (e.g., marked sexual dimorphism) of natural selection.
Emlen, S. T., and L. W. Oring. 1977. Ecology, sexual selection, and the evolution of mating systems. Science 197: 215–223. One of the most influential papers in behavioral ecology, which proposed that mating systems can be classified by their species’ ecology, in particular the spatiotemporal distribution of resources.
Lane, J. E., M.N.K. Forrest, and C.K.R. Willis. 2011. Anthropogenic influences on natural animal mating systems. Animal Behaviour 81: 909–917. A novel and thought-provoking view of how the study of mating systems can be brought into applied relevance.
Mills, S. C., and J. D. Reynolds. 2003. Operational sex ratio and alternative reproductive behaviours in the European Bitterling, Rhodeus sericeus. Behavioral Ecology and Sociobiology 54: 98–104. A classic example of the difficulties of using OSR as a measure of the mating system.
Moya-Laraño, J., M.E.T. El-Sayyid, and C. W. Fox. 2007. Smaller beetles are better scramble competitors at cooler temperatures. Biology Letters 3: 475–478. An empirical illustration of the potential benefits of and difficulties with attempting to demonstrate the influence of climate change on mating systems.
Shuster, S. M., and M. J. Wade. 2003. Mating Systems and Strategies. Princeton, NJ: Princeton University Press. A book devoted to quantifying the opportunity for sexual selection (I) and using it to study and classify animal mating systems.
Thornhill, R., and J. Alcock. 1983. The Evolution of Insect Mating Systems. Cambridge, MA: Harvard University Press. First widely referred use of polyandry as female multiple mating without conditions for the number of mates/males—currently the predominantly used definition for polyandry.
Trivers, R. L. 1972. Parental investment and sexual selection. In B. Campbell, ed., Sexual Selection and the Descent of Man, 1871–1971. London: Heinemann. Based on Bateman’s work, this revolutionary contribution suggested that because females invest more in parental care (including the initial cost of larger eggs—anisogamy), females are the limiting resource, and therefore males compete for them.