Chapter 4
Volatile Components
4.1 General
As already explained, the volatile organic compounds in wine are perhaps their most characterizing feature, both to the wine taster and certainly to the scientist. They are responsible for their so-called ‘bouquet’ on sniffing the head-space from a glass, and the odour/aroma component (palate-aroma) of the overall flavour perceived on drinking. Three sources of these compounds are recognized: (1) primary aromas, i.e. those persisting through from the grape; (2) secondary aromas, i.e. those arising from the vinification process, often a result of yeast or bacterial metabolism; and (3) tertiary aromas, i.e. those arising during subsequent storage of the finished wine as a result of chemical reactions and/or wood extraction especially during long-term storage in wooden barrels.
Recent years have seen a wealth of information regarding the formation of wine flavour compounds by yeasts during fermentation, summarized in a recent review (Ugliano & Henschke, 2009). The alcoholic fermentation involves the conversion of hexose sugars to ethanol and carbon dioxide, and in addition to ensuring yeast maintenance and growth, the glycolytic and associated pathways provide volatile and non-volatile metabolites that contribute to wine flavour. Factors influencing the formation of these compounds are nutrient content of the must, fermentation conditions and the species of yeasts involved in the fermentation. The role of yeasts has been shown to contribute significantly in the development of wine flavour, and the interactions between yeasts and grape compounds influence many aspects of wine quality, ranging from appearance, aroma and flavour of wine to its ‘texture’. Both the fermentation bouquet (compounds typically resulting from fermentation and not necessarily related to the grape variety used) and the varietal character (compounds typical for the grape used for wine-making) are influenced by yeasts. When fermenting in wood, even wood extracted compounds can be modified by yeasts.
The varietal character of a wine is an area of great interest to the wine maker, however, still difficult to define. It may not always be due to compounds directly derived from the grape, but has been reported to be due to yeast derived esters and alcohols (see Francis & Newton, 2005). In addition, the amino acid composition and concentration influences the volatiles produced during yeast fermentation and the varietal aspect of this is still under investigation. The current view is that for most cultivars, varietal aromas are due to quantitative differences in the aroma profiles.
Enhanced knowledge will increase the control wine makers may be able to exert over wine flavour production, by selecting yeasts and using different inoculation techniques. Currently, wine yeast strains are selected to be less susceptible to off-flavour formation and augment components for optimal wine flavour. Characterization of yeasts, including the genetic characterization of metabolic pathways and their regulation, will allow the wine maker more choice in optimizing wine flavour. Easy, rapid and reliable methods for measuring nutrients in grapes and must will increase the control. However, the balance of nutrients in grapes and must is thought to depend on viticultural conditions, so regions influencing this composition in any way may well contribute to the varietal character of a wine. Much more research is needed to fully explore and exploit the role of yeasts, nutrients and its influence on wine flavour and quality. More discussion on the formation of volatiles during fermentation is discussed in Chapter 7.
As a result of sophisticated analytical procedures using gas chromatography developed during recent decades, over 400 volatile compounds have now been detected and many of these also quantified in their amounts present in different wines. Similarly, these techniques enable the volatile composition of the original grapes to be determined, so that the primary aromas of the finished wines can be deduced (not otherwise possible). Some of these same aromas may, however, be produced additionally during the fermentation and storage stages.
Once these volatile compounds have been detected and, preferably quantified, it is possible to relate their presence to the perceived flavour of the wine and indeed to decide which compounds are determining the flavour of a particular wine; be it a Sancerre, Australian Chardonnay, a Bulgarian Cabernet Sauvignon, or whatever.
4.1.1 Sensory perception
The scientific understanding of the perception of volatiles has greatly increased over the last two decades, in particular the the award of the Nobel Prize for Physiology and Medicine in 2004 to Richard Buck and Linda Axel for their pioneering research on the genetics of the perception of odour (Buck & Axel, 1991) has given great impetuous to this research field. A short discussion of the main findings follows below, based on recent reviews of Swiegers et al. (2005); Ache & Young (2005); Mombaerts (2004); Meilgaard et al. (2007).
The perception of volatiles is of great importance to the wine drinker, since a great part of the enjoyment of wine depends on the smell or aroma. The wine industry is well aware that the sensory properties of wine are of great value, obviously the wine needs to be free of any off-flavour, but even more importantly a small difference in smell and taste of a wine can distinguish the wine from the average quality range and elevate it into the premium range, thus commanding a premium price! Nothing is more prestigious than winning a gold award in a well known wine competition.
Our sense of smell, or olfaction, is much more developed than our sense of taste. Our sense of taste seemed to be developed primarily to select energy and to avoid toxic compounds, which are mostly bitter, as discussed in Chapter 3. In contrast, we are able to detect thousands of different compounds and despite the great variation in chemical structure and molecular weight of these volatile compounds, we can differentiate between them and identify their individual smells. Volatile compounds travel from our food or drink via the nose, either nasally by sniffing or retronasally once the food or drink has been placed in the mouth volatile compounds move through passage in the throat, to the olfactory epithelium, which is the size of a postage stamp. Compounds are detected by bipolar olfactory receptor cells, which have cilia exposed where the air we breathe in carries the volatile compounds past the olfactory epithelium. The olfactory receptors to which the volatile components bind are located on the cilia. This binding leads to a number of transduction events thus generating a signal that is transmitted via the neuron of the olfactory cell to the olfactory bulb in the brain. The odour signals are sent to the olfactory cortex and to the higher cortical areas where perception takes place. It is thought that our sense of smell holds a central position in human physiology.
Buck & Axel (1991) discovered that a large family of genes encodes for the olfacory epithelium transmembrane proteins and were thereby the first to elucidate the molecular mechanism of odourant recognition. A wealth of information in this research area has been published since. Sequencing of the human genome revealed that the number of sequences for odour receptors would give only about 400 functional odour receptors, leaving the question: how we can detect and differentiate so many different volatile odour molecules? The answer seems to be that each odour receptor cell is specific for one odour and sends a specific signal to the brain. Despite this specific ability to recognize an aroma molecule, series of related molecules can be detected by one specific receptor cell, such as a series of aliphatic aldehyes. In addition, individual aroma molecules can react with several receptor cells. Hence it is thought that this explains why we can discriminate between so many volatile aromas. However, some volatile compounds can block our perception, although this is a temporary phenomenon. Questions still remain, for example why is there such a great difference in sensitivity for aroma compounds, ranging from mg L−1 to ng L−1 (see Section 4.1.2).
The perception of volatile components in mixtures does seem to hold additional difficulties. It appears that humans have difficulty in distinguishing more than three compounds in a mixture. In addition there are interactions in sensory perception and it seems so far not possible to predict how a blend of different compounds is perceived. Hence, despite our knowledge about smelling and volatile compound composition, the sensory assessment of a particular wine remains a key step in the production of wine!
4.1.2 Partition coefficients
Some of the information discussed in this section is discussed in more detail elsewhere (Pierotti, 1959; Perry, 1980; Clarke, 2001). In practice, many, if not all of these volatile compounds are present in exceedingly small quantity (expressed as mg L−1, ppm; μg L−1, ppb; or even ng L−1, ppt). Their individual threshold flavour/odour values need also to be known. To reach the organoleptic sensors of the nose, any particular compound must be present in the vapour phase, admixed in the air, which is carried to and passes through the front nostrils (as in ‘nosing’ bouquet), or additionally through the back (retro) passages of the throat (retronasal) or both (when actually drinking or ‘slurping’ the wine). In a wine, or an extremely dilute aqueous solution of a volatile substance, contained in a vessel, such as a half-filled wine glass, an approximate equilibrium condition will be established between the amount of the volatile compound ‘j’, present in the aqueous liquid phase, and that in the air/vapour space above, determined mathematically by the partition coefficient, K j,a-w, for this compound. This coefficient expresses the ratio of the weight of the component in unit volume in the air phase to that weight in the same unit volume in the water phase. The higher this value, the higher will be the amount of the compound in the vapour state in the air space for a given amount of the volatile compound in the aqueous phase. As these compounds are present in the aqueous liquid phase (e.g. as in wine) in very small quantities, so we have the concept of infinite dilution, which will be found to simplify enormously the understanding and calculation of this coefficient, including its mathematical derivatives.
K
j,a-w can be determined by direct measurement by GC techniques; one sample is taken from the head-space above the aqueous liquid (e.g. the wine) and another from the aqueous liquid itself. Measurements are expressed per millilitre of each of the air and liquid, and the value of Kj,a-w is merely and conveniently the ratio of the GC peak areas in the two samples. The K
j,a-w (or just K
j for simplicity, or 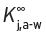 more strictly) in a given circumstance, may also be predicted from other physical property measurements, as will be described. Several other methods of measurement are now available, as described in Section 4.5.
more strictly) in a given circumstance, may also be predicted from other physical property measurements, as will be described. Several other methods of measurement are now available, as described in Section 4.5.
Volatile organic compounds with high boiling points, but low solubility in water (or wine) and other flavourful beverages, have, perhaps surprisingly, very high partition coefficients. In contrast, compounds with low boiling points and high water solubility have low values. They are, in fact, not much more volatile than water, at atmospheric pressure and normal drinking temperatures in which we are interested in assessing flavour. This phenomenon is a consequence of the inherent hydrophobicity of the volatile compound, reflected in the ratio of the number and size of non-polar to polar groups and their positioning in the particular molecule. It reflects the tendency of the molecules of such a compound to escape into the air space from their watery environment but is only true at very dilute solutions, close to infinite dilution. The concentration of their vapour in the air space will then be greater than above the same compound in the pure 100% liquid state (with a low vapour pressure at ambient temperature). This will of course, not be true at temperatures approaching boiling points but this situation is not relevant to the tasting of wines. As an example of an aroma/odoriferous volatile organic compound in wine, we can cite ethyl butanoate (n-butyrate), which shows a measured K
j,a-w at 25°C of 1.87 × 10−2 (Pollien & Yeretzian, 2000). The antagonism to water of such a compound is shown in another useful mathematical derivative, known as its activity coefficient, 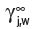 for the compound, ‘j’ in water ‘w’. It is related to the partition coefficient by Equation 4.1, where
for the compound, ‘j’ in water ‘w’. It is related to the partition coefficient by Equation 4.1, where 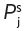 is the vapour pressure of the pure volatile compound at the same temperature.
is the vapour pressure of the pure volatile compound at the same temperature.
(4.1) 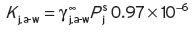
The activity coefficient is a reciprocal measure of the saturation solubility of the compound in water, so that the lower the water solubility, the greater will be its activity coefficient. Activity coefficient is a dimensionless quality; the factor 0.97 × 10−6 ensures that the concentrations of ‘j’ are in the same weight/volume units in both the liquid and the air. From the measured K
j,a-w of ethyl butanoate, and a vapour pressure 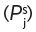 of 17 mm at 25°C,
of 17 mm at 25°C, 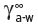 calculates to 1134. Buttery et al. (1971b) described the derivation of this equation, and its application to the volatile compounds in wine is discussed in Section 4.5 and fully described in Appendix I.4.
calculates to 1134. Buttery et al. (1971b) described the derivation of this equation, and its application to the volatile compounds in wine is discussed in Section 4.5 and fully described in Appendix I.4.
Notably, therefore, in any homologous series, of say aliphatic esters, alcohols, ketones, etc., partition coefficients will be at their lowest for compounds at the bottom of the series, with the lowest molecular weights. Partition coefficients will rapidly increase with increasing molecular weight. However, as Buttery et al. (1971b) explained, this does not mean that, in a given homologous series, increasing molecular weight would indefinitely be associated with ever correspondingly increasing concentrations of the volatile compound in the air space. Eventually, such compounds would be found to be so insoluble in water that, at a certain level of concentration (say 30 ppm and less), the greater quantity of the compound present would be in the pure liquid state. They would then exert only a very low actual vapour pressure, with a low corresponding vapour concentration in the air space. It is only in the very dilute aqueous solution condition that high partition coefficients are possible. For example, the maximum aqueous solubility of this same ethyl butanoate is reported as 0.68% (Appendix II, Table II.1) at 25°C, or 6800 mg L−1. Experimental data suggests a content in wine of 0.01–1.0 ppm (Table 4.11 which follows), figures clearly well below its maximum solubility. Similarly, ethyl hexanoate has a calculated maximum solubility (from Pierotti estimates, see Appendix I) of 0.032% at 20°C. A direct solubility measurement figure is not available; 320 mg L−1 is again higher than the quantity reported present in wine.
The concept and measurements of threshold flavour/odour levels for these same compounds in water and in wine is also important, and discussed in more detail in Section 4.1.2. Figures from various investigators suggest 0.1 ppm or less for the minimum detectable level of ethyl butanoate, when sniffed from an aqueous solution, though data is limited and absent for wine itself (see Table 4.11). Ethyl butanoate may, therefore, play some part in determining the perceived flavour of a wine, in which it is present, at higher amounts above the threshold concentration. Even more likely is ethyl hexanoate with a threshold of 0.036 ppm in water, and 0.850 ppm in wine (see Table 4.11).
Notably, volatile compounds of a high or relatively high molecular weight, and with a complex molecular structure, affect the nasal receptors much more efficiently than others, i.e. they have very low flavour threshold flavour levels. The best known of these is 2-methoxy-3-isobutylpyrazine, which is to be found in many vegetable plants, including some grape varieties, and is also present in green coffee beans. It is characterized as having a ‘green-pepper’ odour (green peppers themselves are an important source), with the incredibly low reported threshold figure of 0.002 ppb in water. Its measured partition coefficient (K j,a-w) of 2 × 10−3 is similar to that of ethyl acetate (4 × 10−3). However, the latter compound, which is a simpler molecule, has a much higher threshold, variously reported as 5–60 ppm (Table 4.11). This aspect of flavour impact will also be discussed later, together with the still controversial physiological mechanism of nasal (organo-leptic) perception, and indeed concerning why a given compound has its specific flavour-generating characteristic.
There are four factors still to be considered, however, in the use of threshold levels and partition coefficients for assessing volatile odour/flavour characteristics in a beverage: (1) the temperature of the aqueous solution containing the volatile compound; (2) the presence of any other constituents in substantive amount in the aqueous liquid, e.g. acids, sugars, and ethyl alcohol; (3) concentration of the volatile component in the aqueous solution; and (4) the presence of other volatile substances. The effect of increasing the temperature is usually to increase K j for any given compound. Not unexpectedly, the concentration of the compound in the air space above the solution will usually be higher with a higher temperature, and K j is governed by Equation 4.2, where T is the temperature in °C, and A and B are constants that depend upon the substance.
(4.2) 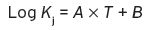
Wine is normally ‘nosed’ and drunk at around 20°C, though, as is well known, white wines are recommended to be tasted and drunk chilled (e.g. 10–15°C) after storing in a refrigerator as necessary. The aroma impact actually would then be expected to be lower than at normal room temperature. Red wines are, however, recommended to be both tasted and drunk at room temperature (even up to 25°C). Of greater significance is the effect of the other factors mentioned above. Sucrose in solution increases the partition coefficients, as has been well described for a number of volatile compounds (Chandrasekaran & King, 1972). Ethyl ethanoate (acetate) in pure water, for example, at 20°C, showed an activity coefficient of 65 in water, but 300 in a 70% aqueous sucrose solution, consequent upon its poorer solubility in the latter. The corresponding K js were 4 × 10−3 and 2.6 × 10−2. In finished wines, the sugar content is, however, very low (<0.3%), though in some sweet wines as high as 60 g L−1. Total soluble acid content (say at 10 g L−1) may also have some effect, but values will differ according to the particular aroma compound being assessed.
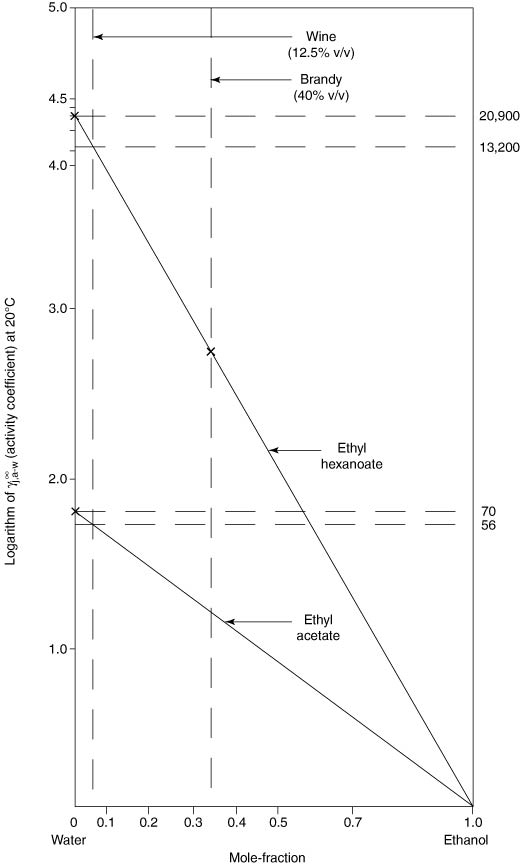
Ethyl alcohol in the aqueous solution, as in wine, at say 10–15% v/v, will have the opposite effect of lowering partition coefficients since, in general, volatile compounds are more soluble in alcohol–water mixtures than in water alone. The effect can be calculated, since (1) the activity coefficients of most of these compounds will be approximately one, in pure ethyl alcohol in which they are completely soluble, and (2) the logarithm of the activity coefficient (log γ) will generally be proportional to the mole-fraction of the constituents of the mixed solution. A graphical plot as in Figure 4.1 shows these relationships for ethyl hexanoate (γ = 20 900 at 20°C, calculated value) and ethyl acetate (γ = 78 at 20°C) in 12% ethyl alcohol (0.04 mole-fraction), and in brandy (40% v/v ethyl alcohol, or 0.35 mole-fraction). An inspection of the plot indicates that the partition coefficient of ethyl hexanoate will be lowered by (13 200/20 900) × 100 = 63%; and similarly ethyl acetate to 56/70 × 100 = 80% (vapour pressures, P s j are unaltered). The threshold levels of these compounds are expected to increase correspondingly in the alcoholic solutions, as indicated in tests (Table 4.4 later in this chapter).
Another important aspect of this partition coefficient effect of ethyl alcohol present in wine is that the very high molecular weight substances may, however, now be brought into solution at higher amounts. Therefore a higher concentration of volatile compound will equilibrate in the air space and consequently contribute also to overall flavour.
There appears to be little satisfactory practical data available to determine whether the measured partition coefficient of a particular component is affected by the presence of other volatile components, of which there may be many in actual beverages. However, recently Pollien & Yeretzian (2001) demonstrated that the partition coefficient of 2-methyl-propanal and -butanal were little different whether determined in pure water or in a 0.5% instant coffee solution (added with its other volatiles present). However, actual threshold values can be markedly affected as shown in the next section.
4.1.3 Threshold flavour/odour levels
To examine properly changes in flavour contribution of different compounds due to the ethyl alcohol and other constituents of wine it is necessary first to examine the various methods of threshold determination that have been used. Guadagni et al. (1963b) carried out considerable experimental work on volatile aldehydes and some other compounds, with a trained panel, and came to the conclusion that the use of a plastic (Teflon) squeeze bottle was the most satisfactory method. Vapour from the head-space over the dilute aqueous solution of the compound of interest, in a half-filled bottle, would be directed into the nostril(s), and its odour sensation compared with that from pure water at the same temperature. Threshold odour levels were thus obtained (with tolerance levels included), which for the homologous series of alkanals were found to be very low, i.e. in the ppb range (e.g. propanal at 9.5 parts × 10−9 ± 1.0 in 1 part water, or 9.5 ppb). The thresholds were rather higher in numerical value and much less reliable when obtained by typical sniffing techniques from the same solution held in a small glass vessel (e.g. propanal showed 12 ± 6 ppb). However, threshold flavour levels (perception or detection thresholds) determined from drinking experiments, from which the lowest concentration of volatile compound is detectable, are really more relevant. Some comparative results are available. Even more relevant are flavour threshold data for different compounds in water containing 10–15% v/v ethyl alcohol, and other non-volatile substances of wine or, better still, in actual wine from which all other volatile compounds have been stripped, leaving only the ethyl alcohol. This is not really feasible or even possible.
Figure 4.1 Lowering of activity coefficients of ethyl hexanoate and ethyl acetate in water–ethanol solutions. Pierotti-estimated values for activity coefficients in water; in ethanol, γ = 1. (Pierotti, 1959).
The concept of the Aroma Index or Unit has been developed, i.e. the ratio, amount of volatile compound present divided by the absolute threshold flavour level, when it may reasonably be expected that any compound showing an Aroma Index of substantially above 1.0 is contributing to the flavour of the wine. In practice, in wine, very few individual volatile substances appear to have a high aroma index; the content of substances with similar organoleptic properties, such as the ‘fruity’ esters, might well be added together for this purpose. The problems connected with the approach are discussed in Chapter 5.
Ribéreau-Gayon et al. (2000) describe the distinction between recognition and perception thresholds. When the former is employed, a descriptive term is used, rather than the mere appearance of a flavour/aroma. Numerous investigators have now compiled data on threshold values of all kinds, in water and other beverages, though generally they are those of perception, or not distinguished.
Units
Some confusion can arise over the usage of different units in expressing threshold levels, and indeed of partition coefficients (though these are usually in non-dimensional units). Threshold levels or values have been traditionally expressed in parts of the given compound in parts per thousand, per million, per billion (thousand million) or even per trillion, parts of pure water solution or other aqueous liquid. Preference is now given to expressing in grams (compound) per litre (aqueous liquid) units. Naturally, grams may be in the sub-units of mg, μg or ng. Equivalencies are illustrated in Table 4.1. There can be some advantage in the use of ppm, ppb, ppt units, as errors can arise with the use of 10−n, where n may not be accurately read, or mis-transposed from the literature, as also with contents expressed in these units. Exponent values of 10 are now often quoted as × E (+ or − 1), etc. In citing actual values, it is desirable to quote them in the units originally given, not least on account of any possible differences between old British and US trillions. Threshold values are usually given as absolute values, and sometimes as difference values (where there is a known pre-existing content of the compound under examination).
Table 4.1 Equivalencies of different threshold levels and content units.
| Parts | Weight/Vol. or g/g Weight/Weight | |
| ppm (parts per million parts of water, 106; or 10−6 parts in one part) |
= | mg L−1(kg) milligrams per litre (kg) i.e. 0.001 g/ L−1 (kg) or 10−3 g/103 g = 10−6 g/g |
| ppb (parts per billion of water, 109; or 10−9 parts in one part) |
= | μg L−1 (μ − million), micrograms per litre (kg) 0.000001 g L−1 10−6 g/10−3 g = 10−9 |
| ppt (parts per trillion, 1012 or 10−12 parts in one part) |
= | ng L−1 nanograms per litre 10−9 g/103 g = 10−12 |
| Percentages | Parts | Weight/Vol |
| 0.1% | 1000 ppm | 1000 mg L−1 |
| 0.01% | 100 ppm | 100 mg L−1 |
| 0.001% | 10 ppm | 10 mg L−1 |
Consistency of threshold odour levels
Reviewing the available data for a range of different compounds in wines, there appears to be a reasonable degree of consistency for figures given by different investigators for the same compound (Table 4.2). Factors of importance are, firstly, the relative skills or biases in the judgements of panels used (see Chapter 5) and, secondly, the purity of the test compound. As described by Meilgaard (1982), some compounds require elaborate technical procedures of purification, to achieve 100% purity. Contaminants can have a much lower threshold than the test compound and thus confuse assessment.
Threshold level difference between sniffing and tasting
It is important to determine whether a threshold is different, when detecting the presence of a compound only by the nose (through the nostrils alone) as with the work of Guadagni et al. (1963b), or actually measured by drinking the beverage or aqueous solution. Ahmed et al. (1978) obtained data in which threshold levels were determined by tasting (i.e. flavour level in water), and compared them with some other values determined by odour sniffing only. Table 4.3 gives Ahmed’s tasting data for a number of different compounds, found in fruit juices, together with data derived purely from sniffing. Flament (2001) has provided a useful compilation of odour/flavour threshold data for most of the volatile compounds in green and roasted coffee. The differences are not large, with some indication that volatile compounds are more readily detected, at least in pure water, when the solution is taken by mouth, rather than in simple sniffing of the head-space gas. The difference has relevance to ‘nosing’ for ‘bouquet’ in fine wines. Similarly, Feneroli’s compilation (Burdock, 2002) gives both values, where available.
Table 4.2 Consistency of reported odour threshold levels data.
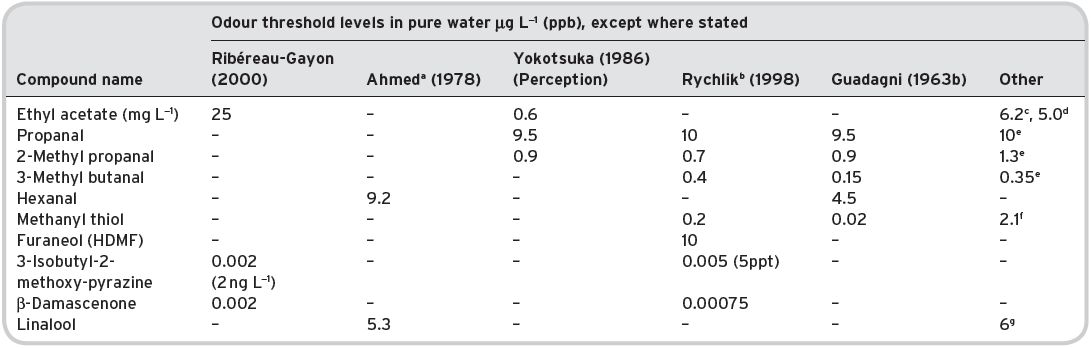
aAlso quoted by Shaw (1986); bquoted by Grosch (2001); cMulders (1973); dFlath et al. (1967); eGrosch (1995); fPerrson & von Sydow (1973); gButtery et al. (1969b).
Table 4.3 Comparison of threshold levels determined by taste/flavour and by odour (sniffing) alone in water.
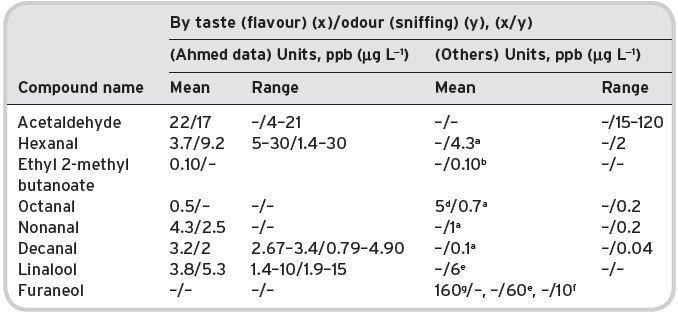
Ahmed data with confidence limits. aGuadagni et al. (1963b); bFlath et al. (1967); dLea & Swoboda (1958); eButtery et al. (1969b); fGrosch (2001); gHuber (1992).
Threshold levels in solutions of dissolved substances in water and in beverages
Ribèreau-Gayon et al. (1978 and 2006) in particular have described comparative tests for some compounds in water, or in a model solution containing substantial amounts of dissolved substances and which would be found in wine. For example, two models were used, (a) synthetic model solution (with 12.5% alcohol with added tartaric acid to bring the pH to 3.0), and (b) the same as (a) but also with 10% added sucrose; the results are shown in Table 4.4.
Even higher threshold levels are experienced when measurements are made in real beverages, which also will contain, in addition to non-volatile compounds, other volatile compounds than the particular one under examination. There is comparative data not only for wine but also for other beverages such as beer, orange juice and Japanese sake.
These determinations will, however, necessarily be carried out on the wine or beverage already containing a quantity of the same compound under examination, and so they do not give absolute values of threshold odour/flavour. Meilgaard (1982 and 1986) described the use of the Difference Threshold figure and explained that the actual value obtained in a given solution depends upon the amount of that compound already present. Thus, quoting the work of Brown et al. (1978) on the threshold for butan-2,3-dione (diacetyl highly purified), determined in a beer already containing 30 μg L−1 (ppb), the perception threshold of added compound was reported to be 100 μg L−1. The threshold was 300 μg L−1, when the beer also contained 300 μg L−1; similarly, the figure 1500 μg L−1 when the beer already contained 2000 μg L−1 of diacetyl. By linear extrapolation, the threshold back-calculates to 81 μg L−1, when the initial diacetyl content in a beer is taken as zero (i.e. an absolute threshold figure). Other data gives a figure in water alone of 15 μg L−1 (Rychlik et al., 1998). The difference between these two absolute values will be a result of the combination of the relatively small physico-chemical effect of the ethyl alcohol as already discussed, and a larger one due to the presence of the other volatile compounds on nasal perception. Meilgaard (1982) explained that the interactions were such that similar odour sensations were largely additive, whilst contrasting aroma substances could be antagonistic, increasing the threshold level of each, e.g. in solutions with octanoic acid and ethyl acetate. The effect of interactions is further considered in Chapter 5 (Section 5.7.1), along with psychological factors relating to other components present.
Table 4.4 Threshold levels in alcoholic model solutions.
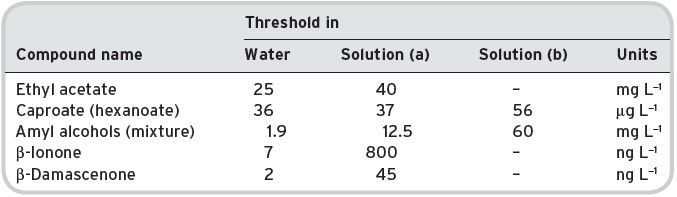
Data from Ribéreau-Gayon (1978 and 2006). Solution (a) water with 12.5% w.v alcohol, with tartaric acid, 10 pH3, (b) as (a) but with 10% with sugar.
Comparative data for different beverages for different representative volatile compounds is given in Table 4.5. Different threshold figures are given according to a range of contents for which they are applicable; in the case of wine, the available data was not precisely related to content in the wine examined, except that it was ‘a white wine’. We can clearly expect much higher thresholds for most components in actual wines, compared with those in plain water (a ratio of 50 or greater).
Data for ethyl acetate and hexanoate (caproate) in water alone and in alcoholic solutions shows the expected increases in threshold level that are observed, following a lowering of volatile compound concentration in the head-space air presented to the olfactory sensors in the nose. This observation is consistent with the figure already calculated for the lowering of partition coefficients (Fig. 4.1 and Section 4.1.2) by the presence of ethyl alcohol.
Table 4.5 Absolute and difference odour/flavour threshold levels for various compounds in different beverages.
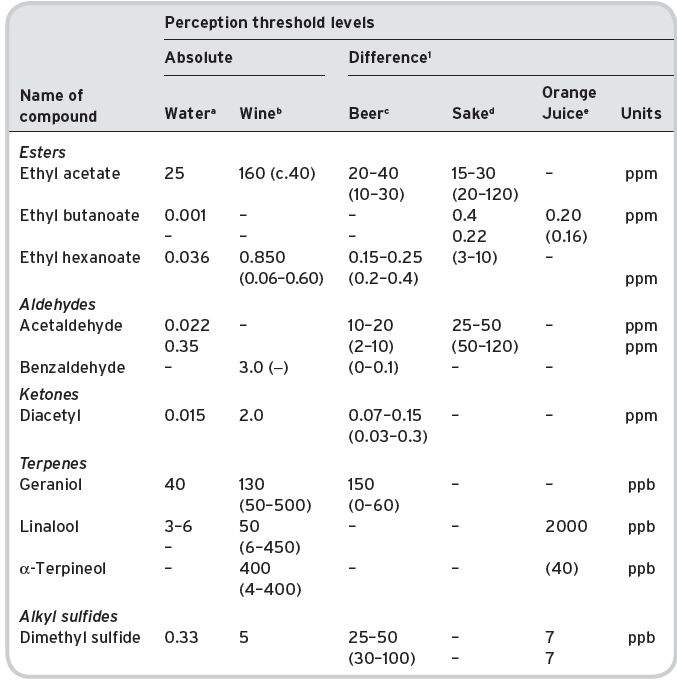
a Various references (see under Tables for Compound Groups; esters, etc.). NB values for ethyl butanoate, very variably reported. b Ribéreau-Gayon et al. (2000). c Meilgaard & Peppard (1986). d Takashashi, K. & Akiyama, H. (1986). e Shaw (1986).
1 Range of content in beverage in brackets, applicable to difference level given.
Relationship of threshold values to partition coefficients
However, the relationship between partition coefficients and threshold odour levels for a homologous series of compounds, even in water is not simple, as an inspection of the reliable data of Guadagni et al. (1963b and 1969) for both variables shows. Comparative data is given in Table 4.6 for aliphatic alkanals, which are, in effect, in infinitely dilute solutions.
Table 4.6 Partition coefficients and threshold odour levels for alkanals in aqueous solution (infinitely dilute).
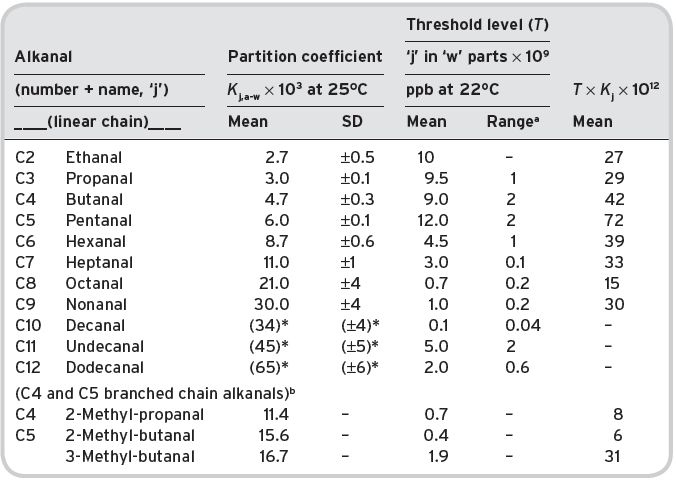
aData from Guadagni et al. (1963b), Buttery et al. (1971b) of 2–6 determinations, as quoted. bData for ‘C4 and C5 branched chain’ from Pollien & Yeretzian (2001), and Rychlik et al., quoted by Grosch (2001). *Data in brackets, extrapolated.
The partition coefficients of these alkanals, C3 to C9 show an excellent logarithmic relationship with carbon number on a linear scale; the values used for C10–C12 compounds have been extrapolated.
Compounds C5–C10 also show a good logarithmic–linear relationship between threshold values and carbon number. Compounds C2–C4 exhibit a separate logarithmic relationship, indicating lower threshold levels than might have been expected, which could be due to the incomplete purity of compounds under test. Purity is much more difficult to achieve with inherently weakly odoriferous, low boiling point substances with high polarity, which are easily contaminated by higher molecular weight and more odoriferous homologues. Familiar substances often have an odour they do not really possess in the completely pure state, thus, acetamide has a ‘mousy’ smell probably due to some contaminant; similarly, aniline can have a powerful smell. Really pure acetaldehyde (ethanal) and propanal may well have higher threshold values, which is suggested by earlier data (Lea & Swoboda, 1958) in the literature. Similarly the data for the compounds C11 and C12 is apparently anomalous, but the most likely explanation has already been given, i.e. increasing insolubility in water to virtually like that of non-polar hydrocarbons.
Alkanals are not of particular relevance to wine flavour (except acetaldehyde), but the data provides a good basis for determining any mathematical relationship that may exist between partition coefficients and threshold values, which could be of relevance to other homologous series of compounds. The threshold levels decrease much more markedly with molecule size, than do the head-space concentrations increase, e.g. the product K j,a-w threshold decreases with increasing molecular weight.
This implies that the organoleptic sensors in the olfactory organs in the nose are much more sensitive to the larger molecules than the smaller.
Also of relevance is that branched-chain alkanals have much lower threshold levels than their corresponding linear chain homologues, in accordance with their partition coefficients, values recently obtained by a new direct method (Pollien & Yeretzian, 2001). In contrast, unsaturated chain aldehydes tend to have higher threshold values, with lower partition coefficients; thus, the threshold for trans-2-hexenal is 17 × 10−9, whilst that for hexanal is 4.5 × 10−9, reflecting a slightly higher affinity for, and solubility in, water.
The relationship between partition coefficients and threshold odour values is shown in Figures 4.2 and 4.3. The data in Figure 4.2 accommodates the precision reported by these investigators, which varies according to the compound by usually around ±10% or less. At the moment, similar relationships cannot be established for other homologous series in other groups of compounds, such as the alkyl alkanoates, alkanols or acids. This is largely because of insufficient and reliable data on threshold odour values, though there is some available (Tables 4.11, 4.12, 4.13 and 4.14 later in this chapter). Buttery et al. (1969b) also showed that the logarithms of partition coefficients plotted against carbon number gave parallel lines for these homologous series on the same graph. Threshold odour levels might be expected to show similar parallel relationships for homologous series, giving scope for estimated values, when actual values are not available.
Volatile compound concentration in the vapour phase
The actual concentration of a given compound in the air conveyed by the nose or retro-nasal passage, to the olfactory receptors, is the figure directly determining its olfactory effect. It can, of course, be calculated, knowing the concentration of the volatile compound in the liquid phase (e.g. water or aqueous solution) and the partition coefficient (K j,a-w) at the given temperature.
However, this figure can be determined directly during GC-olfactometry, when the eluted vapour is being sniffed at the exit port (see Chapter 5, Section 5.5). At the same time, the identity and purity of the test sample can be confirmed or otherwise, using pure reference components. Using aroma dilution experiments, the value can be obtained at a threshold odour point. Thus, butanal (Fors, 1983) shows threshold values varying from 0.013 to 0.042 mg m−3 of air, whilst odour thresholds measured from water are between 9.0 and 37.3 ppb (μg L−1). This data is compatible with a measured partition coefficient of 4.7 × 10−3 (Buttery et al., 1969b). Grosch and his colleagues (1995), quoted by Flament (2001), have provided air concentration data for a number of other compounds as shown in subsequent tables (Tables 4.9, 4.11, 4.12, 4.14, 4.15, 4.16, 4.17, 4.20, 4.21, 4.22, 4.23, 4.24, 4.25, 4.26, and 4.27).
Figure 4.2 Relationship between partition coefficients and threshold flavour/odour levels for alkanals (data from Table 4.6).
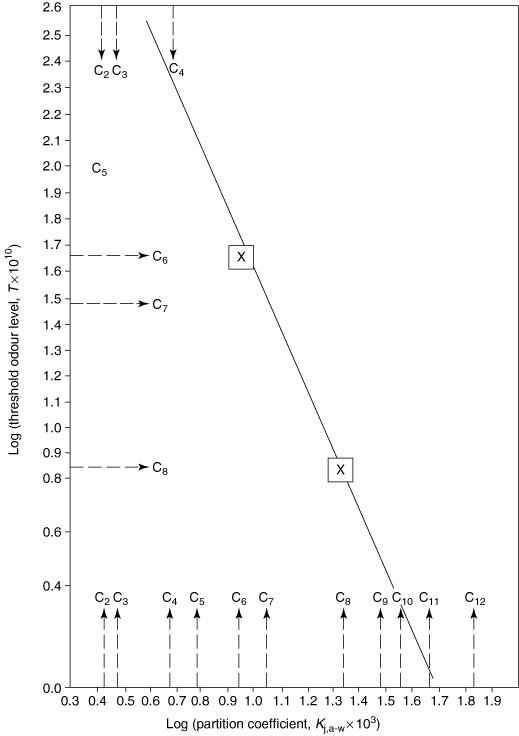
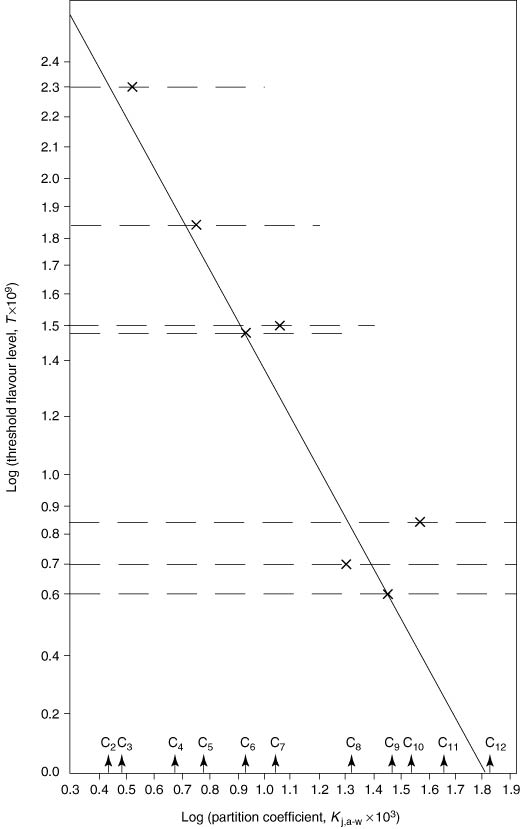
Figure 4.3 As in Figure 4.2 but showing only the flavour in water data from Table 4.14 (alkanals).
4.1.4 Flavour/odour descriptions
Use of word descriptions
Tables 4.11, 4.12, 4.14, 4.15, 4.16, 4.17, 4.20, 4.21, 4.22, 4.23, 4.24, 4.25, 4.26, and 4.27 give, for each of the various chemical compound groups, flavour/odour (i.e. olfactory only) descriptions of the individual volatile compounds found in wines, together with published threshold data (as available). Although flavour descriptions are, of course, somewhat subjective, nevertheless those given have a considerable degree of consensus amongst experts. Notably, however, many volatile compounds can present somewhat variable sensory impressions, depending upon their concentration in the air (or in aqueous solution) at which they are being assessed (Table 4.7). For detailed discussion and examples on this see Francis & Newton (2005).
Although the concentrations of volatile compounds are generally low, the method of presentation varies enormously. For example, compounds may be sniffed, when emerging from the discharge point of GC equipment when testing a particular beverage sample, or they can be sniffed from the static head-space in a squeezable plastic bottle (as described earlier). Of course, the volatile compounds are also perceived during actual drinking. In all these instances, the compounds will be present in very low concentrations, such as in wine and coffee. The odour of a substance, however, is often reported, whilst being assessed in the vapour above a large quantity of the pure liquid, when the head-space vapour concentration may be quite high, unlike for a small quantity of very dilute aqueous solution. Similarly, the perfumer, skilled in assessing and blending odoriferous substances (often called odorants), usually assesses odours of compounds dissolved in alcoholic solutions. Compilations and dictionaries of pharmaceutical compounds (e.g. Merck Index, 18th edn., 2000) and of perfume and flavouring chemicals (e.g. Feneroli, 2002) report the odour and colour of the pure liquids.
Arctander (1967) is particularly associated with a compilation of odour assessments of compounds generally, whilst Flament (2001) has provided a very detailed compilation of all available (and different types of) threshold data for volatile substances in coffee, many of which are also present in wines. Grosch and his colleagues, such as Blank & Semmelroch (1995), have specialized in descriptions of odours eluting from sniffing ports during gas-chromatography of coffee brews. These descriptions may not be identical, for the same compounds, if a wine was being similarly tested. Arctander (1967), in particular, emphasizes the different odour description that can exist according to vapour concentration. This situation is especially evident with the alkanals, such as acetaldehyde to octanal described as having a ‘nauseating’, ‘suffocating’, etc. odour in high concentration, but becoming much more pleasant (e.g. ‘fruity’) from very dilute solutions. The perfumer is well aware of the odoriferous natures of a substance such as skatole, which is ‘putrid’ at high concentration, but possesses useful and desirable odour notes at low concentrations.
Table 4.7 Typical examples of differing sensory (olfactory) impressions of volatile compounds with concentration in aqueous solution.
Descriptions are also available as the odour element of flavour on ‘tasting’ the aqueous solution. A specialized compilation of such data has been made by the Firmenich company in Switzerland, though the text Chemisis has not been published; Flament (2001) has quoted the available information for coffee volatile substances, many of which also occur in wines. Feneroli’s Handbook (2002), for each flavour compound listed, provides information, as far is available, in three ways: (1) smell or odour description (generally from the pure substance); (2) aroma threshold level (detection) in aqueous solution, but source is not always referenced; and (3) taste characteristics at a particular concentration in solution, well beyond the detection threshold value. For example, under ethyl caproate (hexanoate) we have (1) ‘powerful fruity odour with a pineapple-banana note’; (2) 0.3–5 ppb (cp data in Table 4.10, 1 and 36 ppb); and (3) ‘fruity and waxy with a tropical nuance’ at 10 ppm aqueous solution.
Intensity of flavour/odour
A further dimension to be considered in odour characterization is the perceived intensity of an odour at different concentrations, that is including and beyond threshold values. According to Steven’s Law originally promulgated around 1957 (Stevens & Galanter, 1957), R = k × S n where R is the perceived flavour intensity of a constituent, and S its concentration, whilst k and n are constants for a particular compound. The units of S are conventionally g L−1, whilst R can be an intensity figure obtained by reference to a scale (typically, 0–9). The exponent, n, appears in practice to be between 0.12 and 1.7 (usually 0.4–0.7). This implies that doubling the concentration of a given compound does not mean that we will smell the compound twice as strongly; it could well be 20.5 = 1.4 times. However, for n > 1, we will smell the compound more than twice as strongly on doubling the concentration. Teghtsonian (1973), quoted by Meilgaard & Peppard (1986), suggested that n is a function of the range of concentrations under study; the value is higher the narrower the range, though this seems surprising.
These findings have particular implications in the use of Aroma Indices, such as the Flavour Unit concept of Meilgaard (1982), which are discussed in Chapter 5.
4.2 Volatile compounds detected in wines
Lists have been compiled of all the volatile compounds that have been detected and identified by instrumental analysis in wines by numerous different investigators over the last three decades, in a range of different types of wine. Information given at later dates, say after 1980, tends to be more reliable, due to the increasing capability and certainty of gas chromatographic techniques, which have been coupled with mass-spectral data (i.e. GC-MS), and have made use of capillary columns for better resolution. Such comprehensive lists of volatile compounds have been provided by Montedoro & Bertuccioli (1986), and by the T.N.O. Food Research Institute in Zeist in The Netherlands, with their publications (7th edn., 1996). Clearly, not every wine in commerce will contain all these listed compounds; some will derive from specialized wines, like botrytized wines, or are developed on ageing. Indeed, some compounds listed may unfortunately be mere artefacts of the GC technique used and the method of extraction from the sample. Assurance of correct identification needs a careful study of the original published papers. However, reliability is clearly increased by more than one investigator reporting the presence of the same given compound; three or more references has been the criterion for inclusion for the numerous esters reported, which are an important group of substances responsible for wine flavours. Since the methodology has been much improved (see Section 4.5), with most authors showing validation of their method and statistical data on the errors, therefore some compounds recently identified have been added, even when only one publication is available.
The definition of ‘volatile’ compound needs some explanation. Nearly all compounds can be said to be volatile from infinite dilution to some degree. Many relatively involatile compounds can be steam distillable, and therefore appear in an extracted sample in some GC techniques. The definition also describes compounds that are directly detectable by gas chromatographs, without use of derivatization (that is, not made volatile by some chemical combination process).
In the first instance, it is useful to provide information on the numbers of compounds in various groups, and sub-groups of chemical nomenclature. Tables 4.8 and 4.9 provide such information from the listing by Montedoro & Bertuccioli (1986). Table 4.8 details aliphatic compounds (by far the greatest number) and Table 4.9 shows compounds with a heterocyclic ring structure. No distinction is made between primary, secondary and tertiary aromas in the lists, which show the marked predominance of aliphatic esters, with much fewer heterocyclic compounds, except lactones/furanones. The volatile compounds described are those that frequently occur in numerous other beverages but the amounts and proportions in relation to each other can be very different.
Some of the main scientific investigators laying the foundation of the identification of volatile compounds of wine have been:
(1) Ribéreau-Gayon, and many colleagues and research students at the Faculty d’Oenology, University of Bordeaux, and other Universities and Institutes in France.
(2) Amerine, Ough, Webb and many others in the USA, mainly at the University of California, Davis.
(3) Drewart, Rapp, Schreier and others in Germany at the Institut für Rebenzüchtung Geilweilerhof, Siebeldinge and elsewhere.
(4) Bertuccioli and Montedoro, at the Istituto di Industrie Agrarie, University of Perugia and others in Italy.
Research institutes in Australia, South Africa and Spain have also been very active in wine technology and numerous aspects of wine science.
Several investigators have recently devoted and recorded their efforts in detail to specific wine types, such as the Bandol (southern France) wines based upon Mourvèdre grapes, and made valuable comparisons with wines based on Syrah grapes (Vernin et al., 1993). Riesling and Sylvaner wines have been especially studied by Rapp et al. (1985); Spanish white wines by Aldave et al. (1993); and wines based on Cabernet Sauvignon, by Ribéreau-Gayon (1990) and by Boison & Tomlinson (1990). A review by Ebeler & Thorngate (2009) showed the development of the flavour science, initially the emphasis was on determining the major volatile components, while current analytical techniques combining analytical and sensory information is more focused on identifying impact aroma compounds. Another focus is determining the effect on aroma of the matrix and other components in the wine giving so-called interaction effects.
Table 4.8 Volatile compounds found in wines: linear chain and homocyclic.
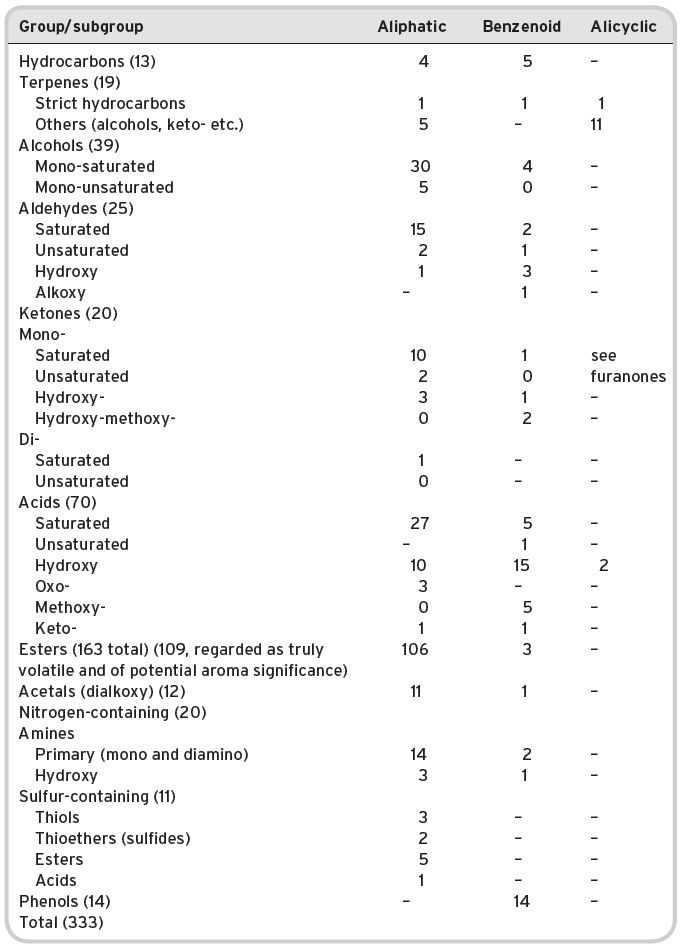
Data from Montedoro & Bertuccioli (1986), all references taken.
Table 4.9 Volatile compounds found in wines: heterocylic (oxygen and nitrogen containing).
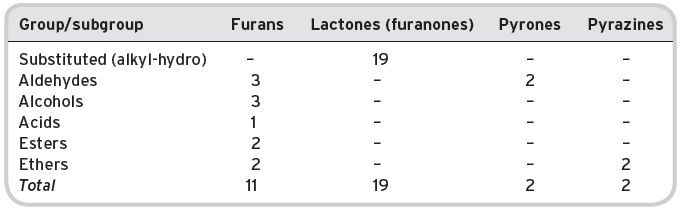
Data from Montedoro & Bertuccioli (1986).
The Spanish work of Aldave et al. (1993) is of particular interest, with its quantitative data on numerous samples over a whole range of different groups of volatiles. The French work of Vernin et al. on Mourvèdre grapes and wines is also of special interest, covering a wide range of volatiles, using valid identification techniques, though they are not fully quantitative. Numerous investigations have been carried out on individual compounds and specific groups of compounds like the terpenes, including quantitative data, often as exercizes in GC analyses by non-wine experts, such as Tressl of the University of Berlin, who has carried out similar determinations on various beverages. More investigators will be found in the study of the composition of grape brandies, Sherries and Port, which is relevant to that of the originating wines. A considerable amount of qualitative work has been reported by other investigators, such as in the IARC monograph (1988) on alcohol drinking, but the information is presented more for potential toxicity interest than flavour. This chapter leads on to the discussion in Chapter 5 of the wines of different grape varieties in relation to composition and individual flavour perception.
Both quantitative and qualitative data is now presented in the tables, grouped by the chemical nature of the compounds found, starting with the aliphatic/benzenoid esters, which are important compounds involved in any wine flavour assessment. Where possible, threshold odour/flavour data from different sources has also been tabulated, and in conjunction with actual content data a very approximate assessment can be made of the likely contribution of each compound to overall flavour, by comparing contents with threshold values.
4.2.1 Types of aroma in volatile compounds
The three main types of volatile compounds found in wines, according to origin, are included in the tables. These are (1) primary aromas (P), compounds already present in the grape and persisting through vinification; (2) secondary aromas (S), which are generated aromas primarily in the fermentation, by the action of the yeast (the main function of which is to produce ethyl alcohol) and bacteria, and make up, qualitatively and quantitatively, the largest amount of volatile compounds present in the wine; and (3) tertiary aromas, (T), which are generated during maturation or ageing processes, either in-cask (or vat/tanks) or in-bottle, subsequent to vinification. Secondary aromas may also include those really generated in pre-fermentation stages, such as in the process of crushing grapes, where notably, such compounds as the hexenals may be produced and largely persist through the remaining stages of vinification. The combination of secondary/tertiary aromas makes the wine markedly different in flavour to that of the grapes/must from which it originates.
Nevertheless, certain primary aromas characterize a wine. These are mainly the terpenes, the ‘grapey’ aromas present in relatively large amounts in the Muscat variety of grape, which has a number of sub-varieties with modified names. They may also be present to a lesser extent in other grape varieties, e.g. Riesling. There are some other primary aromas in grapes, which come through fermentation in the same way. These are often described as ‘varietal’ aromas, i.e. compounds in wine characteristic of a particular grape variety. Recently, the highly flavourful compound 2-methoxy-3-isobutylyrazine has been identified, at above recognition level, in wines made from Cabernet Sauvignon grapes, originating from the grape (mainly located in the skins). It imparts a variously described ‘green’ or ‘bell-pepper’ aroma with about 2 parts per trillion in water, though the threshold value in wine is higher. Nevertheless up to 50 ppt in wine has been detected by the most modern GC-MS techniques. This compound has been identified in a number of other grape varieties.
A ‘blackcurranty’ aroma is especially characteristic of Cabernet Sauvignon wines; its chemical origin is believed to differ from that of the blackcurrant fruit, and according to Marricon (1986) is based upon a complex terpene composition, which brings it in part to primary aroma status. Similarly thioketones and thioterpineols are now believed important. Jackson (2008) mentions also some other characterizing compounds of varieties, such as 4-vinyl guiaicol as ‘spicy’ in Gewürztraminer wines; but contrast Ribéreau-Gayon et al. (2006) with the description of odour ‘smoky/clove-like’. The thioketone 4-methyl-4-mercaptopentan-2-one (guava-like) is particularly associated with Sauvignon Blanc wines and also Chenin Blanc and Colombard wines, with 2-phenyl-ethanol in Muscadine varieties and isoamyl acetate (fruity/bananas, pears) in Pinotage wines. Other specific varietal compounds have been identified recently, with accurate quantification data. The discovery of rotundone, giving the peppery aroma typical in Shiraz, was reported by Wood et al. (2008), and four ethyl esters contributing to sweet, fruity aromas in wines were identified by Campo et al. (2007). Recent reviews on wine flavour are Ugliano & Henschke (2009), Ebeler & Thorngate (2009) and Palaskova et al. (2008). It is, however, not always clear to what extent these substances are present in the original grapes, or are actually produced in the fermentation or by microbiological action from particular precursors in the grapes (see Chapter 7).
Varietal aromas can be present in other Vitis grape species, such as Vitis labrusca, where substances such as methyl anthranilate may be characterizing.
4.2.2 Stereochemical effects in aroma volatile compounds
The effect of slight changes in molecular structure of a given type of compound (e.g. with unsaturated/saturated carbon bonds) on flavour/aroma perception has already been discussed (Section 4.1.2). In recent years, the significance of chirality (i.e. the existence of molecular structures that are mirror-images, known as enantiomers, see Appendix I) on flavour/aroma has been examined (Pickenhagen, 1989; Lettingwell, 2002). In nature, however, biogenesis in fruits/vegetables usually generates only one of the enantomers possible, described as a specific chiral variant by the nomenclature of the letters R and S (see Appendix I), e.g. tartaric acid, with a characteristic optical rotation. Chemical or bacterial reaction may well produce both enantiomers or forms, e.g. lactic acid; as also in other compounds from fermentation processes catalysed by enzymes (e.g. amyl alcohols). Differences in flavour/aroma occur from one to another compound in a given pair.
The most marked differences in flavour/aroma occur with stereoisomers (see Appendix I), which arise when a compound has a ‘cis’ (Z) or ‘trans’ (E) molecular structure, in a molecule containing two carbon atoms joined by an unsaturated bond. The stereoisomers have the same atom groups attached to the two carbon atoms (the atom groups themselves, however, should not be identical). A typical example is 2-nonenal, also with perceived flavour differences, (E), trans, odour threshold 0.5 μg m−3 in air (0.5–1 ppb in water), and (Z) cis, 0.08–0.23 μg m−3 in air.
In lists of volatile compounds, the proper characterization of compounds in respect of possible chirality/stereoisomerism should therefore be included. The presence of asymmetrical carbon atoms is usually apparent from a structural formula, though they can be labelled by use of an asterisk.
4.3 Contents and sensory evaluation data
Tables 4.10, 4.11, 4.12, 4.13, 4.14, 4.15, 4.16, 4.17, 4.18, 4.19, 4.20, 4.21, 4.22, 4.23 and 4.24 record the available data for individual substances with their chemical structures in groups of similar compounds. Many of these compounds, especially esters, have trivial and other names established by long usage, but chemical names according to IUPAC preferred recommendations are usually recorded first. Relevant physical properties of these same compounds are given in Appendix II.
4.3.1 Esters
As already mentioned, esters of all kinds are regarded as especially important to wine flavour, and are usually secondary aromas, arising from the fermentation, and sometimes tertiary aromas arising from ageing, where alcohol–acid rearrangements can occur. Since there are many acids and alcohols in wine, there are many esters possibly formed in wine. Ethyl acetates are the most prevalent in wine, since there is a high concentration of ethanol and primary alcohols are quite reactive. Over 160 esters in wine have been identified, although not all are commonly present above their threshold values.
Structure
Esters result from the combination of organic alcohols R1OH with organic acid, R2COOH [e.g. R1–O–C(=O)–R2] with the elimination of water. R1 and R2 are usually alkyl or sometimes aryl radicals in mono-esters in wines, but some di-acid esters are also present.
Generally esters in wines can be divided in two groups. The first group consists of acetate esters, in which the acid group is derived from acetic acid and the alcohol group is ethanol or a complex alcohol. The second group is the ethyl esters, where the acid group is a medium chain fatty acid and the alcohol group is ethanol.
Presence in wines
Based on peak areas from GC traces, Vernin et al. (1993) found that about 38% of all the volatile compounds detected were esters, mainly aliphatic, in a red wine from Mourvèdre grapes grown in southern France, and similarly (35%) for a Syrah red wine; there were very few esters, in small amount, in the originating must.
Of the numerous esters (163) reported in the list of Montedoro & Bertuccioli (1986), some 109 could be regarded as properly volatile but only 59 of these are multi-referenced (i.e. three or more citations). At different times, 22 of these have been quantified, mainly by Bertrand (1975, unpublished data), as reported by Ribéreau-Gayon (1978) (Table 4.10 which follows). This data, covering a large number of wines, is of interest in that it shows on average a higher content of esters (lower C esters) in white wines compared with red, corresponding to the known effect of lower vinification temperatures used. The data of Vernin et al. (1993) is not fully quantitative, but the relevant amounts of some of the individual esters are of interest, as tabulated below (1984 vintage year, see Table 4.10). None of these compounds identified are new to the list in Tables 4.11 or 4.12 following.
Notably, individual ester content is generally in the ppm range, contrasting with most other volatile components, which are more usually at ppb levels.
However, four esters recently analysed and quantified in a range of wines, have very low thresholds at ppb levels (Campo et al., 2007). They suggested that ethyl 2-methylpentanoate, ethyl 3-methylpentanoate, ethyl 4-methylpentanoate and ethyl cyclohexanoate are formed by esterification reactions with ethanol and the corresponding acids formed by micro-organisms. Their concentrations vary, they are especially high in aged wines and can reach concentrations well above threshold levels.
Table 4.10 Percentages of some of the individual estersa for 1984 wines made from MourvÒdre grapes.
| Diethyl succinate | 12.90% |
| Ethyl lactate | 5.56% |
| Ethyl-3-hydroxy butyrate | 4.72% |
| Ethyl decanoate | 4.68% |
| Ethyl octanoate | 3.78% |
| Methyl-2-hydroxy 2-methylbutyrate | 1.07% |
| Isoamyl lactate | 0.92% |
| Ethyl phenyl acetate | 0.58% |
| Ethyl hexanoate | 0.55% |
| Others (20 in all) | 3.5% |
| Total of 29 | 38.2% |
a Tabulated using data from Vernin et al. (1993).
Francis & Newton (2005) surveyed the literature and compiled a list of compounds likely to contribute to wine aroma, based on their threshold values and the concentration ranges determined in wines. They listed the following esters all considered important in wine (Table 4.13).
Flavour characteristics
Tables 4.11 and 4.12 also give some reported odour/flavour characteristics of these esters. It is generally recognized that the lower aliphatic ethyl esters show fruity notes of different kinds (mostly tropical tree fruit, banana, pineapple, but also apple, pears, etc.) up to about ethyl heptanoate (C2 + C7 = C9); whereas the higher homologues tend towards soapy, oily and candle wax characteristics. Other esters, such as ethyl benzoate, iso-amylacetate and hexyl acetate, will also show important fruity characteristics. However, none of these esters themselves appear to offer a number of other fruity characteristics found in many wines, such as blackcurrants, gooseberry or plums (see Chapter 5).
There is some quantitative threshold odour/flavour data for water around but little in wine at 10–15% alcohol content. It is apparent that some ester compounds will have high partition coefficients, and have very low threshold levels, even in alcoholic solutions, but others will not have so. It is difficult to establish a reasonably scientific basis of flavour assessment, based on Flavour Unit or other values as described in Chapter 5, Section 5.5. Whilst not many individual esters may show FUs or Aroma Indices greater than 1.0, in a given wine, a combination of the certain fruity esters may well give a combined high value, as recognized by wine tasters and, similarly, for the soapy esters from ethyl octanoate upwards to laurate. Ribéreau-Gayon et al. (2006) show how the character of a white wine can be altered, by fermentation temperature and other factors, lower temperatures favouring the formation of ‘fruity’ esters, which are especially significant in young, white wines, contributing to their ‘fruity’ character. With red wines, higher fermentation temperature is used. These factors are further discussed in Chapter 7. The use of SO2 in vinification (Aldave et al., 1993) and clarification procedures is also important.
Table 4.11 Main esters in wines: contents and olfactory characteristics (odour/flavour descriptions and thresholds). Origin, mainly secondary (S).
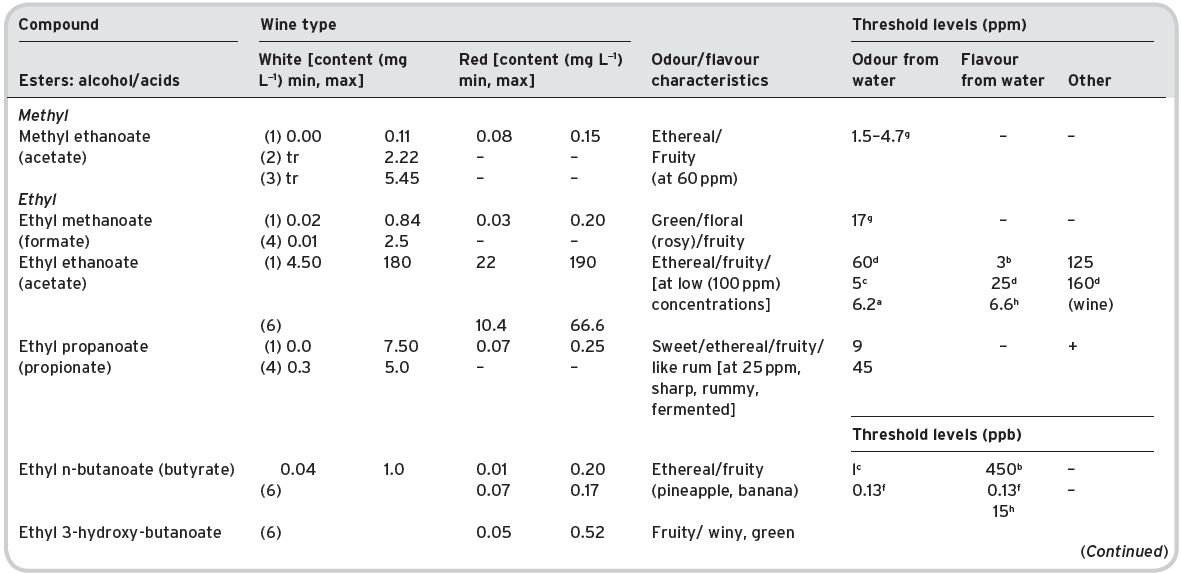
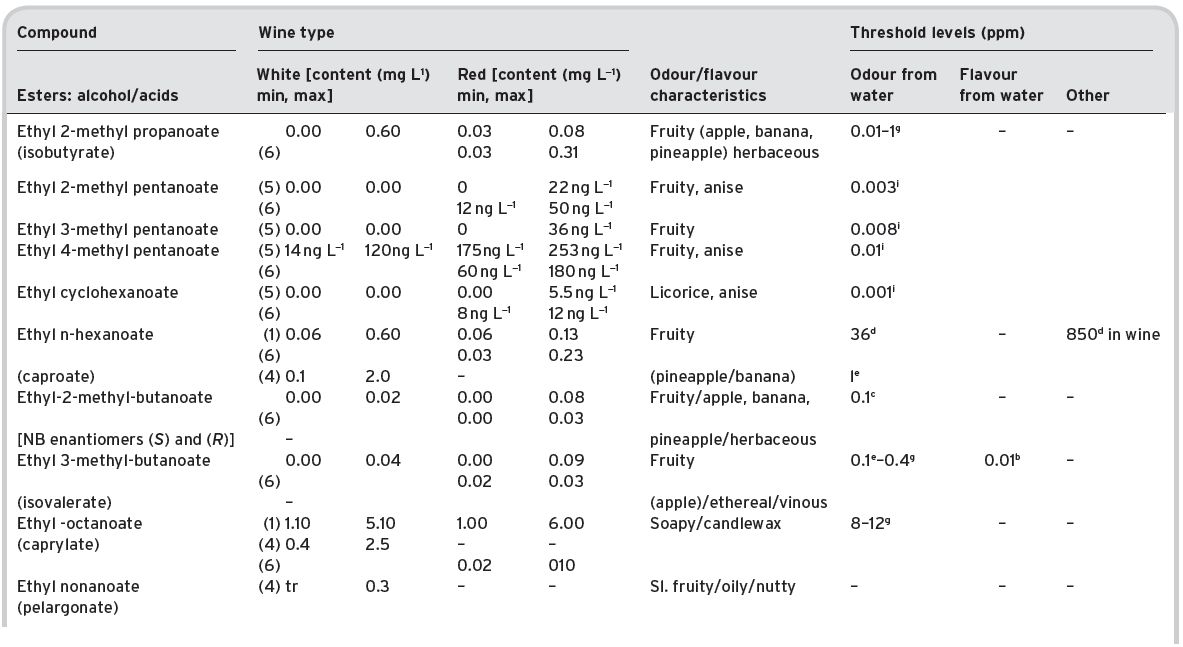
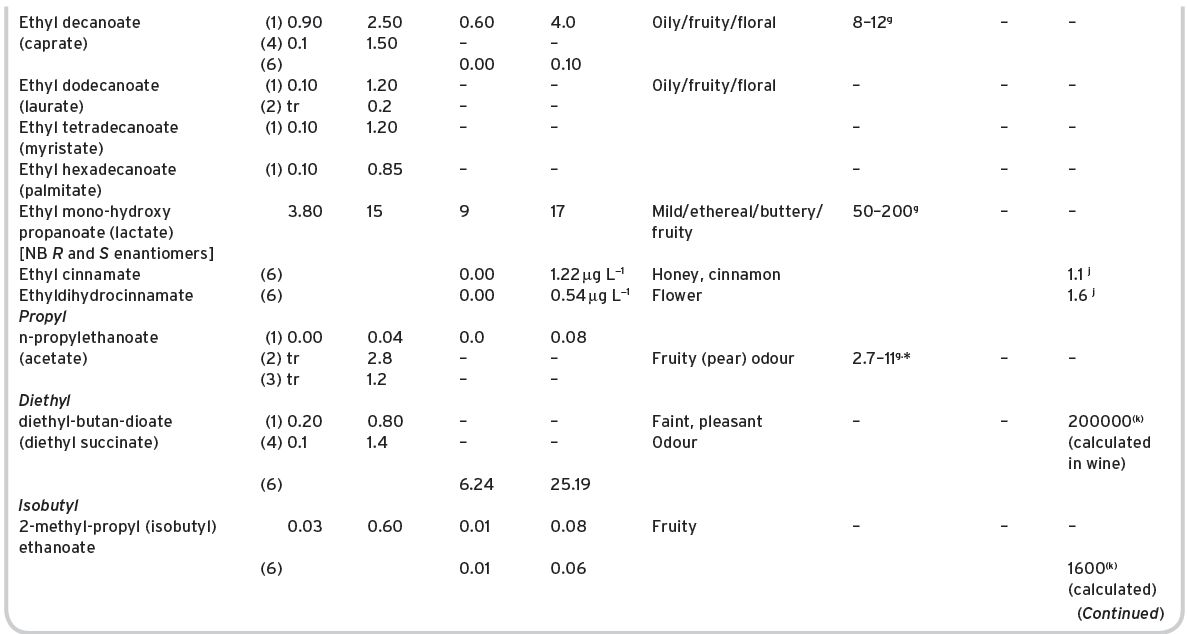
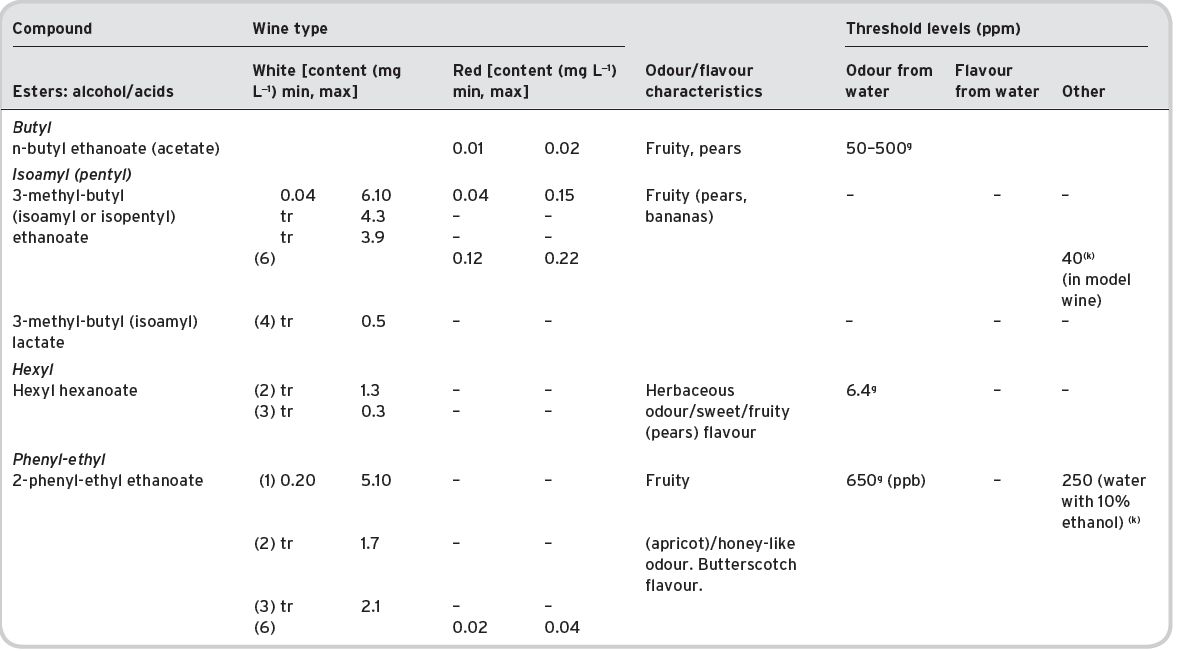
a Mulders (1973). bKeith & Powers (1968). cFlath et al. (1967). dRibéreau-Gayon (1978). eTakeoka et al. (1995). fAhmed (1978). gFeneroli (2002). hSick et al. (1969). iCalculated from Campo et al. (2007). jQuoted by Francis & Newton (2005). kQuoted by Escudero et al. (2007). *Value not consistent with ethyl, butyl and hexyl acetates.
References: (1) Ribéreau-Gayon (1978) quoting Bertrand (1975). (2) Aldave (1993) from 33 samples among the Spanish varieties, Xarel-lo, Macabeo, Parallada. (3) Aldave (1993) from 21 samples among Airen, and Jaen Pardillo, and Viura (4) 54 samples of (2) and (3). (5) Campo et al. (2007). (6) Escudero et al. (2007).
Table 4.12 Other esters in wines identified (multi-referenced), but not quantified: odour/flavour characteristics and threshold flavour values.
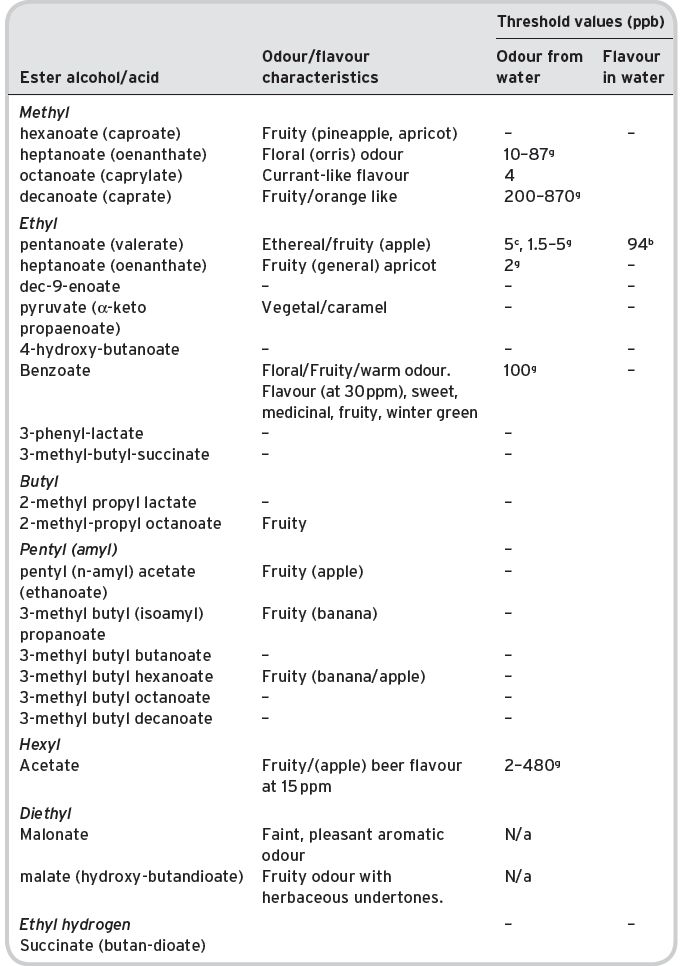
bKeith & Powers (1968). cFlath (1967). gFeneroli (2002). N/a not available.
Table 4.13 Esters likely to contribute to wine aroma, selected on wine concentration and sensory threshold informationa.
| Ethyl esters | Acetate esters | Cinnamic esters |
| Ethyl isobutyrate | Isoamyl acetate | Ethyl dihydrocinnamate |
| Ethyl 2-methyl butyrate | Phenylethyl acetate | trans-Ethyl cinnamate |
| Ethyl isovalerate | Ethyl acetate | |
| Ethyl butyrate | ||
| Ethyl hexanoate | ||
| Ethyl octanoate | ||
| Ethyl decanoate |
aFollowing Francis & Newton (2005).
It will depend on the grape variety, wine-making conditions and maturation which combination of esters will exert the greatest influence on the sensory properties of the wine. Some changes in content occur with malo-lactic fermentation, when practiced (e.g. increase of ethyl lactate).
From this data, it can be seen that ethyl acetate, on account of quantities often encountered (despite a relatively high odour/flavour threshold figure), could be an important flavour component of wines. However, though Ribéreau-Gayon (1978) regards 50–80 mg L−1 of ethyl acetate as being desirable in wines, with 125 mg L−1 a threshold flavour level, he considered that 160 mg L−1 contributes a ‘hard’ flavour, with an unpleasant pungent tang. Ethyl acetate is also listed as a potential off-flavour contributor (Chapter 5). Below threshold at 120 mg L−1 it can give a hot flavour in red wines, giving a bitter after taste. Acetic acid originates largely from undesirable spoilage (micro-organisms) by Acetobacter.
Ethyl lactate generally appears at relatively high amounts, especially after ageing, when lactic acid was formed in any malo-lactic acid fermentation, and/or from adventitious growth of lactic acid bacteria. It is not thought, however, that it contributes much to wine aroma, which is supported by a study of its physical property characteristics, as given in the Table II.1, in Appendix II along with those of all other esters present. The aroma effect of ethyl lactate in Tannat wines has been especially studied by Lioret & Versini (2002) in the Canary Islands. Similarly, diethyl succinate is another wine ester, again increasing in amount after ageing and, as already noted, representative of a high percentage of the wine distillate aroma (Vernin et al., 1993).
The recently described branched esters ethyl 2-, 3- and 4-methylpentanoate and one cyclic ester ethyl cyclohexanoate are thought to be important contributors to sweet-fruity aroma in wines (Campo et al., 2007). They are present in red and white wines, in particular in aged wines they reach concentrations well above their low thresholds (ppb levels) concentrations.
More studies focused on determining the contribution of the individual aroma compounds wine aroma, using analytical and sensory techniques as well as statistical analyses, for example Escudero et al. (2007) concluded that in the range of wine analysed, the berry fruit character could be related to the effect of nine fruity esters (ethyl butyrate, ethyl hexanoate, isoamyl acetate, ethyl 2-methylpropanoate, ethyl 2-methylbutyrate, ethyl 3-methylbutyrate, ethyl cyclohexanoate, ethyl 2-methylpentanoate, ethyl 4-methylpentanoate). It is likely that different groups of esters will contribute fruity flavours to different wines.
Table 4.12 gives some details of the ester compounds that have also been identified, but not quantified in the 1975 Bertrand data, which may have flavour significance in wine – many contributing ‘fruity’ characteristics. As most of these esters result from fermentations by yeasts (though not necessarily S. cerevisiae) they occur also in beer and such beverages as sake (rice wine). The composition of brandies (distilled product of grape wine) is also highly relevant.
4.3.2 Aldehydes
Presence in wine
Of the 25 aldehydes reported as being present by Montedoro & Bertuccioli (1986), only seven have three or more references to their detection. Ribéreau-Gayon et al. (2006) list 18 aldehydes (mostly alkyls) in wine, but state that, with the exception of acetaldehyde (ethanal) present at around 0.1 g L−1, or 100 mg L−1, they are only present in traces or in very small amounts. Whilst aldehydes can be present in grapes, under the conditions of vinification they will be largely oxidized to the corresponding alcohols. Acetaldehyde itself will be present as a fermentation product in differing amounts, dependent directly upon the quantity of sulfur dioxide also present, with which it combines. Acetaldehyde also combines with excess alcohol to form acetals. Only free acetaldehyde can be accorded flavour significance, e.g. as positively in Fino Sherries. Excess acetaldehyde confers ‘flatness’ in wines.
Hexanal, and the two hexenals [(trans) (E)-hex-2-enal and cis (Z)-hex-3-enal], the ‘leaf aldehydes’, are reported present in grapes and musts, together with 3-hexanol and trans-hex-2-enol by several investigators (Vernin et al., 1993) in Mourvèdre wines. Their presence is due to the crushing of grapes, prior to vinification, when enzymatic oxidation of linoleic/linolenic acid can occur (Chapter 7). However, it is also stated that this wine flavour is a result of the use of unripe grapes (Ribéreau-Gayon et al., 2006) and noted especially in the use of Grenache and Cabernet Sauvignon grapes. During fermentation, these aldehydes are transformed into the corresponding alcohols, which have a similar ‘grassy’ flavour at low concentration. As shown by Vernin, some amount of these aldehydes may remain unconverted. Octanal has been particularly mentioned in connection with Cabernet Sauvignon wines. Another aldehyde, (Z)-2-Nonenal, has recently been identified in a range of Spanish red wines, made from different grape varieties (Ferreira et al., 2009), described as green and metallic.
Flavour characteristics
These higher aldehydes, if present, have strong flavours/odours, in particular nonen-2-al, which occurs in two stereoisomers, E (trans) and Z (cis) of which the former is the more important. It is of interest as being substantially present in green arabica coffee (Grosch, 2001), though negligible amounts remain in the subsequent roasted coffee. It has also been identified in malted barley (Meilgaard & Peppard, 1986), though again not in the subsequent brewed beer. In the latter case, it is converted into nonan-1-ol and nonan-l-yl acetate.
Nonanoic acid is of much less sensory interest. Nonanal has been reported present in wines but until recently, not the corresponding unsaturated aldehyde. However, Ferreira et al. (2009) identified (Z)-2-Nonenal in a range of Spanish wines. There was no information on formation, concentration or sensory contribution to the wines. It was detected in relatively high concentrations in all wine samples, giving a green, metallic note.
Except, therefore, for acetaldehyde the sensory significance of these alkyl aldehydes is regarded as low. Acetaldehyde, above its threshold level and in free form, is usually regarded as an off-odour (‘flatness’); at high concentrations, it is characterized as pungent, even nauseating, but in dilute solution there are a number of more pleasant descriptions. A trace of acetaldehyde gives a recognisable apple like smell. It contributes significantly to the flavour of Sherry and of brandies.
Several aromatic aldehydes (or benzene derivatives) are of wine flavour importance, such as those developed during ageing in oak barrels. Thus, there are vanillin and cinnamic aldehyde, which are often recognized but little quantitative information appears available (further discussed in Section 4.4). Benzaldehyde (bitter almond) is a potential defect in wine but characteristic of certain grapes, such as Gamay. 2-Furfural and (5-hydroxy-methyl)-2-furfuraldehyde, resulting from carbohydrate oxidation, have also been reported, and increase in amount in in-bottle aged wines.
Only two aldehydes, acetaldehyde and phenylacetaldehyde, are listed as potential significant contributors to wine aroma by Francis & Newton (2005).
Detailed olfactory information is available for aldehydes (Table 4.14).
4.3.3 Ketones
Presence in wines
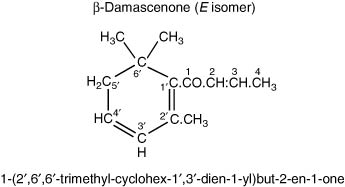
Of some 20 different ketones, only seven are multi-referenced in the listing by Montedoro & Bertuccioli (1986). β-Damascenone and α,β-ionones can be listed under terpenes, as also by Ribéreau-Gayon et al. (2006), but here (Table 4.15) they are listed simply as ketones.
Flavour characteristics
Diacetyl (buta-2,3-dione) may reach content levels to produce a sweet, buttery or butterscotch odour, in the range 1–4 mg L−1, though it can be regarded in ‘spoiled’ wines as an off odour (at up to 7.5 mg L−1). Low concentrations may impart yeasty, nutty, toasted aromas (see a review by Bartowsky & Henschke, 2004). They quoted that taste threshold levels for diacetyl are wine dependent, ranging from 0.2 to 2.8 mg L−1, thought to be due to other compounds present in wines. Control of its formation is another factor (see Chapter 7).
Table 4.14 Aldehydes in wines: content, general flavour/odour characteristics and threshold odour/flavour data.
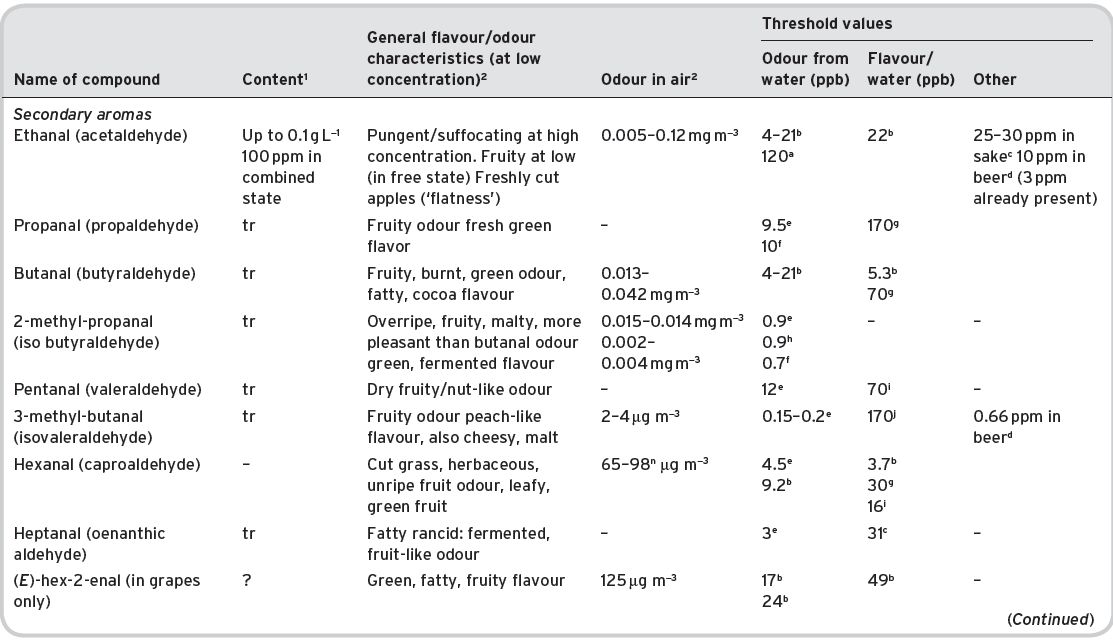
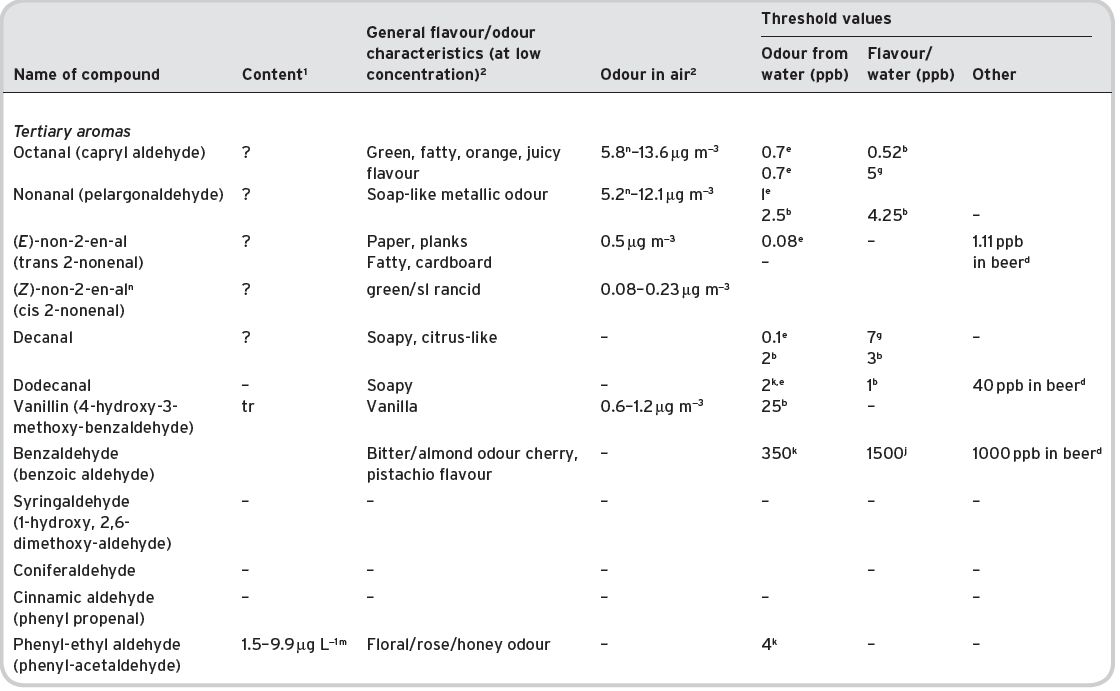
References: (1) Data from Ribéreau-Gayon et al. (2000). (2) Data from Flament (2001). aMulders (1973); bAhmed (1978) also quoted by Shaw (1986); cTakahashi & Ayiyama (1993); dMeilgaard (1982); eGuadagni et al. (1963b); fGrosch (1995); gLea & Swoboda (1958); hYakotsuka (1986); i Sick et al. (1971) ; jKeith & Powers (1968); kButtery et al. (1971a); lParliment (1981); mEscudero et al. (2007); nFerreira et al. (2009).
Table 4.15 Ketones in wines: contents, general olfactory characteristics and threshold data.
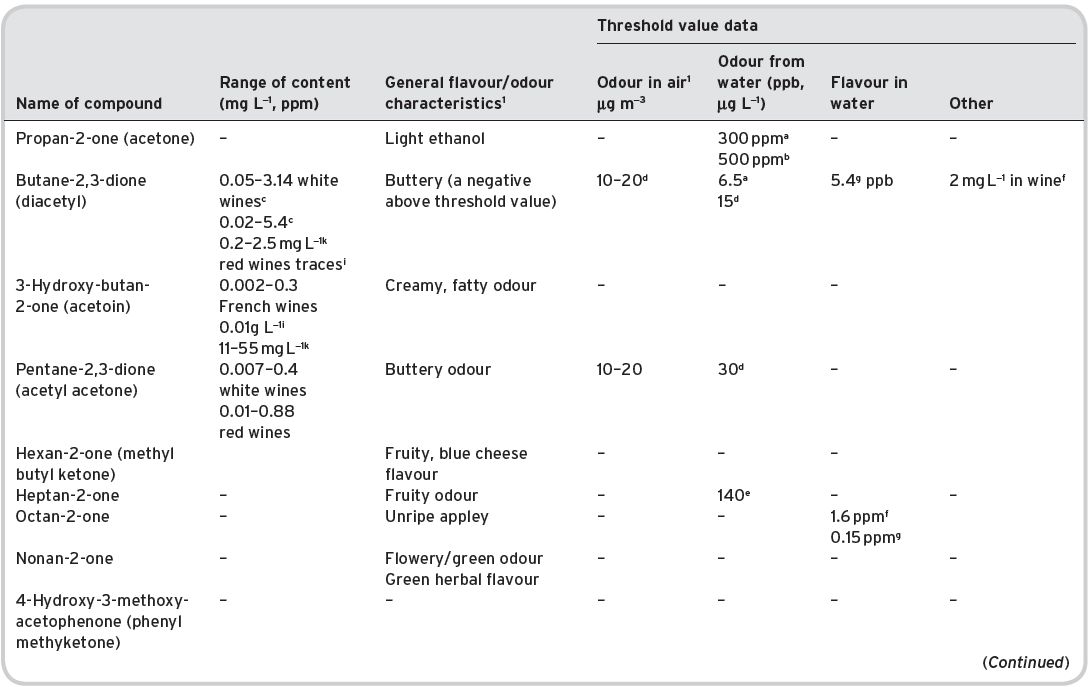
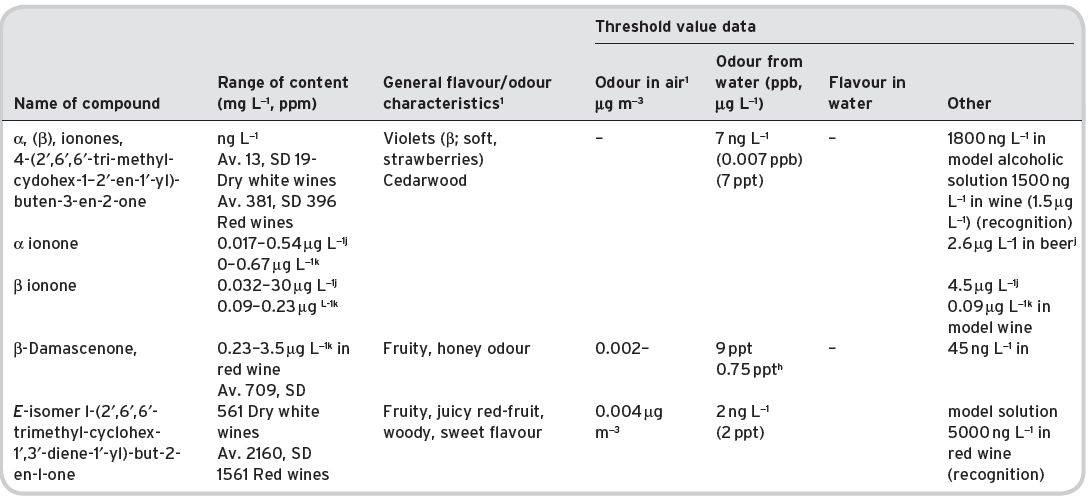
Av. = average value. SD standard deviation of range. References: 1Data from Flament (2001). aMulders (1973); bPerssons et al. (1973); cNykanan & Soumaileinan (1983); dBlank et al. (1992), quoted by Grosch (2001); eButtery et al. (1969a); fLea & Swoboda (1958); gSiek et al. (1969), quoted by Flament (2001); hData of Rychlik et al. (1998), quoted by Grosch (2001); iRibéreau-Gayon et al. (2000). jQuoted by Swiegers & Pretorius (2005). kEscudero et al., 2007, for five red wines. All other data for ionones/damascenone from Chattonnet & Dubordieu (1997), quoted by Ribéreau-Gayon et al. (2006).
Acetoin (3-hydroxybutan-2-one; or acetyl methyl carbinol in some literature) has a similar slightly milky odour, and may be perceptibly present in wines. The other simple aliphatic ketones, though present and formed during fermentation, are not considered to have much flavour significance in wines.
The complex ketones, β-damascenone and α,β-ionones (so-called isoprenoids derived by oxidative degradation from carotenoids) are present, partly as a result of crushing the grapes. β-Damascenone has a variably described odour, sometimes rose-like, and is believed to contribute to the aroma of wines from grape varieties such as Chardonnay, but it is probably present in all wines. A study on the aroma impact of β-damascenone in red wines by Pineau et al. (2007) places doubt on how large its contribution on wine aroma is. They studied 23 French wines from six different regions and comprising eight different grape varieties and determined a concentration range from 545–2307 ng L−1, whilst they quote a range based on literature between 1–1.5 μg L−1. They also accurately analysed the sensory threshold value of β-damascenone. The threshold in water/ethanol solution was 50 ng L−1, but in wines these values were significantly higher (model white wine 140 ng L−1, model red wine 850–2100 ng L−1 and red wine 7000 ng L−1). Hence the perception threshold appears to depend significantly on the matrix used and in red wine it ranges probably between 2–7 μg L−1, within the range of the value quoted in red wine in Table 4.15. Hence they concluded that there may not be a direct impact of β-damascenone on red wine aroma, although they suggested that it may enhance the fruity notes in particular of ethyl cinnamate and caproate, which need to be further investigated. Escudero et al. (2007) also suggested a flavour enhancing role for β-damascenone. In contrast, the detection threshold of β-damascenone in gas chromatography olfactometry is very low, which may have led to the conclusion in other research papers that β-damascenone does contribute to red wine aroma.
Also, it has been shown by Grosch (2001) to be present in green coffee, and an important contributor to the flavour of coffee brews from roasted coffee, since its threshold level is very low (in water). Similarly, α- and β-ionones occur in Riesling grapes, again, having a somewhat variably described odour, but notably, of violets. Considerable data on these ketones in wine has been obtained by Chatonnet & Dubourdieu (1997), and reported by Ribéreau-Gayon et al. (2006). The latter tabulate content and threshold information for 12 white wines and 64 red wines, showing that the content of both these ketones is much higher in red wines than in white (especially β-ionone). Both these compounds are present in considerable amount in brandies (4.I and 4.II).
4.I
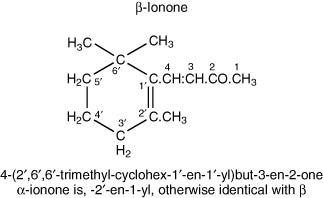
4.II
Certain ketonic substances are associated with the ageing of wines, such as the oak-lactones, but these are described under lactones/furanones (Section 4.3.6).
Based on a literature survey, Francis & Newton (2005) listed 2,3-butanedione, acetoin, β-damascenone and β-ionone as potental significant aroma ketones in wines.
4.3.4 Acetals
Acetal (1,1-diethoxyethane) is formed by the reaction of acetaldehyde with ethanol and regarded as flavour significant. Similarly, other acetals are formed from other alcohols and aldehydes; though only 12 acetals are mentioned by Montedoro & Bertuccioli (1986) up to 20 are suggested as having been detected in wines by Jackson (2008) and Ribéreau-Gayon et al. (2006). They have an herbaceous-like character but are regarded to have little significance in wine flavour (except Vin Jaune, of the Jura) and they are more important in Sherry and aged Ports (Chapter 6), in which conditions for their formation are more favourable.
4.3.5 Alcohols
Some 39 alcohols (plus ethyl alcohol) have been identified and listed by Montedoro & Bertuccioli (1986) but only 16 are multi-referenced. Ribéreau-Gayon et al. (2006) list 28 different alcohols (mainly alkyl). Contents of various alcohols in wines have been determined, and are shown in Table 4.16, together with some sensory description information, and threshold values.
Presence in wines
There is some detailed quantitative information (Aldave et al., 1993) on young Spanish white wines (Table 4.17).
The data of Vernin et al. (1993) on Mourvèdre grape wine shows the proportions of the different alcohols present in a distillate, following GC analysis, as shown in Table 4.18.
Methanol is normally present at low concentrations, 0.1–0.2 g L−1 quoted by Jackson (2008), between 30 and 35 mg L−1 quoted by Ribéreau-Gayon et al. (2006) and does not contribute to the sensory properties of wine. These concentrations are too low to pose a toxicity risk. Even its contribution to esters formed in wine is small. Methanol is formed due to pectin degradation and as the pectin levels in grapes are low, the amount of methanol formed is low. The amount formed depends on the maceration used, hence red wines have higher concentrations than rosé or white wines. Ethanol is of course the most abundant alcohol in wine and discussed in Section 3.2.
Most higher alcohols, having more that two carbon atoms, are formed during yeast fermentation, and often constitute about 50% of the volatile aroma compounds in wine, excluding ethanol. Typical concentrations in wine are 150–550 mg L−1 (quoted by Ribéreau-Gayon et al., 2006). Spoilage by yeasts and bacteria can increase the concentration of higher alcohols.
The alcohols (C4 upwards), mainly the branched chain compounds, such as 2-methylpropan-1-ol, and the 2- and 3-methylbutanols, together with the normal straight chain compounds, such as butan-1-ol, as well as the benzenoid alcohol phenylethanol are known as fusel oils, and are usually present in fair quantity. The most important of these are the amyl alcohols, C5H11OH, of which there are three main isomers, pentan-1-ol (normal amyl), 3-methyl butan-1-ol (isoamyl) and 2-methyl-butan-1-ol (optically active amyl alcohol). The word ‘amyl’ comes from the Latin ‘amylum’ and means starch, so these alcohols are known as fermentation alcohols. Other pentanols such as the -2, and -3-ols are believed present, but only in traces.
The quantity of fusel oils present has been related (like esters) to the vinification conditions by Ribéreau-Gayon (1978) (quoting the works of Soufleros, 1978; Doctoral Thesis); that is, lower fermentation temperatures (e.g. 20°C and pH 3.4) produce higher amounts of total alcohols (i.e. 201 mg L−1) than higher temperatures. Similarly, higher amounts are also produced by not clarifying juice by racking before fermentation. See also Chapter 7.
Table 4.16 Higher alcohols in wines: contents, general olfactory characteristics and threshold value data
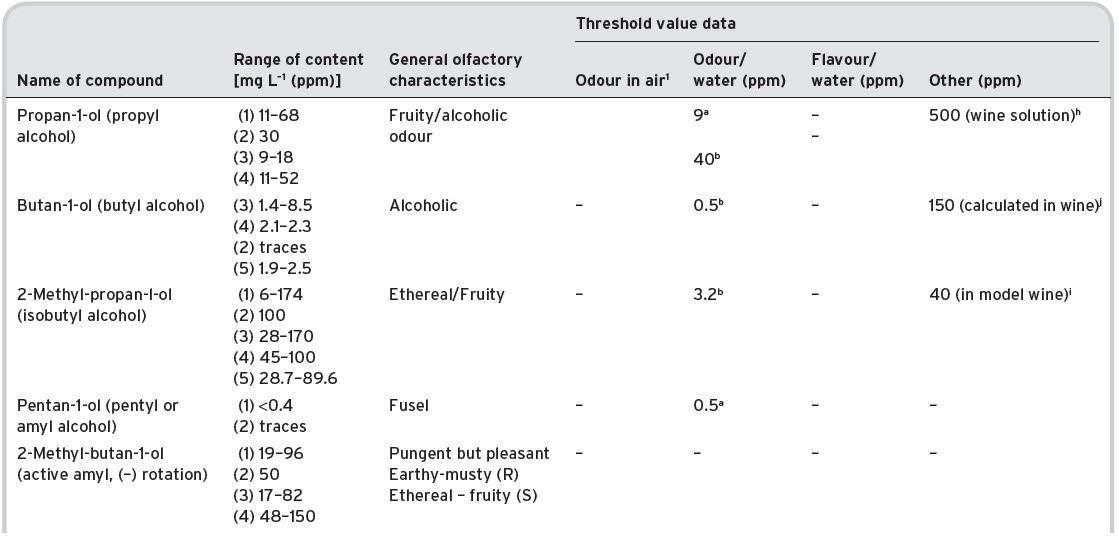
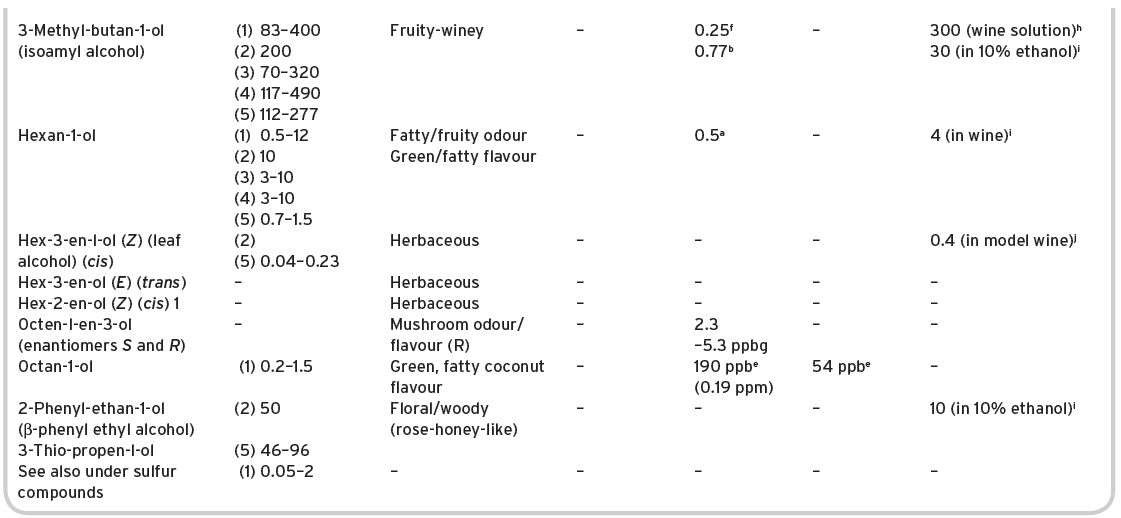
References: (1) Amerine & Ough (1983); (2) Ribéreau-Gayon et al. (2000); (3) Bertrand (1975) for white wine; (4) Bertrand (1975) – red wine quoted by Ribéreau-Gayon et al. (1978). (5) Escudero et al. (2007), range of five5 red wines.
a Flath (1967); b Mulder (1973); c Yakotsuka (1986); d Ribéreau-Gayon (1978); e Ahmed (1978); f Buttery et al. (1971a); g Grosch and Würzenberger (1985), quoted by Flament (2001). h Ouoted by Swiegers & Pretorious (2005). iQOuoted by Swiegers et al. (2005). jOuoted by Escudero et al. (2007).
Table 4.17 Higher alcohols found in Spanish white wines (additional data of Aldave et al., 1993).
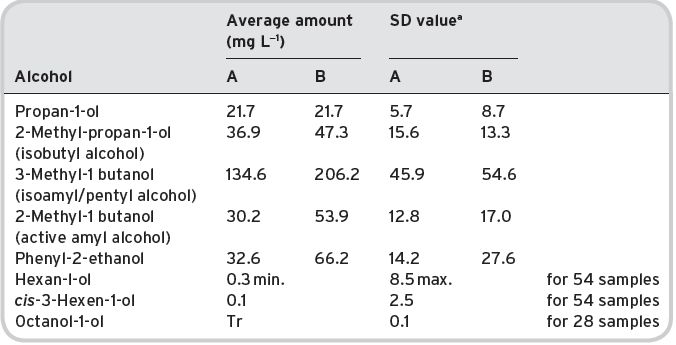
a SD Standard deviation of mean value. Group A: 33 samples from Xarelelo, Meccbeo and Paralleda grapes used for sparkling wines. Group B: 21 samples, Airen, Jean, Pardillo and Viura grapes.
Table 4.18 Percentages of alcohols in a distallate following GC analysis of wine made from MourvÒdre grapes in 1984a.
| 2- and 3-Methyl butanols | 31.60 |
| 1-Butanol | 3.32 |
| β-Phenylethanol | 2.77 |
| 2-Methylpropanol | 0.84 |
| 1-Hexanol | 0.47 |
| Benzyl alcohol | 0.27 |
| cis-3-Hexen-1-ol | 0.20 |
| trans-3-Hexen-1-ol | 0.17 |
| trans-2-Hexen-1-ol | 0.13 |
| 2-Pentanol | 0.30 |
| 1-Penten-ol | 0.05 |
| Total (11) | 39.95% of all volatile compounds present |
aTaken from data in Vernin et al. (1993).
Table 4.19 Numbering of atoms in linear and cyclic lactones.
| No in linear chain | No in ring |
| 1 | 2 |
| 2 | 3 |
| 3 | 4 |
| 4 | 5 |
Flavour characteristics
Higher alcohols generally are thought to contribute to the complex aroma of wines, however, they are not considered to be desirable at high concentrations and may mask the fruitiness of the wine. These fusel alcohols have a characteristic pungent odour. At a high concentration (>300 mg L−1) these are negative quality factors but at lower levels they add to the desirable aspects of wine flavour. Their esters, in particular isoamylacetate, may contribute a fruity banana note to young wines.
Hexanol is known as ‘leaf’ alcohol, and has a ‘grassy’ flavour that is reflected in the flavour of wines in which it is present in a sufficient amount, together with cis- and trans-3-hexen-1-ol and trans-2-hexen-1-ol. It is often originally present in the grape must, due to enzyme action on linoleic acid, resulting from grape crushing techniques (see also Section 4.3.2). Vernin et al. (1993) have compared content in Mourvèdre must with wine and generally showed a decrease in the amount of hexanol and the enols, as a result of fermentation. Oct-1-en-3-ol is reported to be present in wines, due to Botrytis action (Chapter 7) and has a mushroom aroma.
4.3.6 Lactones and furanones
Lactones/furanones are widely distributed in nature, particularly in the fruit of plants. The organic substances known as lactones are particularly associated with Sherry but some lactones originate from ageing in oak barrels.
Molecular structures
Chemically, lactones are inner anhydrides of hydroxy acids, resulting from the splitting off of one molecule of water. The so-called γ- and δ-lactones in older literature are the most easily formed compounds. They are generated from aliphatic hydroxy-acids with the hydroxy group in the 4 or 5 position (4.III).
4.III
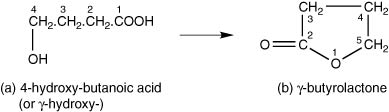
NB Numbering of the atoms has changed as in Table 4.19 below. (Other groups can be attached on linear carbon = 4–ring carbon-5.)
γ-Lactones have also been known as ‘-olides’; thus γ-butyrolactone was also known as ‘butanolide’ but they are now more correctly described as furan derivatives, as can be expected by the basic furan ring present. Thus, γ-butyrolactone is now ascribed as dihydro-3(H)-furan-2-one.
The term ‘3(H)’ means that a hydrogen atom has been moved from the 2- to the 3-position in the ring (as the result of carbonyl formation) and that there are two additional hydrogen atoms present, at positions 4 and 5, in addition to those in a simple furan ring (4.IV).
Table 4.20 Lactones/furanones in wines: contents and olfactory characteristics (descriptions and threshold data).
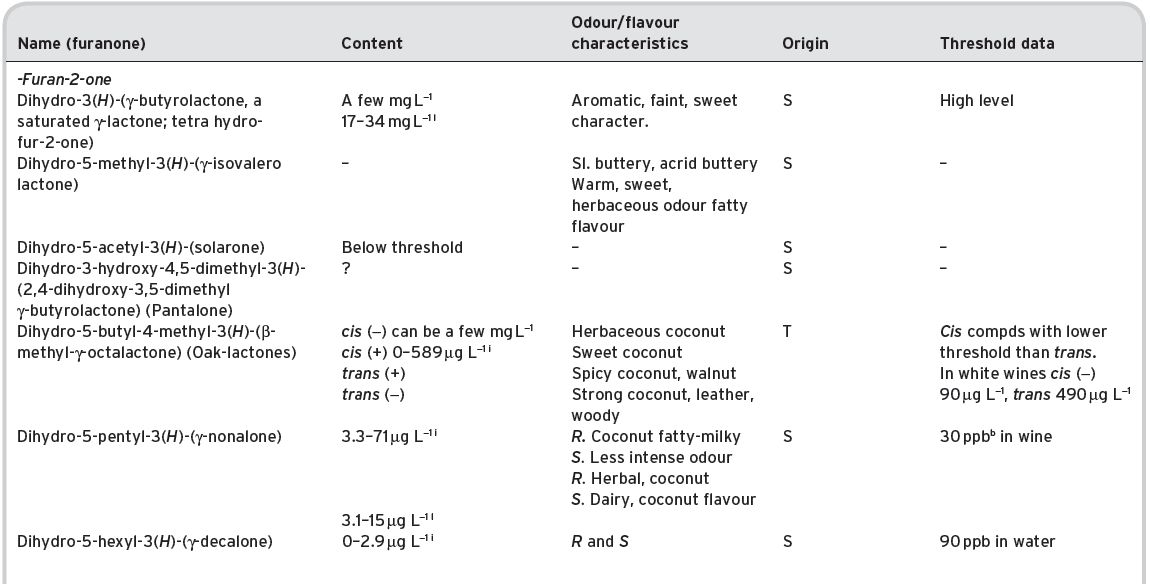

References: aRibéreau-Gayon et al. (2000). bNakamura et al. (1988). cKeith & Powers (1968). dSemmelroch & Grosch (1996). eRychlik et al. (1998). fButtery (1999). gHuher (1992). hBruche (1995). iquoted by Francis & Newton (2005). jFerreira et al. (2003). lkCamara et al. (2006). lEscudero et al. (2007) determined in red wines.
4.IV
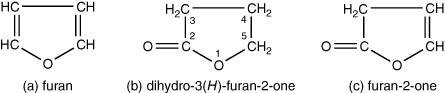
Dihydro compounds are so-called saturated γ-lactones and are also referred to as tetrahydro-furanones.
The symmetry of the furan ring also means that furan-2-one is identical with furan-5-one and similarly, for substitutes at positions 3 and 4.
A compound such as 4-hydroxyisovaleric acid (4.V) forms a furanone (4.VI).
4.V
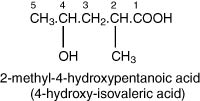
4.VI
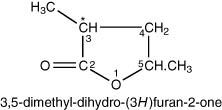
Some lactones are derived from unsaturated hydroxy-acids.
Several other chemical reactions lead to the formation of furanones, and where the beta position is at C3 (or C4) they are thus known as furan-3-ones, which are not, strictly speaking, lactones.
Stereoisomers of these lactones are possible with cis and trans geometric forms, and optical variants with flavour implications (see the next section on Flavour characteristics).
When linkage takes place with a hydroxyl- group in the 5 (linear) position, the resulting lactone is the δ-lactone type, now forming a 6-membered ring, i.e. a pyran ring. Thus, the ‘5’ (linear) position may be in a benzene ring (4.VII and 4.VIII).
These substances may still be referred to as either γ or δ-lactones, but their origins can be determined from their furanone/pyranone structure.
4.VII 4.VIII
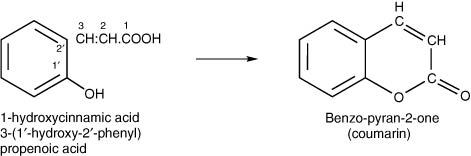
Presence in wines
Some 20 lactones have been identified in wines and are listed by Montedoro & Bertuccioli (1986) and several others have been recently identified, both in grapes and extracted from oak barrels during ageing. Most lactones/furanones appear to be formed during fermentation. γ-Butyrolactone is present at about 1 mg L−1 (Ribéreau-Gayon et al., 2006), but it is not considered an important flavour component in wines. Certain lactones are believed to be present in certain kinds of grapes, thus:
(1) Riesling and Muscat grapes; 2-vinyl-dihydrofuran-2-one.
(2) HDMF or furanol is believed present particularly in Merlot wines, but also in others, including Vitis lambrusco wines.
(3) Sotolon is particularly associated with Botrytis infected grapes and wines (Noble Rot) occurring at above the sensory threshold of 7.5 ppb (Mauake et al., 1992), also identified in the Vin Jaune from the Jura, France (Ribéreau-Gayon et al., 2000).
(4) Sotolon has also been found in fortified Port wines (Fereira et al., 2003). In Madeira wines sotolon levels identified were up to 2 mg L−1 in wines aged 25 years (Camara et al., 2004).
However, Vernin et al. (1993) report few lactones/furanones in the Mourvèdre and Syrah grape must and wines they examined. There appears to be relatively little quantitative information and it is thought that, unlike in Sherries, their flavour importance in wines is low. Lactones tend to be high-boiling compounds and their solubility in water is relatively high with consequent low volatility. Based on a literature review, Francis & Newton (2005) concluded that the following lactones may contribute to the aroma of some wines since their concentration was near or above threshold concentration: cis-oak lactone, γ-nonalactone, γ-decalactone, γ-dodecalactone, 4-hydroxy-2,5-dimethyl-3(2H)-furanone and sotolon.
Nevertheless, the so-called oak-lactones, sometimes referred to as whisky lactones, present in aged wines have aroused considerable interest, with concentrations at several mg L−1. The main oak-lactone is 5-butyl-4-methyl-dihydro-3(H)-furan-2-one. There are two geometrical isomers (cis and trans) and two optical isomers for each, (+) and (−), with differing sensory characteristics. Ribéreau-Gayon et al. (2006) report in detail the findings of Chatonnet (University of Bordeaux) and others on these characteristics, and furthermore on their extraction from oak barrels including the effect of barrel treatment (fresh, steamed and toasted).
Table 4.21 Volatile acids in wine: contents, general olfactory characteristics and threshold values data.
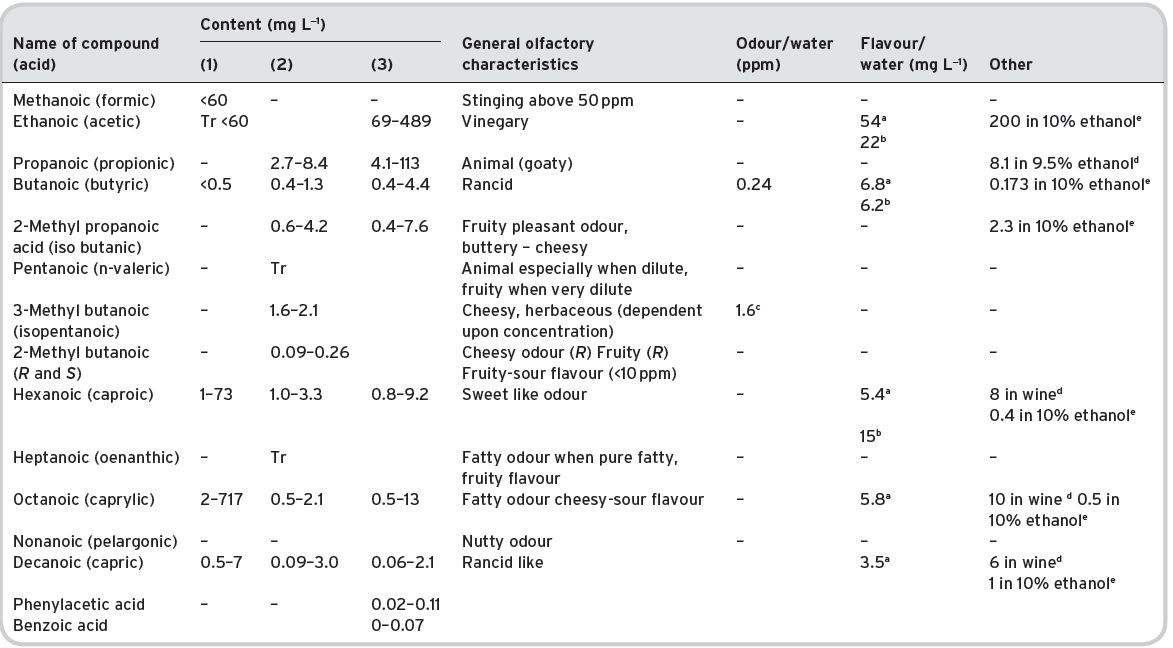
References: (1) Amerine & Roessler (1983). (2) Escudero et al. (2007) determined in red wines. (3) Quoted by Francis & Newton (2005).
aPatton (1964); bSick et al. (1971); cKeith & Powers (1968). dSwiegers & Pretorius (2005). eQuoted by Francis & Newton (2005).
Some of these lactones/furanones have also been found in green coffee, roasted coffee and coffee brews (Grosch, 2001).
Flavour characteristics
Lactones/furanones show a range of flavour characteristics. Though of similar chemical structure, sotolon (an unsaturated 3-hydroxy-2-one) and HDMF or furanol (also unsaturated, 4-hydroxy-3-one) have very different odour/flavour characteristics (see Table 4.20). Thus, the latter isomers have a fruity/caramel odour impression, whilst the former has some spicy notes. The 2-ones tend to be fatty and herbaceous, especially the lower molecular weight compounds, the higher molecular weight compounds include coconut notes. The trans oak-lactone is distinctive in showing spicy notes.
Many flavour investigators have described enantiomers of each compound as having often quite different odour/flavour characteristics. Unless they were well separated when assessing their flavour, there may be some anomalies in their descriptions.
Recently, HDMF has been shown to be an important flavour contributor to roasted coffee and its brews, with sotolon and other furanones important in both green and roasted coffee (Grosch, 2001).
A listing of these compounds is provided in Table 4.20 together with olfactory information, as available.
4.3.7 Acids
The main concentration of acids are non-volatile and are discussed in Chapter 3. Montedoro & Bertuccioli (1986) list some 116 acids as having been identified in wine, but of these only some 14 are volatile liquid substances, while the remainder like the hydroxy benzoic acid are generally crystallizable solids with higher melting points. Though some acids may have recognizable odours, these may be due to lower boiling point contaminants. In addition, these acids will be rather soluble in water. Of the 14 separately identified nearly all are multi-referenced (three or more). Some quantitative data is included in Table 4.21.
Presence in wines
Ribéreau-Gayon et al. (1982, quoted by Ribéreau-Gayon et al., 2006) analysed all fatty acids in the table to be present in trace amounts. In a Mourvèdre wine, Vernin et al. (1993) identified all these acids up to octanoic acid but in this aroma complex only three acids, butanoic, 3-methyl-butanoic and hexanoic, were included in a total GC peak area assessment, consisting of 0.78% of the total. In their examination of 13–16 young Spanish white wines Aldave et al. (1993) only reported quantitative information on octanoic acid, averaging 1.3 mg L−1 in wines made where SO2 had not been used and 2.6 mg L−1 in wines made with SO2. Patton (1964) has described some work on the odour thresholds of fatty acids. Escudero et al. (2007) analysed the acids in five different Spanish red wines and Francis & Newton (2005) published a list based on a literature survey, including odour threshold values (see Table 4.21).
Table 4.22 Amines found in wine: contents together with general odour character and threshold values.
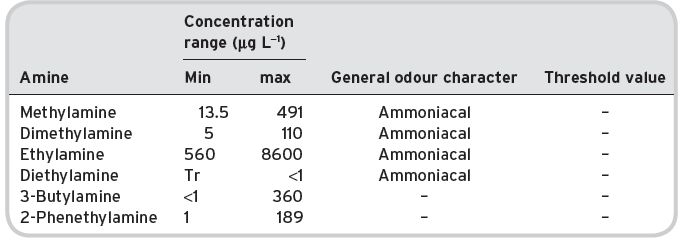
Content data from Ough et al. (1981), quoted in Montedoro & Bertuccioli (1986).
Flavour characterisitcs
At concentrations near their threshold value, fatty acids contribute to the complexity of wine but at higher concentrations they have a negative impact. High acetic acid concentrations indicate spoilage but yeasts will also form some, so acetic acid will constitute the volatile acidity in wine. Propanoic and butanoic acid can be associated with bacterial spoilage. Hexanoic, octanoic and decanoic acids are formed by yeasts and seem to have generally negative aroma descriptors in wines.
4.3.8 Nitrogeneous compounds
Montedoro& Bertuccioli (1986) identified some 31 different amines and amides in wines, though many are non-volatile and mainly single referenced. In addition, Ough (1981) has quantified their presence in wine, as shown in Table 4.22, with odour data. Note that the concentrations of these listed components are all very low, i.e. μg L−1.
Their concentration decreases during fermentation, since yeasts can metabolize amines. They can also be formed by yeasts. Their contribution to wine flavour has not been established but they can contribute a harsh flavour to beer (see Jackson, 2008).
4.3.9 Phenols
As noted in Chapter 3, phenolic and polyphenolic compounds abound in grapes, musts and wines but our interest here is only in volatile phenolic compounds.
Presence in wines
Some 17 phenols were listed by Montedoro & Bertuccioli (1986). Tressl (1976) listed 13, together with their content, which were present in the order of μg per litre, rather than the expected mg per litre. Later information on certain of these phenols has been obtained by Chatonet (1992, 1993) (quoted by Ribéreau-Gayon et al., 2006) (Table 4.23). A detailed method for accurate determination of ethyl phenols in eight wines ranged from 394–1292 μg L−1 for 4-ethylphenol, 42–107 μg L−1 for 4-ethylguiacol and 0–99 μg L−1 for 4-ethylcatechol (Carillo & Tena, 2007).
Flavour characteristics
Of these phenols, vinyl-4-phenol, vinyl-4-guaiacol, ethyl-4-phenol and ethyl-4-guaiacol are regarded by Ribéreau-Gayon et al. (2006) as being especially important in an olfactory defect known as ‘phenol’ character, which occurs, unfortunately, relatively frequently. Changes in wine-making, in particular maturation, contribute to their occurrence. Recent research regarding their formation, sensory characteristics and occurrence in wines is reviewed by Suarez et al. (2007) and discussed in Chapter 7. Vinyl compounds may be formed during fermentation, whereas ethyl compounds are much more likely to develop during barrel ageing (further mentioned in Chapter 7).
In addition to the phenolic medicinal character, several such compounds have also been described as having a ‘smoky’ or ‘tarry’ or even ‘bacon-like character’, though not many appear to be present in sufficient quantity to affect flavour. Their origin is described in Chapter 7.
Detailed sensory information is included in Table 4.23.
4.3.10 Terpenes
Terpenes and their derivatives (generally called terpenoids) are widely distributed in nature, and occur in different amounts in grapes; they persist through vinification and can be thus described as essentially primary aromas, or even varietal. It is not possible here to describe all the different types of terpenoids, including bicyclic forms (not present in grapes).
Chemical structure
Terpenes consist of a wide range of substances, which have been regarded as deriving from a basic structure of a linear chain of five carbon atoms, as in isoprene (C5H8): CH2=C(CH3)CH=CH2.
Table 4.23 Volatile phenols in wines: content, together with sensory information.
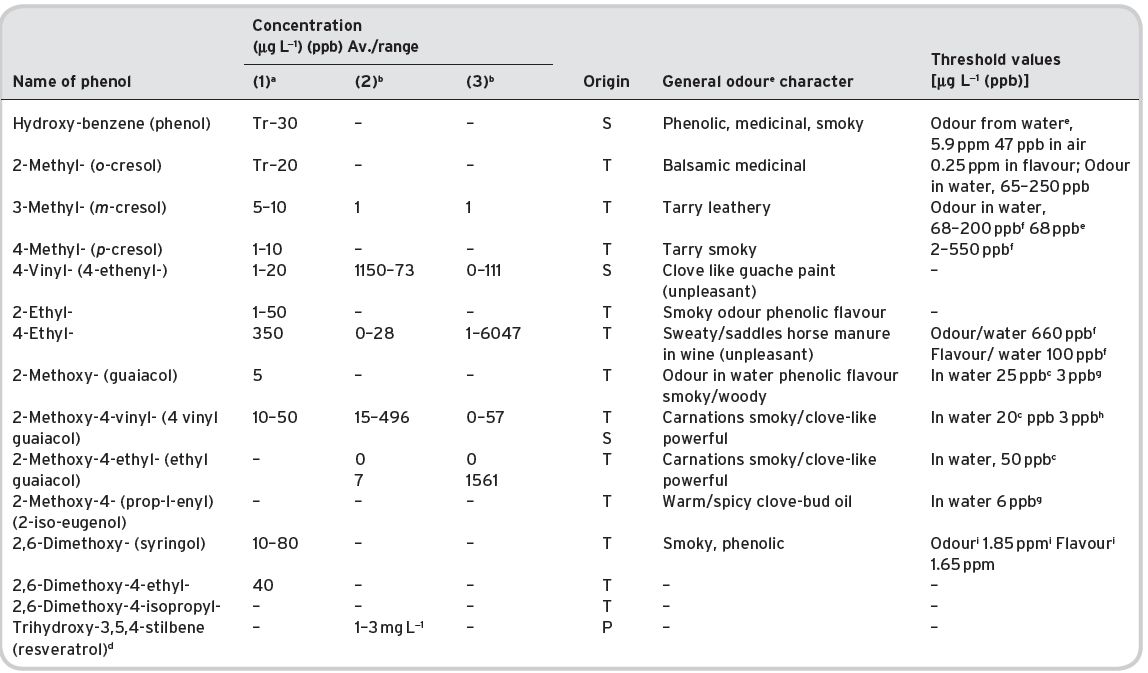
aData from Tressl et al. (1976). bData from Chatonnel et al. (1992 and 1993) for (2) white wines and (3) red wine, quoted by Ribéreau-Gayon et al. (2000). cData from Rychlik (2001) quoted by Grosch (2001). dData from Ribéreau-Gayon (1978). eMaga (1978). fKim Ha & Lindsey (1991). gButtery et al. (1971a). hButtery et al. (1976). iWasserman (1966).
Table 4.24 Main types of terpenes in wines: contents according to grape variety and location (μg L−1).
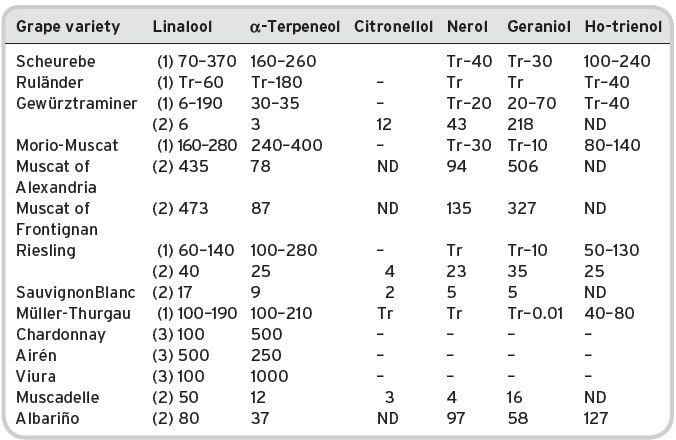
Data: (1) Schreier et al. (1977). (2) Ribéreau-Gayon et al. (2006) quoting others. (3) Aldave et al. (1993).
There are many terpenes, which all have the basic formula of C10H16 with two double bonds but there are also compounds with a cyclic structure (monocyclic terpenes). The basic ring structure is shown by 4.IX.
4.IX
The main compounds of wine flavour interest are the hydrocarbons themselves, but more particularly their alcohols, both with linear and monocyclic structures.
The structure (4.IXa) conforms generally with an older numbering system for the carbon atoms, while 4.IXb has the IUPAC system of numbering the carbon atoms of the ring separately, e.g. those for which attached radicals have separate numbers, as will be seen in the compounds to be described (e.g. α-terpineol).
These structural formulae also indicate a relationship to the hydrocarbon p- (or 1,4) cymene, which is 4-isopropyl-1-methylbenzene and to hexa-hydrocymene (menthane). Some chemical names of the unsaturated compounds may show a menthene connection. Straight chain compounds are then regarded as having a molecular structure opened up at the C1 and C6 carbon atoms, with a slightly different numbering carbon system. Structurally, many may contain asymmetric carbon atoms (e.g. at C8), so that optical isomers exist, though in nature, a particular form is usually prevalent (+), or (−). Geometrical isomers (Z and E), due to presence of double bonds, may also occur. Terpenes therefore have complex molecular structures.
The correct chemical names and structures of the important terpene compounds found in wines, are as follows:
- Straight chain monoterpenols.
- Linalool, 3,7-dimethyl-1,6-octadien-3-ol (4.X).
4.X
The structure as illustrated to show relationship with a corresponding cyclic compound otherwise, in a linear form (4.XI).
4.XI
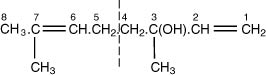
Enantiomers result from the C3 position, whilst geometrical isomers result from the C6 (and C2) position. Isomerism can occur at the double bond C1 position to C2:
- Geraniol, 3,7-dimethyl-octo-2–6-dien-1-ol (4.XII).
4.XII
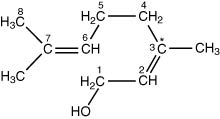
Nerol is a structural isomer (-8-ol).
- Citronellol, 3,7-dimethyl-octa-6-en-1-ol (4.XIII); (+) and (−) isomers known.
4.XIII
- Ho-trienol, 3,7-dimethylocta-1,5,7-trien-3-ol (4.XIV).
4.XIV
- Cyclic terpenols: α-terpineol (terpineol-8), 2-(4′-methyl-3′-cyclohexene-l′-yl) propan-2-ol or p-menth-1-en-8-ol (4.XV); both (+) and (−) isomers are known.
4.XV
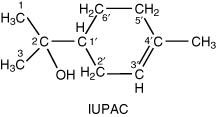
Presence in grapes/wines
In the Bertuccioli–Montadoro (1986) list, 19 different terpenoids have been identified in wine by different investigators, many, however, with only one reference. Ten had been well investigated and their content quantified. According to Ribéreau-Gayon et al. (2006), about 40 different terpenes have now been identified in grapes but only six compounds (linalol, α-terpeneol, citronellol, nerol, geraniol and ho-trienol) are regarded as having flavour/aroma significance in wines. Ebeler & Thorngate (2009) in their review report that over 50 monoterpenes having been identified in grapes and wines. Rotundone, a complex terpene recently identified, contributes significantly to Shiraz, Mourvèdre, Durif and possibly some Cabernet Sauvignon wines (Wood et al., 2008).
They exist in grapes in three forms: volatile forms are free monoterpene alcohols or oxide, a variable proportion is non-volatile and complexed to glycosides, or may occur as di- or tri-ols, in which forms they are nonvolatile and will not contribute to aroma. They are largely present in the skins of grapes. There is little detailed information on the content of any separate stereoisomers.
Table 4.25 Terpenes in wines: olfactory characteristics (odour/flavour descriptions and threshold values).
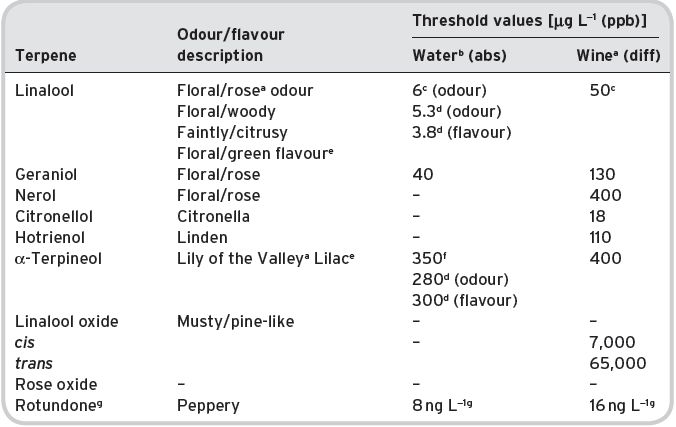
aRibéreau-Gayon et al. (2006); bShaw (1986); cButtery et al. (1969a); dAhmed (1978); eChemisis (1989); fButtery et al. (1971a); gWood et al. (2008).
Their content in grapes varies with different varieties/cultivars of Vitis vinifera and is highest in the Muscat variety but they are also present significantly in some ‘noble’ varieties, e.g. Riesling. Monoterpenes contribute to the varietal character of Viognier, Albariño and Muscadelle. Though other ‘neutral’ grapes may have zero or low content such as the white grape Chardonnay as already noted, grapes for red wines usually have a low terpene content, except Black Muscat. During ageing, the types and proportion found in wine change; Table 4.24 gives some available data on contents from different sets of investigations, and is discussed further in Section 4.4.
The marked content of terpenes in a Muscat wine is notable, though other varieties of grape have high contents of linalool. Ribéreau-Gayon (1978) has also provided some data on terpene contents in Muscat grapes themselves, e.g. average 0.4 mg L−1 of linalool and 0.8 of geraniol, as the main contributors, whilst α-terpineol is at a low level (0.06). Changes in terpene content can take place on ageing (see Section 4.4).
Data on young white Spanish wines (i.e. not aged) of their terpene content from Aldave et al. (1993) for some different grape varieties grown in Spain are included in this table, though the reported contents seem rather high by comparison. The method of determination is not given.
Other components identified by two or more investigators are limonene (a hydrocarbon) and the linalool oxides. β-Damascenone and the α- and β-ionones are included in lists of ketones.
Table 4.26 Methoxypyrazines in wines: contents and sensory characteristics (flavour description and threshold data).
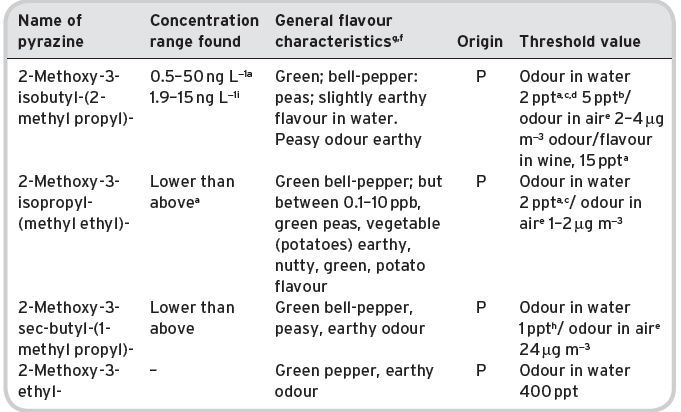
Data: aRibéreau-Gayon et al. (2000); bRychlik et al. (1998); cSeifert et al. (1970); dParliment (1981); eWagner et al. (1999); fChemisis (1995); gFlament (2001); hMurray & Whitfield (1970); iCullere et al. (2009).
- Limonene, 4-isopropenyl-l-methyl-l-cyclohexene, with R(+) and S(−) enantiomers present.
Flavour characteristics
Terpenes are complex substances, which tend to have floral aromas. Threshold values are probably high in wines, but the cumulative or even synergistic effect of a number of different terpene compounds may well determine their actual flavour contribution. Some available information is shown in Table 4.25. The effect of the presence of stereoisomers on flavour/threshold levels is not fully known, or much reported for wines.
Linalool has quite a low threshold level even in grape must but any transformation into, or presence of, its oxides will show terpene compounds with a much higher threshold level, especially the trans-isomer and so is sensorially less important.
A bicyclic sesquiterpene, rotundone, has been identified to be a potent aroma compound, responsible for the typical pepper aroma in Shiraz grapes and wines (Wood et al., 2008). Its correct name is (-)-rotundone, the S stereoisomer, so the correct full chemical name is (3S)-3,4,5,6,7,8-Hexahydro-3,8α-dimethyl-5α-(1-methylethenyl)azulen-1(2H)-one (personal communication, 2010, Alan Pollnitz). Interestingly, these authors also identified this compound for the first time in peppercorns, to which it gives the pepper aroma. Detection threshold levels are very low, in red wine 16 ng L−1 and in water 8 ng L−1. Grape samples analysed for rotundone ranged from 10–60 ng kg−1. In wines the levels were from 29 ng L−1 in a sensory non-peppery year to 145 ng L−1 from wine in a peppery year, well above the reported threshold concentration. Indeed, the authors reported an excellent correlation between sensory panel ratings of peppery intensity and the rotundone concentration, further support of its aroma contribution. Rotundone was also found above threshold levels in wines made from other grapes, Mourvèdre (134 ng L−1), Durif (128 ng L−1) and lower levels in some Cabernet Sauvignon wines.
4.3.11 Pyrazines
Methoxy-alkyl-pyrazines are well known substances found in plants with important flavour/odour characteristics, of high intensity and low threshold values.
Chemical structure
They are known constituents, together with other pyrazines (4.XVI) of simpler molecular structure, in roasted coffee, generated largely through roasting, though some are present in the original raw coffee beans (Grosch, 2001).
4.XVI
Presence in grapes/wines
According to Ribéreau-Gayon et al. (2006), 2-methoxy-3-isobutyl-pyrazine (strictly speaking, 2-methoxy-3-(2-methylprop-1-yl)pyrazine) was first identified in grapes (Cabernet Sauvignon) by Bayonave et al. in France around 1975, and also in wine. Subsequently it has been identified in a number of wines, but is only of particular interest in wines from Sauvignon Blanc and Cabernet Franc grapes. Ribéreau-Gayon et al. (2006), however, consider that the concentrations of these pyrazines are significantly above their recognition threshold levels but that their presence is more apparent when the grapes are under-ripe. Therefore, they conclude that pyrazines are not welcome or appreciated in ‘red Bordeaux wines’ and that at higher concentrations the herbaceous character spoils the the wine aroma. The other methoxy-pyrazines are much less influential for flavour as can be seen from the data assembled in Table 4.26. Relevant physical data can be found in Appendix II.
Flavour characteristics
Quantitative content data is limited. Part of the reason is the very low concentrations in wine having an odour impact. Ebeler & Thorngate (2009) quote concentration ranges in wines from 4 to 30 ng L−1, depending on growing conditions, maturity and grape variety. Concentrations of 2-sec-isobutyl-3-methoxypyrazine and and 3-isopropyl-2-methoxypyrazine were reported at least eight times lower. In a recent study using multidimentional gas chromatography-mass spectrometry authors were able to achieve detection limits in wines between 0.09 and 0.15 ng L−1 (Cullere et al., 2009). They reported that the 36 wines and 17 must samples analysed contained between 1.9 and 15 ng L−1 2-methoxy-3-isobutyl-pyrazine.
Table 4.27 Sulfur compounds in wines: contents, general flavour/odour characteristics and threshold value data.
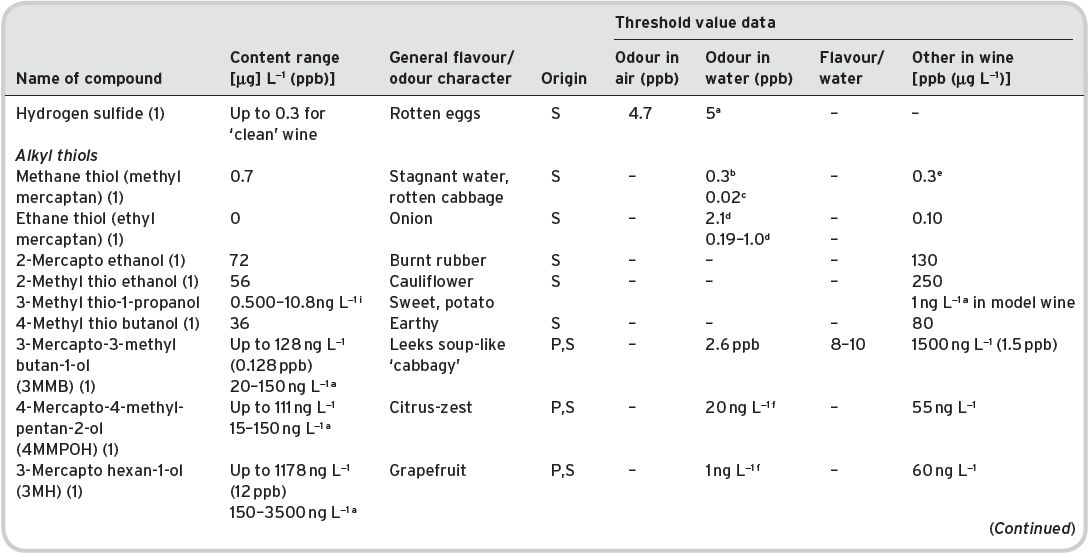
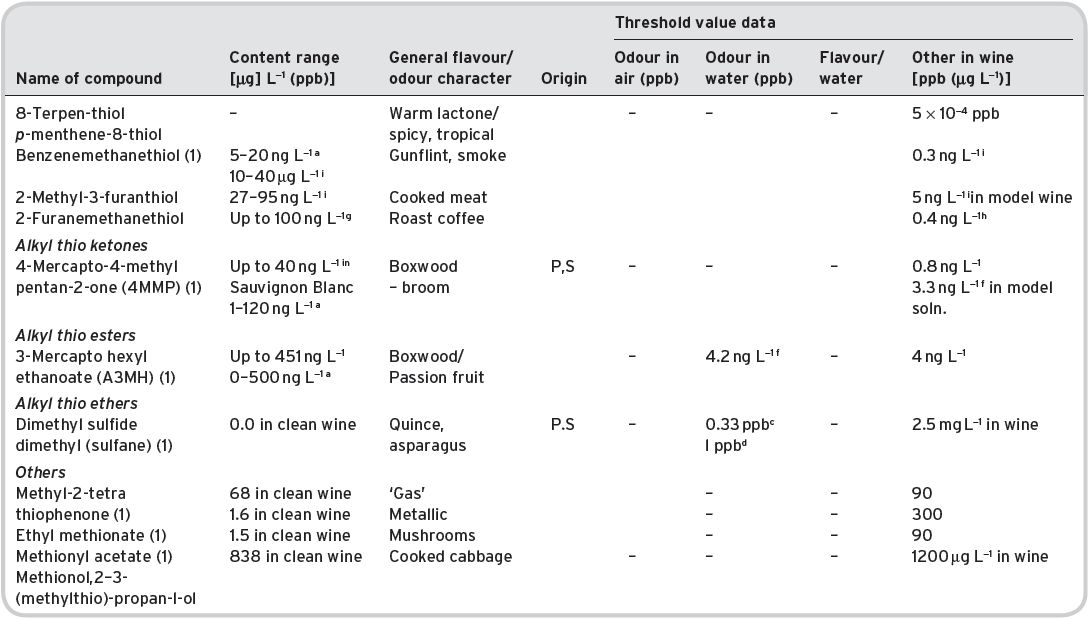
References: (1) Ribéreau-Gayon et al. (2006); aRibéreau-Gayon et al. (2006); bShankaranarayana et al. (1982); cGuadagni et al. (1963a); dPersson & Sydow (1973); eSemme Ravel et al. (1995); fQuoted by Swiegers & Pretoruius (2005); gTominaga & Dubourdieu (2006); h Tominaga et al. (2000); i Quoted by Francis & Newton (2005).
Whilst the 2-methoxy-3-isobutyl compound is described as having a ‘green pepper’ aroma, in fact the more characterizing feature, as with the others, is ‘green’, ‘earthy’ and ‘peasy’. Good threshold data is available.
4.3.12 Sulfur compounds
Various sulfur compounds have been found in wines, ranging from simple alkyl thiols or mercaptans to the more complex to thiolactones and terpenthiols. The latter have been identified in recent years and found to confer strong flavours with very low threshold levels, especially when compared with their oxygen-containing oxy counterparts.
Chemical structure
These compounds may be divided into simple alkyl thiols, where the OH (hydroxyl) group in an alcohol is replaced by the –SH (mercapto-) group, which applies also to certain terpen-thiols (4.XVII). Additionally, mercapto-groups may also be present in alkyl alcohols, ketones, aldehydes and esters. Other compounds may have –S– groups (thio-) as in simple thioesters or sulfides), or also in alcohols, aldehydes, ketones and esters. Further complexity can come in the form of disulfides (–S–S–) or even trisulfides (–S–S–S–).
4.XVII
Presence in wines
The more complex sulfur compounds, which have the more desirable flavour notes, have not only been detected but also quantified. Some mercapto compounds (described as 4MMP, A3MM, 3MM, 4MMPOH and 3HMB) by Ribéreau-Gayon et al. (2006) have varietal character. They are particularly associated with Sauvignon Blanc and their contents are shown in Table 4.27 together with their full chemical names. They also describe their presence in differing amounts in Alsace wines, based upon Gewürztraminer, and Riesling and Muscat grapes.
Some more sulfur containing compounds have been determined in wines, contributing positively to wine character. Tominaga et al. (2003) identified benzenemethanethiol in red and white wines, in particular in Sauvignon Blanc, Semillon and Chardonnay. Two other highly odoriferous sulfur compounds, 2-methyl-3-furanthiol and 2-furanmethanethiol were isolated and quantified by a method specially adapted for these compounds (Tominaga et al., 2000; Tominaga & Dubourdieu, 2006). The concentrations of benzenemethanethiol, 2-furanmethanethiol and ethyl 3-mercaptopropionate increase during bottle ageing in Champagne wines and contribute their typical flavour quality (Tominaga et al., 2003a). A number of sulfur compounds have also been associated with wines made from grapes affected by Noble Rot (see Chapter 7). The sulfur thiols, including hydrogen sulfide, are often present, as also shown in Table 4.27 together with other thio compounds contributing to wine flavour.
Flavour characteristics
Sulfur compounds of the thiol and thio-type are generally associated with flavour defects in wine (Table 4.27, under general odour/flavour characteristics). These are divisible into ‘light’ sulfur compounds (low boiling points) such as dimethyl sulfide and ‘heavy’ compounds (higher boiling points), such as methionol, both responsible for reduction odours (formed during bottle-ageing), as discussed by Ribéreau-Gayon et al. (2006), when concentrations present can exceed threshold levels. Sulfur compounds have low or even very low threshold levels.
Some new information suggests that some sulfur compounds at suprathreshold concentrations can be perceived as pleasant in some wines (see the review by Ugliano & Henschke, 2009). For example dimethylsulfide can contribute to pleasant corn, molasses and asparagus in some wines, and quince, truffles and metallic in other wines. In some red wines dimethylsulfide enhanced the berry fruit aromas. There are also suggestions that some sulfur compounds at near threshold concentrations can contribute to the varietal character of some wines, for example sulfides, disulfides, benzothiazole and thioalcohols were determined at higher concentrations in some Merlot wines. More data are needed to confirm this positive contribution of sulfur compounds at very low concentrations.
Of particular interest are those mercapto-compounds previously described in this Section, characterizing the Sauvignon Blanc aroma. The individual odour/flavour characteristics together with threshold values are given also in Table 4.27. Some of these compounds in particular, 4-mercapto-4-methyl-pentan-2-one (4MMP) can reach levels well in excess of its threshold to give the ‘boxwood’, ‘broom’ aromas often described in Sauvignon Blanc wines. Interestingly, 4MMP has been described as giving a ‘catty’ flavour in beer (Meilgaard & Peppard, 1986).
Another sulfur compound, 3-mercaptohexan-1-yl acetate, is associated with some tropical and citrus fruit flavours, such as passion fruit and grape fruit, and indeed with blackcurrant flavour.
p-Menthene-8-thiol (by a substitution of –SH for OH in α-terpineol) has a very intense flavour, with an extremely low threshold figure, with a marked complex smell of a ‘spicy/ tropical note with a warm lactone background’, over the floral notes of the terpenol compound.
Tominaga et al. (2003) identified benzenemethanethiol, giving a smokey character to wines. Its sensory threshold is very low and its concentration in wine was determined 30 to 100 times higher than the threshold. In particular Chardonnay wines contained 30–40 ng L−1.
Furfurylthiol has been identified in red Bordeaux wines, white Petite Manseng as well as in toasted barrel staves (Tominaga et al., 2000). Further isolation and quantification gave very low threshold values, and a contribution of 2-methyl-3-furanthiol to a cooked meat note in red wines, whilst 2-furanmethanethiol contributed to coffee notes in certain wines Tominaga et al., 2000; Tominaga & Dubourdieu, 2006).
Although the concentrations in wine are low, the contribution of many sulfur compounds can be significant in wines due to their very low threshold values. Francis & Newton (2005) listed seven compounds that could be significant in wine (3-mercapto hexyl ethanoate, 4-mercapto-4-methyl pentan-2-one, 3-mercapto hexan-1-ol, 2-Methyl-3-furanthiol, 3-methylthio-1-propanol, benzenemethanethiol, dimethylsulfide).
4.4 Changes during maturation
Changes in composition of the non-volatile compounds, but including the volatile acids (like acetic acid) responsible in part for acidity, have been discussed in Chapter 3, Sections 3.8.1 and 3.8.2. Wines can be fermented as well as stored in oak vats, and although storage will give the largest changes, some effects of fermentation in vats have been documented. During maturation slow chemical reactions in wine occur. Some do not require any other input than time, others require oxygen (oxidative changes) or oak (oak wood compound extraction). In-bottle maturation only allows the anaerobic changes that typify the ‘in-bottle’ aged bouquet, whilst ‘in-barrel’ maturation allows also oxidative and extractive changes. Consequently, information on changes in the volatile components composition needs to be sub-divided into sections on ‘in-barrel’ (also ‘in-vat’ and ‘in-tank’) and ‘in-bottle’ storage.
4.4.1 Fermentation and storage of wines ‘in-vat (tank)’ and ‘in-barrel (cask)’
Fermentation
During fermentation in oak, some compounds extracted from the wood can be changed by enzymic action of the fermenting yeast, for example the reduction of vanillin to vanillic alcohol (see review Ugliano & Henschke, 2009), thus giving a less woody character to a wine fermented in an oak vat. Yeasts can metabolize ferulic and p-coumaric acids to aromatic phenols, contributing to off-flavours (see Chapter 7).
Fermentation in vats is also an established method of conditioning new barrels, although time consuming and costly. Phenols and ellagitannins are readily extracted, but tend to combine with wine compounds and precipitate and hence are lost with the removal of lees. Desirable aromatic compounds such as oak lactones dissolve more slowly. Other methods are available; barrel preparation, care and hygiene are pertinent for quality wine maturation, discussed in depth by Jackson (2008) and Ribéreau Gayon et al. (2006).
Storage
The additional aromas that develop after fermentation are conveniently referred to as tertiary aromas and are derived from two sources. Firstly, there is a slight oxidation of some existing components, either primary or secondary, by the dissolved oxygen present and absorbed by wine during storage. Ribéreau-Gayon (1978) has used the term ‘oxidative bouquet’ arising in this type of ageing. Certain non-oxidative changes may also take place. Secondly, we have the chemical/physical extraction from the wood of the barrels when used, usually oak, producing so-called oak aroma. The characteristics of different types of oak barrels have been described in Chapter 3. Thirdly, oak absorbs compounds. These aspects are briefly discussed next. However, given the number of variables, it appears not an easy matter to mature in oak in order to enhance the aroma quality of a wine. A quote from Ribéreau-Gayon et al. (2006) says it all: ‘Wood is capable of enhancing a wine’s intrinsic qualities and may hide certain faults. Used unwisely, barrel ageing may produce disastrous results.’
Oxidation is characterized by increases in aldehydic compound content (including acetaldehyde itself, by oxidation of ethyl alcohol) to give potential rise to quince, apples, dried nuts, buttery, rancid and Madeira flavour notes. These flavours are especially associated with brandy and fortified wines. Such changes are likely, however, to be influenced by the amount of sulfur dioxide present at any time (a reducing agent), irreversibly reducing oxygen content and other factors. Sulfur dioxide should not, though, be present in sufficient quantity to generate thiol compounds (e.g. methyl/ethyl mercaptan), which have unpleasant odours. As described above (Section 4.3), acetaldehyde (ethanal) is not regarded as a desirable component of ordinary table wines – if present in free form at great excess over its threshold it produces so-called ‘flatness’.
The general nature of the oxidative changes occurring during in-vat or cask storage has been discussed in Chapter 3 in relation to non-volatile compounds. This discussion, including the concepts of oxidation–reduction potential, as reviewed by Ribéreau-Gayon et al. (2006), is relevant to the volatile compounds, in particular acetaldehyde.
However, the precise mechanism whereby particular volatile substances are generated such as ethanal, from ethanol and acetoin from butan-2-diol, is not clear, i.e. whether by the direct addition of hydrogen through dehydrogenase catalysis, or through hydrogen peroxide intermediate action. For further information, see Appendix I.
During storage in oak barrels, a range of flavour/aromas can develop as a result of extractives from the wood, dependent upon the grape variety/vinification conditions, the type of oak (e.g. American or French oak, type of drying, degree of ‘toasting’) and the age/condition of the barrels. Quantitative data is somewhat scarce for content in actual wines, but aldehydes, ketones, furanones (oak-lactones) and phenols are clearly identified, which have been included in the corresponding tables of Section 4.3. Regarding flavour, a wine is often referred to as ‘oaky’, but separate flavour distinctions are made with the threshold level data available, as also shown in the tables. Particular aged flavours are associated with certain grape varieties (more fully described in Chapter 5), thus there are ‘vanilla/coconut’ flavours of Chardonnay wines, ‘cedar’ for Cabernet Sauvignon and ‘toasted/spicy’ for Rioja red wines. The exact origin of these compounds from the oak wood is not always clear, involving some chemical changes in the actual extractives – thus, degradation of lignin extracted from wood to produce aldehydes such as vanillin.
Jackson (2008) has recently overviewed oak and its use in wine flavour. An ‘oaky’ flavour can also be introduced by fermentation in an oak barrel, or fermentation in the presence of oak chips. Pérez-Coello et al. (2000) have studied the chemical and sensory changes in white wines (American and European). The wines gained a distinctive oak aroma and lost their fresh and green apple aromas. The quantity of oak-lactones (cis and trans) was found to increase with increasing concentration of wood chips. More cis oak-lactone was extracted from American oak than from two of the three European ones tested.
However, Ribéreau-Gayon et al. (2006) comment unfavourably on the separate use of oak chips and of other wood extractives, for artificial ageing. They reported a high degree of extraction of cis β-methyl-γ-octalactone and vanillin when using chips. Robinson (1995) also comments on their use, saying ‘that they are not necessarily a bad thing’; ‘providing consumers with the sort of flavours for a fraction of the cost’; but not ‘able to provide the physical properties of barrel fermentation and maturation’.
An overview on the use of oak chips listed numerous factors relevant when using oak chips to flavour wine (Campbell et al., 2006), such as size of fragments, chip heating time and temperature, timing of oak addition and the time with wine, pretreatment of chips with sulfur dioxide and shelf life of chips. Chip size is an obvious parameter, with smaller chips giving faster extraction due to the larger surface area. However, the chips are heated before use and the combination heat and extraction gives both qualitative and quantitative differences in extraction. The order of treatment can have a significant effect on the extraction of compounds in wine, for example oak shaved and then heated gave a much larger extraction of vanillin than wood heated and then shaved.
Time after oak maturation may also influence the oaky character of a wine. Wilkinson et al. (2004), who showed that the odourless and open-ring precursor of cis-oak lactone, 3-methyl-4-hydroxyoctanoic acid, takes time to close. Hence the full oak aroma of cis-oak lactone extracted from shavings may not become apparent until some time after removal of the shavings. The development of the oak lactone flavour in vats will also take time to establish.
Adsorption of compounds by oak is particularly relevant for water and ethanol (see also Chapter 3), but Ramirez-Ramirez et al. (2004) reported that significant amounts of fruity esters can be adsorbed by wood from model wines. However, more studies are needed to assess the effect of selective adsorption of oak on the aroma of wine.
Further insight (R. de Heide, 1986) comes from the study of grape brandy storage, where storage in oak barrels is arranged over many years, where even sugars (such as xylose, arabinose) can be obtained by acid hydrolysis of the hemicelluloses and then to furfural derivatives over many years. Vanillin and syringaldehyde increase markedly with time and similarly, so do γ-lactones, phenolic acids, ethyl esters of higher aliphatic acids. Oak-lactones are of particular interest in brandy (distilled wine), since a large amount was noticed in brandies aged in American oak barrels compared with those in French.
Maga (1978) has published an extensive review of the contribution of wood to the flavour of alcoholic beverages. Whisky is a particular spirit, the flavour of which is strongly determined by oak barrel storage.
4.4.2 ‘In-bottle’ ageing
Compositional changes relate almost entirely to the volatile compound content, especially noticeable in the so-called ‘bouquet’ of the wine. Here, Ribéreau-Gayon (1978) uses the term ‘reductive bouquet’, since during bottle-ageing chemical changes take place in the absence of oxygen (though there will be residual SO2); of course, there is no possibility of extractives in glass bottles. Ribéreau-Gayon et al. (2006) associate reductive changes when the oxidation–reduction potential has fallen below 200 mV, that is, after any initial oxygen has disappeared (see also Chapter 3). A wine’s bouquet is formed by complex reactions corresponding to the formation of reducing substances and harmonization of aromas.
Reactions during bottle ageing depend on the storage temperature, at 12°C wine develops slowly and at 18°C the process is faster. A constant storage temperature helps to keep the bottles well sealed and it is pertinent that the corks do not dry out and keep the wine airtight. Higher temperatures (up to 25°C) may accelerate ageing, although it is thought that the characteristic bouquet does not develop. Wine should be kept in the dark.
Rapp & Marais (1993) have provided a useful report of the chemical changes that they found occurring in a Riesling and other white wines in storage by comparing vintages of different years. Comparison was made also with a wine stored in a freezer (−30°C), and a cellar-stored wine from the same fermentation in 1976, and then examined in 1983. The main changes found are listed below.
Changes in ester content
These consist of:
- a decrease in alkyl acetates, e.g. ethyl acetate, fruity esters such as isoamyl and isobutylacetates;
- an increase in mono- and dicarboxylic ethyl esters of other acids, e.g. diethyl succinate (noted by others), and ethyl hexanoate (also fruity).
These ester composition changes are a re-establishment of chemical equilibrium with time, according to the percentage contents of the acid, alcohol and esters present immediately after fermentation. In particular, young white wines tend to contain an excess of fruity esters. During ageing the content of higher alcohols hardly changed. These changes will be greater for white wines, rather than red, since lower fermentation temperatures during white wine vinification produces higher contents of these fruity esters, as already noted.
Substances produced by carbohydrate degradation
An increase in 2-furfural content was noted (dehydration in acid aqueous solution) over a number of years; also 2-formyl-pyrrole as a Maillard reaction product, though higher temperatures would be required.
Sulfur compounds
Dimethyl sulfide (DMS) is an important volatile compound in beer, formed during fermentation, but in wines it forms only during bottle ageing. These investigators examined its formation in wines made from Chenin Blanc, Riesling and Colombard grapes. The concentration increased with both time (up to 16 weeks) and temperature (examined up to 30°C), and was found to correlate well with taste panel findings on so-called ‘maturation’ bouquet. There were considerable differences in the effect of temperature and storage on the different cultivars. They believed that the presence and content of DMS is an important consequence of ageing in relation to the bouquet formed.
The concentrations of benzenemethanethiol, 2-furanmethanethiol and ethyl 3-mercaptopropionate increase during bottle ageing in Champagne wines, and contribute their typical flavour quality (Tominaga et al., 2003a).
Changes in terpenoids
During ageing, terpenes become involved in a very complex set of reactions:
(1) Decreasing concentration of the monoterpene alcohols (linalool, geraniol and citronellol).
(2) Increasing concentration of the isomeric linalool oxides (four in number), nerol oxides, trimenol, α-terpineol, hydroxylinalool and hydroxycitronellol.
Linalool is one of the main terpenes in Riesling, which gradually decreases in amount whereas the oxide content increases. Linalool has a floral rose odour, whilst α-terpineol has a floral lilac odour and the linalool oxides have a musty pine-like aspect. For example, a Riesling with a stated linalool content 400 μg L−1 (or 0.4 mg L−1) contains only 50 μg L−1 after three years, which is below its odour threshold of 100 μg L−1. This decrease of zconcentration is, reportedly, noticeable in the bouquet. There is an increase of linalool oxide content, which has, however, a much higher threshold perception level than linalool itself. Odour perception is also influenced by an increase in α-terpineol. Quantitative changes of some eleven terpenes in a Riesling wine during storage are also reported.
Formation of substances from carotene breakdown
Riesling wines during ageing develop a strong, petrolly kerosene-like aroma, which is detrimental to wine quality, if present at too high a level. It is more apparent in wines from hot wine-producing countries (e.g. South Africa) than from cooler European countries. The substance responsible is believed to be 1,1,6-trimethyl-1,2-dihydronaphthalene (TDN) (4.XVIII), which has a threshold of 20 ppb (20 μg L−1) in wine. Postulated possible formation mechanisms are, essentially, from carotenoids, by enzymatic or acid catalysed reactions. Other volatile compounds are generated from carotene, i.e. C9, C10, C11 and C13 isoprenoids, such as β-damascenone, α- and β-ionone, vitispiranes (see also Section 4.3 on ketones). However, the carotene content is not the determining factor, since Chenin Blanc grapes have a high carotenoid but low terpene content and a low TDN level on ageing. Ribéreau-Gayon et al. (2006) claim that the concentration of this substance can reach 200 μg L−1.
4.XVIII
Aldave et al. (1993) in reviewing the literature on the shelf-life of young white wines, with particular relationship to Spanish wines, covers similar information to that given by Rapp & Marais (1993).
Ribéreau-Gayon et al. (2006) have also reported changes occurring during in-bottle ageing, especially of red wines. They also mention that the important esters, ethyl esters of higher alcohols with their ‘fruity’ flavours such as hexyl acetate, tend to decrease on ageing, thus being responsible for ‘fading’ effects.
A study on accelerated ageing of model wines with added aroma precursors showed continuous increases in vanillin derivatives, furan linalol oxides, β-ionone, and some compounds with a possible negative impact 4-ethylphenol and guiacol (Loscos et al., 2010). Numerous other compounds increased and then decreased in concentration. Varietal differences could be observed and remained present even after accelerated ageing. Once more information regarding aroma changes during ageing is available, possibly the time required for maturation can be predicted.
4.5 Aroma detection and quantification
The main method for the identification of the volatile aroma volatiles is based on gas chromatography, used to separate the compounds in a sample, followed by a detection method aiding the identification of the compounds. Prior to analysis, there is the sample preparation, a very important part of the procedure. Great progress over the last decade has been made in both the preparation of reliable samples, as well as the separation and detection techniques. Techniques for the identification of compounds, at low concentrations, have also improved.
4.5.1 Gas chromatography
Gas chromatography (GC) allows the analysis of volatile compounds, or those made volatile by derivatization. The equipment basically consists of an inlet for the sample, a column on which the sample is separated and a means of detecting the volatile compounds. A controlled gas flow through the column and a gradually increasing temperature are used to separate the volatile compounds over the column. Great advances have been made in GC equipment, allowing the identification and quantification of very low concentrations of compounds. The rapid development of computer hard- and software, used to program GC equipment, and handle enormous data sets, has contributed to the wealth of information now available on aroma compounds in wines. Some aspects will be discussed next. The sensitivity of the equipment requires careful sample preparation, avoiding the introduction of artefacts and maximizing the accuracy of the quantification.
Volatile aroma compounds in wines (or grapes) are separated, detected and quantified by gas-chromatography techniques. A mass spectrometry detector can be added and allows the accurate identification of compounds corresponding to the peaks shown in a GC trace. GC coupled to a mass spectrometer (MS) allows the tentative identification of eluting volatile compounds, by matching the fragmentation pattern and molecular ion obtained by MS with specialist reference libraries of such information by using a computer. Peak area determination, by comparison with a peak from a known amount of a pure compound, enables quantification of the compound in the original sample and so, therefore, for all the compounds detected from peaks. Internal standards are routinely used to minimize errors in quantification.
These techniques are now well described in the literature, including analyses of particular beverages, with improvements taking place continuously. An in-depth overview of the development and contributions mass spectrometry has made to advances in knowledge of grape and wine constituents is given by Hayasaka et al. (2005), including discussions on sample inlet and preparation techniques. In its infancy mass spectrometry was suitable to analyse volatile compounds, hence mostly suited to analyse aroma compounds and taints, provided samples of sufficient quality (purity) and quantity could be prepared. However, a combination of more sophisticated inlet techniques and increased sensitivity and selectivity has allowed the analysis of ever smaller samples. In addition the development of new techniques in mass spectrometry has also allowed the analysis of charged compounds, such as anthocyanins, and non-volatile compounds, such as proteins, phenols. Significant contributions in the analysis of pyranoanthocyanins were also made using mass spectrometry.
Nuclear magnetic resonance (NMR) techniques are also useful in identification but see Flament (2001). Although the sensitivity of GC and GC-MS techniques has greatly improved, sample preparation remains an important step.
4.5.2 Sample preparation
It is pertinent to mention here that the means of preparation of the sample for testing and the equipment used for testing, should be separately studied. The sample presented to the GC equipment is often a so-called head-space sample, which is a volume of gas/vapour drawn off from above the head-space of the liquid beverage (with which it is in equilibrium). In the purge-and-trap method this gas is trapped in a solid absorbent and then released (by controlled heating) to the GC equipment for analysis of its content. These methods are clearly directly relatable to the vapour/gas that passes to the human nose (olfactory region) and therefore to the perceived aroma/flavour. However, there can be a disadvantage to this method, since it may not be possible to process sufficient vapour to ensure that even extremely small quantities of particular compounds are detected. These compounds may have extremely low threshold flavour levels and, therefore, be potentially important contributors to flavour.
Despite possible drawbacks with sensitivity and selectivity, the sample preparation methods have been much improved both in quality of the sample and ease of preparation, in particular the use of solid-phase extraction techniques has increased. It avoids the use of solvents and allows samples without solvent induced artifacts. Solid-phase extraction is widely used to extract, clean and concentrate samples, has proven to be robust and can be automated. For example a paper validating a solid-phase micro-extraction method of Pinot Gris and Chardonnay wine aroma compounds using headspace analysis followed by gas chromatography and mass spectrometry of with automated sample handling (Howard et al., 2005) showed good sensitivity and reproducibility for esters, alcohols and terpenes. Detection limits were in the μg L−1 range, covering aroma compounds and concentrations typical in wines. This progress in sample preparation technique no doubt has contributed to publications with detailed qualitative as well as quantitative data, often accompanied by data showing reproducibility and errors. However, further advances in the technique can still be made if the selectivity of the method can be improved, in order to achieve more selective samples of greater amounts.
The alternative method, which has been widely used, is to extract the volatile compounds by a solvent, followed by distilling and condensing. A liquid concentrate is thus obtained for presentation to the GC equipment. In the Likens–Nicholson combined extraction–distillation apparatus, pentane–methylene chloride is a popular solvent (Vernin et al., 1993). Other solvents, such as the freons have been described for use with wine volatiles (Rapp & Marais, 1993; Fisher et al., 2000), along with operation under vacuum to lower the extracting temperature in extraction–distillation (Grosch, 2001). The main advantage of distillation is the large quantities of volatile compounds that can be handled, but care is needed in assessment since some of the compounds subsequently detected may merely be artefacts of the extraction–distillation operation. Again, it is important to assess how the composition of the distillate vapour corresponds to what is presented to the olfactory organs when drinking the wine to assess its flavour. For example, much work on coffee has been conducted by exhaustive extraction of the roasted coffee, rather than of actual brews, which have been prepared by simple aqueous extraction, and have lower and different yields of volatile compounds.
Numerous variants of design in the GC equipment have been produced by different commercial suppliers. The important aspect is the choice of ‘column’ – capillary columns are now regarded as essential and appropriate for effective separations of components.
The measurement of partition coefficients has a different aim from identifying aroma compounds in wine, for the latter the experimenter introduces a concentration step to ensure sufficient concentration of the volatile is present to be analysed and detected. For partition coefficient measurement the actual concentration is relevant and headspace analyses have been used, often based on a form of static air sampling method. The accurate determination of such measurements is time consuming. Martin et al. (2009) proposed a quick new method with which they demonstrated the measurement of congeners in wine. Their method involved was based on distillation in a type of equilibrium cell, and data obtained were comparable with other methods. The availability of more accurate partition coefficients would help in the theoretical prediction of flavour in wines and other foods.
4.5.3 Olfactometry
Recent reviews by Plutowska & Wardencki (2007 and 2008), Francis & Newton (2005) Palaskova et al. (2008) describe the chromatography methods using olfactometry, a measuring method of volatile aroma compounds using the nose, currently used to analyse the aroma compounds in alcoholic beverages, and assess their sensory properties. This method allows the separation and identification of volatile compounds in wines and combined with olfactometry it can reveal which components give typical wine aroma descriptors. The human detection methods linked to such analyses gives direct information regarding the actual smell of the samples, and various methods are available to quantify the aroma thus determined. Although the detection methods used for gas chromatographic analyses are very sensitive, the article emphasized that sometimes compounds detected with the nose are not detectable with instrumental detectors, illustrating the enormous sensitivity of the nose.
These techniques have been applied to a wide range of investigations, such as giving so-called odour profiles of wines of a particular region, early detection of compounds giving possible off-flavours, monitoring terpene concentration in wine, role of fermenting yeast on wine aroma and monitoring the undesirable effect of oxidation of white wines. One technique reviewed was developed to determine the aftertaste of a wine, involving sampling the oral cavity of persons just having evaluated the sensory properties of wine. It is anticipated that the evolvement of ever more sophisticated and sensitive methods, including the use of the nose, will lead to a better understanding of the aroma volatiles in wines contributing to their sensory properties.
Another method of detecting aroma compounds has been developed by different commercial companies, often in conjunction with university engineering departments and is referred to as electronic nose or artificial nose. Detection is based upon changes in the electric resistances of odour sensors incorporated in a sensor chamber exposed to the vapour. A typical sensor chamber may contain up to six different exchangeable metal oxide surfaces (with 18 surfaces in total) of semi-conducting character. Computer software is needed to analyse the signals received but the information obtained does not yet compare in detail with that from GC methods.
In contrast to the use of gas chromatography, where individual aroma compounds are analysed, sensor based instruments analyse the aroma as a whole, without dividing it into individual aromatic compounds (Wardencki et al., 2009). Instrumentation is relatively cheap, and analysis can be done very fast. This method is more like the human sensory analysis, still used to analyse drinks, despite drawbacks, such as low repeatability, reproducibility and compounds cannot be quantified or qualified. Sensors are being designed to be analogues of the protein receptors in the olfactory epithelium. The electronic system which transfers the electric signals from the sensors is comparable with human sensory neurons, using artificial neural networks. Hence it is thought that electronic and human noses function similarly, despite the many differences in the processes involved. Considering its sensitivity, speed of analysis and reproducibility, future developments of this method will iron out the current drawbacks and in time electronic noses may play a significant role in assuring wine aroma quality.
4.6 Chemical structure and physical properties
The tables in Appendix II set out the chemical structure (molecular weight and molecular formula), together with reported physical properties, that are or may be relevant to their study in wine, focusing on apparently important volatile compounds already described in Sections 4.2 and 4.3.
The significance of the partition coefficient of a particular aroma substance in very dilute aqueous solution (K
j,a-w), together with the related relative volatility 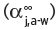 , has been discussed in Section 4.1. Methods to determine these quantities are described by Clarke (1990), Buttery et al. (1969b, 1971b) and detailed descriptions of gas chromatographic procedures are given by Buttery (1969b), and Gretsche et al. (1995), together with some determined values. Unfortunately, published data by direct determination of K
j at given temperatures for different compounds are very few compared with the number of compounds reportedly present in wine. Recently, a new direct method has been published (Pollien & Yeretzian, 2001), and results presented for some 15 different volatile compounds, along with comparison of values made by other methods.
, has been discussed in Section 4.1. Methods to determine these quantities are described by Clarke (1990), Buttery et al. (1969b, 1971b) and detailed descriptions of gas chromatographic procedures are given by Buttery (1969b), and Gretsche et al. (1995), together with some determined values. Unfortunately, published data by direct determination of K
j at given temperatures for different compounds are very few compared with the number of compounds reportedly present in wine. Recently, a new direct method has been published (Pollien & Yeretzian, 2001), and results presented for some 15 different volatile compounds, along with comparison of values made by other methods.
However, estimates can be made as already discussed. Methods of estimation are fully described in Appendix I.7.
Bibliography
General
American Chemical Society Symposium Series. Flavour subjects No. 388 (1989): 490 (1992); 596 (1995); 714 (1998); 794 (2001), American Chemical Society, Washington.
Burdock, G.A. (ed.) Feneroli’s Handbook of Food Flavour Ingredients (2002), 4th Edn. CRS Press, Orlando, FL.
Jackson, R.S. (1994) and 2nd Edn. (2000) Wine Science, Principles, Practice, Perception. Academic Press, San Diego.
Ribéreau-Gayon, P., Glories, Y., Maujean, A. & Dubourdieu, D. (2006) Handbook of Enology, Volume I, Wine and Wine Making. John Wiley & Sons, Ltd, Chichester.
Ugliano, M. & Henschke, P.A. (2009) Yeasts and Wine Flavour. In: Wine Chemistry and Biochemistry (eds. M.V. Moreno-Arribas & M.C. Polo), pp. 313–392. Springer ScienceBusiness Media, LLC.
Sensory perception
General
Ache, B.W. & Young, J.M. (2005) Olfaction: Diverse species, conserved principles. Neuron, 48, 417–430.
Buck, L. & Axel, R. (1991) A novel multigene family may encode odorant receptors: A molecular basis for odor recognition. Cell, 65, 175–187.
Meilgaard, M., Civille, G.V. & Carr, B.T. (2007) Sensory Evaluation Techniques. 4th Edn. CRC Press, Florida.
Mombaerts, P. (2004) Genes and ligands for odorant, vomeronasal and taste receptors. Nature Reviews Neuroscience, 5, 263–278.
Swiegers, J.H., Chambers, P.J. & Pretorius, I.S. (2005) The genetics of olfaction and taste. Australian Journal of Grape and Wine Research, 11, 109–113.
Identification and contents
General
Amerine, M.A. & Roessler, E.B. (1983) Wines: Their Sensory Evaluation, loc. cit. Chapter 1. WH Freeman & Co., San Francisco.
Nijssen, L.M. (Ed.) (1996) Volatile Compounds in Food. Qualitative and Quantitative Data. 7th Edn., TNO Nutrition and Food Research Institute, Zeist, The Netherlands.
Vernin, G., Pascal-Mousselard, H., Metzger, J. & Párkányi, C. (1993) Aromas of Mourvèdre wines. In: Shelf-Life Studies of Foods and Beverages (ed. G. Charalambous), Developments in Food Science No. 33, pp. 945–974. Elsevier, Amsterdam.
References
Aldave, L. (1993) The shelf-life of young white wines. In: Shelf-Life Studies of Foods and Beverages (ed. G. Charalambous), Developments in Food Science, 33, pp. 923–944, Elsevier, Amsterdam.
Bartowsky, E.J. & Henschke, P.A. (2004) The ‘buttery’ attribute of wine -diacetyl- desirability, spoilage and beyond. International Journal of Food Microbiology, 96, 235–252.
Buttery, R.G., Seifert, R.M., Guadagni, D.G. & Ling, L.C. (1971a) Characterization of additional volatile components of tomato. Journal of Agricultural and Food Chemistry, 19, 524–9.
Camara, J.S., Marques. J.C., Alves, M.A. & Ferreira, A.C.S. (2004) 3-Hydroxy-4,5-dimethyl-2(5H)-furanone levels in fortified Madeira wines: relationship to sugar content. Journal of Agricultural Food Chemistry, 52, 6765–6769.
Camara, J.S., Alves, M.A. & Marques, J.C. (2006) Changes in volatile composition of Madeira wines during their oxidative ageing. Analytica Chimica Acta, 563, 188–197.
Campbell, J.I., Pollnitz, A.P., Sefton, M.A., Herderich, M.J. & Pretorius, I.S. (2006) Factors affecting the influence of oak chips on wine flavour. Wine Industry Journal, 21, 38–42.
Carillo, J.D. & Tena, M.T. (2007) Determination of ethylphenols in wine by in situ derivatisation and headspace solid-phase micro extraction-gas chromatography- mass chromatography. Analytical Bioanalytical Chemistry, 387, 2547–2558.
Cullere, L., Escudero, A., Campo, E., Cacho, J. & Ferreira, V. (2009) Multidimensional gas chromatography-mass spectrometry determination of 3-alkyl-2-methoxypyrazines in wine and must. A comparison of solid phase extraction and headspace solid phase extraction methods. Journal of Chromatography A, 1216, 4040–4-45.
Ebeler, S.E. & Thorngate, J.H. (2009) Wine chemistry and flavour: Looking into the crystal glass. Journal of Agricultural and Food Chemistry, 57, 8090–8108.
Escudero, A., Campo, E., Farina, L., Cacho, J. & Ferreira, V. (2007) Analytical characterization of the aroma of five premium red wines. Insights in the role of odour families and the concept of fruitiness of wines. Journal of Agricultural and Food Chemistry, 55, 4501–4510.
Ferereira, A.C.S., Barbe, J.C. & Bertand, A.T. (2003) 3-Hydroxy-4,5-dimethyl-2(5H)-furanone: A key odorant of the typical aroma of oxidative aged Port wine. Journal of Agricultural and Food Chemistry, 51, 4356–4363.
Francis, I.L. & Newton, J.L. (2005) Determining wine aroma from compositional data. Australian Journal of Grape and Wine Research, 11, 114–126.
Loscos, N., Hernandez-Orte, P., Cacho, J. & Ferreira, V. (2010) Evolution of the aroma composition of wines supplemented with grape flavour precursors from different varietals during accelerated wine ageing. Food Chemistry, 120, 205–216.
Loiret, A. & Versini, G. (2002) Aroma variation in Tannet wines: Effect of malo-lactic fermentation on ethyl lactate level. Italian Journal of Food Science, XIV, 2, 175–180.
Montedoro, G. & Bertuccioli, M. (1986) The flavours of wines, vermouth and fortified wines. In: Food Flavours, Part B (eds. I.D. Morton & A.J. Macleod), Elsevier Science Publishers BV, Amsterdam.
Nykanan, L. & Soumaileinan, M. (1983) Composition of wines, quoted by the IARC Monograph, Alcohol Drinking (1988) Vol. 4, pp. 79–86. International Agency for Research into Cancer, Lyon.
Palaskova, P., Herszage, J. & Ebeler, S.E. (2008) Wine flavor: chemistry in a glass. Chemical Society Reviews, 37, 2478–2489.
Ramirez-Ramirez, G., Chassagne, D., Feuillat, M., Voilley, A. & Charpentier, C. (2004) Effect of wine constituents on aroma compounds sorption by oak wood in a model system. American Journal of Enology and Viticulture, 55, 22–26.
Rapp, A. & Guntert, M. (1985) Vitis, 24(2), 87–98.
Rapp, A. & Marais, J. (1993) The Shelf-life of Wine: Changes in aroma substance during storage and ageing of white wines. In: Shelf-Life Studies of Foods and Beverages, (ed. G. Charalambous), Developments in Food Science, 33, pp. 891–921, loc. cit. Elsevier, Amsterdam.
Ribéreau-Gayon, P. (1978) Wine flavour. In: Flavour of Foods and Beverages (eds. G. Charalambous & G.E. Inglett), pp. 355–380. Academic Press, New York.
Ribéreau-Gayon, P. (1990) Rev. Fr. Oenol., 123, 25–33.
Ribéreau-Gayon, P., Glories, Y., Maujean, A. & Dubourdieu, D. (2006) Handbook of Enology, Volume I, Wine and Wine Making, Ch. 1. John Wiley & Sons, Ltd, Chichester.
Schreier, P., Drawert, F. & Junker, A. (1977) Gaschromatographische bestimmung der inhaltsstoffe von gärungagetranke. Part X. Chem. Mikro. Technol. Lebensm. 5, 45–52, quoted by Montedoro & Bertuccioli (1986).
Suarez, R., Suarez-Lepe, J.A., Morata, A. & Calderon, F. (2007) The production of ethylphenols in wine by yeasts of the genera Brettanomyces and Dekkera: A review. Food Chemistry, 102, 10–21.
Swiegers, J.H. & Pretorius, I.S. (2005) Yeast modulation of wine flavour. Advances in Applied Microbiology, 37, 131–175.
Tominaga, T., Blanchard, L., Darriet, P. & Dubourdieau, D. (2000) A powerful aromatic volatile thiol, 2-furanmethanethiol, exhibiting roast coffee aroma in wines made from several Vitis vinifera grape vaireties. Journal of Agricultural and Food Chemistry, 48, 1700–1802.
Tominaga, T., Guimbertau, G. & Dubourdieau, D. (2003a) Role of certain volatile thiols in the bouquet of aged Champagne wines. Journal of Agricultural and Food Chemistry, 51, 1016–1020.
Tominaga, T., Blanchard, L., Darriet, P. & Dubourdieau. D. (2003) Contribution of Benzenemethanethiol to smokey aroma of certain Vitis vinifera grape varieties. Journal of Agricultural and Food Chemistry, 51, 1373–1376.
Tominaga, T. & Dubourdieau, D. (2006) A Novel method for quantification 0f 2-methal-3-furanthiol and 2-furanemathanethiol in wines made from Vitis vinifera grape varieties. Journal of Agricultural and Food Chemistry, 54, 29–33.
Tressl, R., Remer, R. & Apetz, M. (1976) Volatile phenolic components in beer, smoked beer and Sherry. Z. Lebensm – Unters – Forsh, 162, 115–122, quoted by Montedoro & Bertuccioli (1986).
Wilkinson, K.L., Elsey, G.M., Prager, E.P. & Sefton, M.S. (2004) Rates of formation of cis- and trans- oak lactone from 3-methyl-4-hydroxyoctanois acid. Journal of Agricultural and Food Chemistry, 52, 4213–4218.
Flavour descriptions and odour values
General texts
Arctander, S. (1967) Perfume and flavour chemicals, quoted by I. Flament (2001), Coffee Flavour Chemistry. John Wiley & Sons, Ltd, Chichester.
Belitz, H.D. & Grosch, W. (1999) Food Chemistry. pp. 319–377. Springer-Verlag, Berlin.
References
Chemisis Data base on organoleptic properties of perfume and flavour chemicals, Firmenich, S.A., Geneva, unpublished, quoted by I. Flament (2001), loc. cit.
Fors, S. (1983) Sensory aspects of volatile Maillard reaction products and related compounds. American Chemical Society Symposium Series, Series 215, pp. 185–286. American Chemical Society, Washington.
Grosch, W. (2001) Volatile compounds. In: Coffee: Recent Developments (eds. R.J. Clarke & O.H. Vitzthum), pp. 68–89. Blackwell Publishing Ltd., Oxford.
Lettingwell, J.C. (2002) Chiralty and flavour perception, http://www.lettingwell.com/chiralty/chiralty.htm (last accessed: 1 May 2010).
Meilgaard, M. (1982) Prediction of flavour differences between beers from their chemical compositions. Journal of Agricultural and Food Chemistry, 30, 1009–1017.
Meilgaard, M.C. & Peppard, T.L. (1986) The flavour of beer. In: Food Flavours, Part B (eds. I.D. Morton & A.J. Macleod), pp. 99–170, loc. cit.
Motodo, S. (1979) Formation of aldehydes from amino acids by polyphenoloxidase. Journal of Fermentation Technology, 57, 395–399, quoted by Flament (2001).
Pickenhagen, W. (1989) Enantioselectivity in odour perception. In: Flavour Chemistry, American Chemical Society Symposium Series, No. 714, pp. 151–157. American Chemical Society, Washington.
Shaw, P.E. (1986) The flavour of non-alcoholic fruit beverages. In: Food Flavours, pp. 337–368, loc. cit.
Odour/flavour thresholds
General texts
Buttery, R.G. (1999) Flavour chemistry and odour thresholds. In: Flavour Chemistry: Thirty years of progress (eds. R. Teranishi, E.L. Wick & J. Hornstein), pp. 353–365. Kluwer Academic/Plenum, New York.
Campo, E., Cacho, J. & Ferreira, V. (2007) Solid phase extraction, multidimensional gas chromatography determination of four novel aroma powerful ethyl esters. Assessment of their occurrence and importance in wine and other alcoholic beverages. Journal of Chromatography A, 1140, 180–188.
Flath, R.A., Black, D.R., Guadagni, D.G., McFadden, W.H. & Schultz, T.H. (1967) Identification and organoleptic evaluation of compounds in Delicious apple essence. Journal of Agricultural and Food Chemistry, 15, 29–35.
Huber, U.A. (1992) Homofuranol. Perfume and Flavour 17(7/8), 15–19.
Keith, E.S. & Powers, J.J. (1968) Determination of flavour threshold levels and sub-threshold, additive and concentration effects. Journal of Food Science, 33, 213–218.
Kim Ha, J. & Lindsay, R.C. (1991) Volatile alkyl phenols and thiophenol in species-related characterizing flavours of red meats. Journal of Food Science, 56, 1197–1202.
Mulders, E.J. (1973) Odour of white bread. Part IV. Z. Lebensm. Unters. Forsch., 151, 310–317.
Murray, K.E. & Whitfield, F.B. (1970) 2-Methoxypyrazines and the flavour of green peas. Chemistry and Industry (London), 973–974.
Nakamura, S., Crowell, E.A., Ough, C.S. & Totsuka, A. (1988) Quantitative analysis of 7-nonalactone in wines and its threshold determination. Journal of Food Science, 53, 1243–1244.
Parliment, T.H., Clinton, W. & Scarpellino, R. (1973) Trans-2-Nonenal. Coffee compound with novel organoleptic properties. Journal of Agricultural and Food Chemistry, 21, 485–487.
Parliment, T.H. (1981) The chemistry of aroma. Chemtech, 25(8), 38–47.
Perrson, T. & von Sydow, E. (1973) Aroma of canned beef. Journal of Food Science, 38, 377–385.
Seifert, R.M., Buttery, R.G., Guadagni, D.G., Black, D.R. & Harris, J.G. (1970) Synthesis of some 2-methoxy-3-alkyl pyrazines with strong bell-pepper-like odours. Journal of Agricultural and Food Chemistry, 18, 246–249.
Semmelroch, P. & Grosch, W. (1996) Studies in character impact compounds in coffee beans. Journal of Agricultural and Food Chemistry, 44, 537–543.
Sick, T.J., Albin, J.A., Satter, L.A. & Lindsey, R.C. (1971) Comparison of flavour thresholds of aliphatic lactones, with those of fatty acids, esters, aldehydes, alcohol and ketones. Journal of Dairy Science, 34, 344–346.
Swiegers, J.H., Bartowsky, E.J., Henschke, P.A. & Pretorius, I.S. (2005) Yeast and bacterial modulation of wine aroma and flavour. Australian Journal of Grape and Wine Research, 11, 139–173.
Takeoka, G.R., Buttery, R.G., Turbaugh, J.G. & Benson, M. (1995) Odour thresholds of various branched esters. Lebensmittel Wissenschaft und Technologie B, 153–156; also (1998) 31, 443–448.
Wasserman, A.E. (1966) Organoleptic evaluation of three phenols present in wood smoke. Journal of Food Science, 31, 1005–1010.
References
Ahmed, E.M., Dennison, R.A., Dougherty, R.H. & Shaw, P.E. (1978) Flavour and odour thresholds in water of selected orange juice components. Journal of Agricultural and Food Chemistry, 26, 187–191.
Buttery, R.G., Seifert, R.M., Guadagni, D.G. & Ling, L.C. (1969a) Characterization of some volatile constituents of bell-peppers. Journal of Agricultural and Food Chemistry, 17, 1322–1327.
Flament, I. (2001) Coffee Flavour Chemistry. John Wiley & Sons, Ltd, Chichester.
Grosch, W. (1995) Instrumental and sensory analysis of coffee volatiles. Proceedings of 16th ASIC Colloquium (Kyoto), ASIC, Paris, 147–156.
Guadagni, D.C., Buttery, R.G., Okano, S. & Bun, H.K. (1963a) Additive effect of sub-threshold concentrations of some organic compounds associated with food aromas. Nature, 200, 1288–1289.
Guadagni, D.C., Buttery, R.G. & Okano, S. (1963b) Odour threshold of some organic compounds associated with food flavours. Journal Science Food and Agriculture, 14, 761–765.
Lea, C.H. & Swoboda, P.A.T. (1958) The flavour of aliphatic aldehydes. Chemistry and Industry (London), 1289–1290.
Maga, J.A. (1978) Simple phenol and phenolic compounds in food flavour. CRC Critical Reviews Food Technology, 7(2), 147–192.
Patton, S. (1964) Flavour thresholds of fatty acids. Journal of Food Science, 29, 629–680.
Pineau, B., Barbe, J.C., van Leeuwen, C. & Dubourdieu, D. (2007) Which impact for β-damascenone on red wines aroma? Journal of Agricultural and Food Chemistry, 55, 4103–4108.
Rychlik, M., Schieberle, P. & Grosch, W. (1998) Compilation of odour thresholds, odour qualities and retention indices of key odorants. Quoted by W. Grosch, (2001), loc. cit.
Shankaranarayana, M.L., Raghavan, B., Abraham, K.O. & Natarajan, C.P. (1982) Sulphur compounds in flavours. In: Food Flavours (eds. I.D. Morton & A.J. McCleod). Elsevier Science, Amsterdam.
Stevens, S.S. & Galanter, M. (1957) Ratio scales and category scales for a dozen perceptual conditions. Journal of Exp. Psychology, 84, 377–411.
Takahashi, K. & Ayiyama, H. (1993) Shelf-life of Sake. In: Shelf-Life Studies of Foods and Beverages (ed. G. Charalambous), loc. cit.
Wagner, R., Czerny, M., Bielochrodsley, J. & Grosch, W. (1999) Structure-odour activity relationships of alkyl pyrazines. European Food Research and Technology, 208, 308–16, quoted by Flament (2001).
Wood, C., Siebert, T.E., Parker, M., Capone, D.L., Elsey, G.M., Pollnitz, A.P., Eggers, M., Meier, M., Vossing, T., Widder, S., Krammer, G., Sefton, M.A. & Herderich, M.J. (2008) From wine to pepper: Rotundone, an obscure sesquiterpene, is a potent spicy aroma compound. Journal of Agricultural and Food Chemistry, 56, 3738–3744.
Yokotsuka, T. (1986) Chemical and microbiological stability of Shoyu (fermented Soya sauce). In: Handbook of Food and Beverage Stability (ed. G. Charalambous), pp. 518–621. Academic Press Inc. Orlando; quoting Sasaki, M. & Nunomura, N. (1981) Journal of the Chemical Society Japan (no. 5), 736–745.
Physical properties
General texts
Clarke, R.J. (2001) Instant Coffee – Physical properties. In: Coffee: Recent Developments (eds. R.J. Clarke & O.G. Vitzthum), pp. 133–137. Blackwell Publishing Ltd., Oxford.
Perry, J.H. (1980 and later editions; ed. with D.W. Green, 1997, 7th Edn.) Chemical Engineers’ Handbook. McGraw-Hill, New York.
Pierotti, G.J. (1959) Activity coefficients and molecular structure. Industry and Engineering Chemistry, 51, 95–102.
References
Buttery, R.G., Guadagni, D.E. & Ling, L.C. (1969b) Volatilities of aldehydes, ketones and esters in dilute solution. Journal of Agricultural and Food Chemistry, 17(2), 385–389.
Buttery, R.G., Bomben, J.L., Guadagni, D.E. & Ling, L.C. (1971b) Some considerations of the volatilities of organic flavour compounds in foods. Journal of Agricultural and Food Chemistry, 19(No 6), 1045–1048.
Chandrasekaran, S.K. & King, C.J. (1972) Determination of activity coefficients. A.I.Ch.E. Journal, 18, 513–520.
Clarke, R.J. (1990) Physical properties of the volatile compounds of coffee. Café, Cacao et Thè, XXXIV (No 4), 285–294.
Cullere, L., Escudero, A., Campo, E., Cacho, J. & Ferreira, V. (2009) Multidimensional gas chromatography-mass spectrometry determination of 3-alkyl-2-methoxypyrazines in wine and must. A comparison of solid phase extraction and headspace solid phase extraction methods. Journal of Chromatography A, 1216, 4040–4-45.
Ferreira, V., San Juan, F., Escudero, A., Cullere, L., Fernandez-Zurbano, P., Saenz-Navajas, M.P. & Cacho, J. (2009) Modeling quality of premium Spanish red wines from gas chromatography – Olfactometry data. Journal of Agriculture and Food Chemistry, 57, 7490–7498.
Gretsche, C., Grandjean, G., Maering, M., Liardon, K. & Westfall, S. (1995) Determination of the partition coefficients of coffee volatiles using static head-space. In: Proceedings of the 16th ASIC Colloquium (Kyoto), pp. 326–31, ASIC, Paris, France.
Hayasaka, Y., Baldock, G.A. & Pollnitz, A.P. (2005) Contributions of mass spectrometry in the Australian Wine Research institute to advances in knowledge of grape and wine constituents. Australian Journal of Grape and Wine research, 11, 188–204.
Howard, K.L., Mike, J.H. & Riesen, R. (2005) Validation of a solid-phase microextraction method for headspace analysis of wine aroma components. American Journal of Enology and Viticulture, 56, 37–45.
Martin, A., Carillo, F., Trillo, L.M. & Rosello, A. (2009) A quick method for obtaining partition factor of congeners in spirits. European Food Research and Technology, 229(4), 697–703.
Plutowska, B. & Wardencki, W. (2007) Aromagrams – Aromatic profiles in the appreciation of food quality. Food Chemistry, 101, 845–872.
Plutowska, B. & Wardencki, W. (2008) Application of gas chromatography-olfactometry (GC-O) in analysis and quality assessment of alcoholic beverages – A review. Food Chemistry, 107, 449–463.
Pollien, P. & Yeretzian, C. (2001) Measurement of partition coefficients. Proceedings of the 19th ASIC Colloquium on Coffee, CD-ROM, ASIC, Paris.
Wardencki, W., Biernacka, P., Chmiel, T. & Dymerski, T. (2009) Instrumental techniques for the assessment of food quality. Proceedings of ECOpole, 3, 273–279.