2. How phenotypic selection works
3. Measuring phenotypic selection
4. Phenotypic selection in the wild
5. Misunderstandings about phenotypic selection
In this chapter, we describe the strength and patterns of natural selection in the wild. We focus on phenotypic selection because natural selection acts on the phenotypes of individual organisms. We begin by explaining what phenotypic selection is and how it works. We then explore how scientists study phenotypic selection in natural populations and discuss general patterns that have emerged from such investigations. Finally, we address common misunderstandings about selection and identify profitable avenues for future research.
fitness. The extent to which an individual contributes its genes to future generations relative to other individuals in the same population; a good operational definition of fitness is an individual’s relative reproductive success.
heritability. In the broad sense, the fraction of the total phenotypic variation in a population that can be attributed to genetic differences among individuals; in the narrow sense, that fraction of the total phenotypic variation that results from the additive effects of genes.
natural (phenotypic) selection. A difference, on average, between the survival or fecundity of individuals with certain phenotypes compared with individuals with other phenotypes.
phenotype. The outward characteristics of organisms, such as their form, physiology, and behavior.
quantitative trait. A trait that shows continuous rather than discrete variation; such traits are determined by the combined influence of many different genes and the environment.
selection gradient. A measure of the strength of selection acting on quantitative traits: for selection on a single trait, it is equal to the slope of the best-fit regression line in a scatterplot showing relative fitness as a function of phenotype; for selection acting on multiple traits, it is equal to the slope of the partial regression in a scatterplot showing relative fitness as a function of all phenotypes.
sexual selection. A difference, among members of the same sex, between the average mating success of individuals with a particular phenotype and that of individuals with other phenotypes.
In the introduction to On the Origin of Species, Darwin wrote, “a naturalist, reflecting on the mutual affinities of organic beings, on their embryological relations, their geographical distribution, geological succession, and other such facts, might come to the conclusion that each species had not been independently created, but had descended… from other species. Nevertheless, such a conclusion, even if well founded, would be unsatisfactory, until it could be shown how the innumerable species inhabiting this world have been modified…” (emphasis added). Thus, Darwin recognized that no theory of evolution would be complete if it failed to provide a plausible mechanism that could explain how living things change over evolutionary time. Darwin’s theory of evolution by natural selection provided such a mechanism. Yet, Darwin’s theory goes beyond explaining how living things change over time; it also explains the important concept of adaptation: the tendency for living things to evolve traits that make them so apparently well designed for survival and reproduction. Because of this broad explanatory power, Darwin’s theory ranks among the most important ideas in the history of human thought.
Although the central concept of Darwin’s theory is natural selection, Darwin never attempted to measure selection in nature. Moreover, in the century following the publication of On the Origin of Species, selection was generally regarded as too weak to be observed directly in natural populations. Partly for these reasons, some early evolutionists even questioned selection’s efficacy in driving evolutionary change.
This view that selection is weak and cannot be measured has changed dramatically. Beginning in the 1930s, evolutionists demonstrated mathematically that natural selection alone could power evolutionary change and adaptation. Moreover, in the past three decades, selection has been detected and quantified in hundreds of populations in nature. These data demonstrate that not only does selection occur routinely in nature, but that it is often sufficiently potent to bring about substantial evolutionary change in a relatively short time period. Indeed, selection is now viewed as the cause of adaptive evolution within natural populations.
Phenotypic selection takes place when individuals with particular phenotypes survive to reproductive age at higher rates than do individuals with other phenotypes, or when individuals with particular phenotypes produce more offspring than do individuals with other phenotypes. In either case, selection results in differential reproductive success, where some individuals have more offspring than others. Thus, phenotypic selection requires phenotypic variation, where individuals differ in some of their characteristics, and differential reproduction, where some individuals have more surviving offspring than others because of their distinctive characteristics. Those individuals that have more surviving offspring are said to have higher fitness (note that an individual’s fitness is measured as how well the individual performs relative to other individuals in the same population). Ultimately, phenotypic selection can lead to changes in the genetic makeup of populations over time—evolution. In particular, when the phenotypic characteristics under selection are heritable—that is, when the variations among individuals are, at least in part, passed from parents to offspring—selection will cause the population to change in these characteristics over time. Thus, evolution by natural selection requires three conditions: variation, differential reproduction, and heredity. Indeed, when these three conditions are satisfied, evolution by natural selection is a certain outcome.
Numerous factors in the environment can cause selection, including biological agents (such as an individual’s competitors, predators, and parasites) and nonbiological agents (such as the weather). The specific phenotypic traits on which agents of selection act are termed targets of selection. As we will see, however, selection often acts on multiple traits simultaneously in the same individual, making it a challenge to determine precisely which trait represents the actual target of selection.
Although phenotypic selection always favors an increase in fitness, it does not invariably bring about the evolution of greater trait values. In particular, when selection acts on quantitative (i.e., continuously distributed) traits, three different modes of selection are possible, each of which produces a distinctive pattern of trait evolution (figure 1). With directional selection, fitness consistently increases (or decreases) with the value of the trait. When directional selection acts on a trait, it changes the value of that trait in the population. Directional selection also tends to reduce variation, although often not dramatically. With stabilizing selection, individuals with intermediate trait values have highest fitness. Stabilizing selection does not tend to change the mean trait value. It does, however, reduce variation by disfavoring individuals in the tails of the trait’s distribution. Finally, with disruptive selection, individuals with extreme trait values have highest fitness. As with stabilizing selection, disruptive selection does not tend to change the mean trait value. Unlike stabilizing selection, however, disruptive selection increases variation by favoring individuals in the tails of the trait’s distribution.
All three modes of selection drive evolution by eliminating individuals with low fitness and preserving individuals with high fitness. Moreover, as noted earlier, if the trait of interest is heritable, then evolution will result, but the trait distribution in the evolved population will differ depending on the mode of selection (see figure 1). In particular, for traits under positive directional selection, the population will evolve larger trait values (illustrated in figure 1), whereas for those under negative directional selection, the population will evolve smaller trait values. For traits under stabilizing selection, the population will evolve a smaller range of trait values as the average trait value becomes more common in the population. Finally, for traits under disruptive selection, the population will evolve a wider range of trait values, possibly leading to the evolution of discrete, alternative phenotypes (see figure 1).
Given this background, we now turn to the issue of how to measure the mode and strength of phenotypic selection.
Suppose we are interested in measuring possible selection acting on some trait in a population. The first step is to estimate the fitness associated with different trait values. Ideally, we would identify individuals with different trait values and measure their overall fitness. In practice, however, most investigators measure only one component of fitness, such as survival, mating success, fecundity, or (even less directly) a trait that correlates with these fitness components, such as body size. Once we estimate fitness, we then fit a regression line (i.e., the best-fit line) through the data points relating fitness to phenotype. From the slope and shape of this regression line, we can determine the strength and mode of selection acting on our trait of interest. When this fitness function is described by a straight line (indicating directional selection; figure 1), the fitness (w) of the trait (z) can be estimated by the simple linear regression equation:
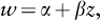
Figure 1. Three different modes of selection (directional, stabilizing, and disruptive) that may act on a quantitative trait (i.e., a trait that shows continuous rather than discrete variation). The top panel shows the distribution of beak sizes in a hypothetical population of birds before selection; the middle panels show fitness associated with different beak sizes during different modes of selection; and the bottom panels show the distribution of beak sizes following each form of selection. Note that for different modes of selection, the shape of the line relating fitness to phenotype varies (middle panels), as does the resulting pattern of trait evolution (bottom panels).
where α is the y-intercept of the fitness function and β is the fitness function’s slope. In this case, β measures the strength of directional selection. By contrast, when the fitness function has curvature (indicating stabilizing and disruptive selection; figure 1), quadratic regression is required to estimate the strength of selection. Here, fitness is estimated by:
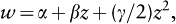
where γ measures the amount of curvature in the fitness function. In this case, γ measures the strength of quadratic selection. When β = 0 and γ is significantly negative (i.e., when the fitness function contains an intermediate performance maximum), we conclude that stabilizing selection is acting on the trait of interest. By contrast, when β = 0 and γ is significantly positive (i.e., when the fitness function contains an intermediate performance minimum), we conclude that disruptive selection is acting.
Figure 2. Spadefoot toad tadpoles (Spea bombifrons and S. multipticata) are highly variable in resource use and feeding morphology as represented by two extreme morphotypes: (A) an omnivore morph, which feeds mostly on detritus, and (B) a carnivore morph, which specializes on fairy shrimp. The mode of selection operating on feeding morphology varies for different species and populations as revealed when the fitness of individual tadpoles is plotted on phenotype for (C) S. bombifrons from mixed-species ponds, (D) S. multiplicata from mixed-species ponds, and (E) S. multiplicata from single-species ponds. Each panel (C–E) shows cubic spline regression estimates bracketed by 95% confidence intervals.
To illustrate how each mode of selection may be manifest in natural populations, consider a recent study of spadefoot toad tadpoles by Pfennig and colleagues (2007). Tadpoles of two species from the southwestern United States, Spea bombifrons and S. multiplicata, are highly variable in resource use and feeding morphology as represented by two extreme morphotypes: an omnivore morph (figure 2A), which feeds mostly on the pond bottom on detritus (decaying organic material), and a carnivore morph (figure 2B), which feeds mostly in the water column on fairy shrimp. In some ponds, there is a clear dimorphism in feeding morphology; in other ponds, individuals with intermediate phenotypes may be most common.
The mode of selection operating on feeding morphology varies for different species and populations. In mixed-species ponds (i.e., ponds containing both species), the most carnivore-like S. bombifrons tadpoles are largest (figure 2C; body size serves as a suitable proxy for fitness because larger individuals have higher survival, mating success, and fecundity in this system). Thus, directional selection favors more carnivore-like S. bombifrons. Presumably, this pattern reflects selection on S. bombifrons to express resource-use phenotypes that minimize their overlap with S. multiplicata for food; S. multiplicata tend to be more omnivore-like than S. bombifrons.
A different mode of selection was detected among S. multiplicata in mixed-species ponds. In this species, stabilizing selection appears to favor individuals with intermediate phenotypes (figure 2D). Presumably, carnivore phenotypes in these individuals are selectively disfavored; earlier work had shown that S. multiplicata carnivores are competitively inferior to S. bombifrons. Yet why does selection not favor omnivores, which are as distinct as possible from S. bombifrons? Presumably, selection acts against S. multiplicata omnivores in mixed-species ponds because omnivores metamorphose later and at a smaller body size than carnivores. Because mixed-species ponds typically contain relatively high shrimp densities, those S. multiplicata that express an intermediate feeding morphology—and can thereby supplement their detritus diet with, but not specialize on, the more nutritious shrimp resource—may be selectively favored. Thus, in mixed-species ponds, selection appears to favor S. multiplicata that are as carnivore-like as possible, but that are not so carnivore-like that they overlap with S. bombifrons in resource use.
Finally, a third mode of selection was detected among S. multiplicata in single-species ponds (figure 2E). Here, disruptive selection favors extreme feeding morphologies. In these ponds, individuals expressing extreme phenotypes would most likely have fewer (and, in the case of extreme omnivores, perhaps lower-quality) resources available. Nevertheless, compared with the majority of the population that may be intermediate in phenotype (and in resource use), individuals expressing extreme phenotypes would also most likely have fewer competitors with which to share those resources. Thus, relative to intermediate individuals, the fitness of extreme omnivores and carnivores may be high.
Although the above example illustrates the general approach that is widely used for measuring phenotypic selection in the wild, a critical assumption behind this approach is that variation in the measured trait causes the observed variation in fitness. However, rather than acting directly on the trait of interest (through direct selection), selection may be acting on other, unmeasured traits that are correlated with the measured trait (through indirect selection), generating a spurious correlation between the focal trait and fitness. One way to reduce the problem of indirect selection is to experimentally alter the trait of interest and then evaluate the effects of the manipulation on subsequent fitness (phenotypic engineering).
To illustrate the latter approach, consider the following example. Male long-tailed widowbirds, Euplectus progne, are endowed with a half-meter-long tail. Malte Andersson hypothesized that these extraordinary tail feathers are selectively favored because females find them attractive; i.e., long tail feathers are favored by sexual selection. To test this hypothesis, Andersson predicted that experimentally augmenting a male’s tail feathers should enhance the male’s fitness. Andersson captured male widowbirds and then shortened the tails of some by removing a segment of tail feathers, only to glue them onto another bird’s tail, thereby lengthening the latter bird’s tail. He also had two control groups: one in which the male’s tail feathers were cut off and glued back on, and another in which the males were handled in the same way but no tail feathers were removed.
The results of this phenotypic manipulation were dramatic. The tail-lengthened males were much more attractive to females than those that had suffered the loss of a portion of their tail feathers. Moreover, the tail-lengthened males also did better than controls. These data therefore indicate that tail length is a target of selection, with females acting as the selective agent.
Because it can expand the range of phenotypic values and reduce the problem of correlated traits, phenotypic engineering is especially useful for determining whether a trait is under direct selection and what mode of selection might operate on it. However, because phenotypic engineering often involves altering trait expression beyond the range of trait values observed in natural population, such manipulations do not help researchers estimate the strength of selection on natural populations in the wild, which is the topic we turn to next.
Numerous studies have used the above approaches to measure phenotypic selection in natural populations. Moreover, many of these studies measured selection acting on multiple traits on the same individual. Such data are particularly valuable because they allow us to distinguish direct selection on traits from the indirect effects of correlated traits. To estimate direct selection, we use a statistical approach known as multiple regression analysis. Multiple regression resembles simple linear regression (introduced in the previous section) except that fitness is regressed on multiple traits simultaneously, allowing us to measure the strength of direct selection acting on each trait after statistically controlling for the effects of correlations among other traits. Specifically, fitness (w) is estimated by:
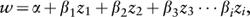
where βi is the partial regression slope associated with trait zi This parameter, termed the linear selection gradient, measures the strength of direct selection acting on trait zi. (Note that for selection on a single trait, the linear selection gradient is equal to the slope of the simple linear regression, as described in the previous section.) To allow comparisons among different types of traits and organisms, we can standardize the linear selection gradient by the amount of variation in the trait (e.g., by the standard deviation) to obtain a standardized measure of selection, βs.
Kingsolver and colleagues recently reviewed studies that used these approaches to measure selection gradients in natural populations. They identified 993 estimates of directional selection (βs), obtained from a diversity of organisms, ecological settings, and traits. Because positive and negative values of βs occur with similar frequency, they used the absolute values, |βs|, as an index of the magnitude of directional selection. The median value (50% of the values above and 50% below) of |βs| was 0.16, with a small fraction of values greater than 0.50, indicating strong selection. To put this strength of selection in perspective, imagine a population that experiences persistent directional selection of this magnitude (βs = 0.16) onatraitthathasatypical heritability of 0.3. In less than 70 generations, the population mean would exceed the initial range of variation in the population. In other words, phenotypic selection in many natural populations is sufficiently strong to cause substantial evolutionary change in a fairly short period of time on an evolutionary time scale.
Another important issue to resolve is the relative magnitude of natural selection (i.e., selection resulting from variation among individuals in survival or fecundity) compared to sexual selection (i.e., selection caused by variation among individuals in mating success). The available data on directional selection gradients suggest that sexual selection is typically stronger than natural selection. Indeed, the median magnitude of sexual selection is more than twice as great as that of natural selection. Thus, competition for mates may be important for rapid evolution in nature.
What are the patterns of quadratic selection in the wild? Kingsolver and colleagues (2001, 2007) identified 574 measures of the strength of quadratic selection, γ. They found that 50% of the values of γ are between –0.1 and +0.1, implying that the magnitude of quadratic selection is often modest. Moreover, the frequency distribution of γ is symmetric about zero, with negative and positive values equally common, which suggests that stabilizing selection is not more common than disruptive selection. Because disruptive selection is generally thought to be relatively rare in nature, this result is particularly surprising. It is possible that this result reflects sampling bias: only 16% of the values of γ in the literature are significantly different from zero. Thus, most studies do not have the sample size or statistical power to quantify quadratic selection of the magnitude that may be typical in natural populations. Alternatively, this result may reflect the true pattern of quadratic selection in nature; i.e., disruptive selection may actually be relatively common. The possible widespread occurrence of disruptive selection may reflect a ubiquitous agent of selection in nature: competition for resources, such as food. Because competition tends to decrease individual fitness, natural selection is generally thought to favor traits that lessen competition’s intensity. One way for selection to do so is to favor evolutionary divergence between initially similar phenotypes through density-dependent or frequency-dependent disruptive selection (e.g., see figure 2E).
Thus, to summarize, phenotypic selection is common in nature, and it is often sufficiently strong to cause substantial evolutionary change in a relatively short time period. Moreover, sexual selection tends to be stronger than natural selection. Finally, stabilizing selection appears to be no more common than disruptive selection. However, because few studies have focused on quadratic selection specifically, it is difficult to say how common or how strong disruptive selection is relative to stabilizing selection in natural populations.
Phenotypic selection is often misunderstood. We therefore highlight and clarify four common misunderstandings.
Selection and evolution are not the same, although the two concepts are often incorrectly equated. Selection is a process that produces evolution, whereas evolution is the historical pattern of change through time. Phenotypic selection (the process) can lead to evolution (the pattern), but it is only one of several processes that can do so (the others are mutation, gene flow, nonrandom mating, and genetic drift). Moreover, if a trait lacks heritable variation, selection will not produce evolution.
A common misconception is that individual organisms evolve following selection. It is true that phenotypic selection acts on the phenotypes of individual organisms. However, after the selection event, none of the selected individuals are expected to change in any way. What does change are characteristics of the population. Thus, populations evolve; individual organisms do not.
Phenotypic selection may indirectly cause the phenotypes of individual organisms to change. Specifically, agents of selection often alter the developmental expression of traits through a process known as phenotypic plasticity. When phenotypes are plastic, individuals that are genetically identical may express radically different phenotypes if they develop in different environments. For example, the spadefoot toad tadpoles in figure 2 are born as omnivores but may develop into carnivores following a change in their diet. In many species, individuals often exhibit heritable variation in their tendency to respond to environmental cues through phenotypic plasticity, indicating that plasticity itself is subject to natural selection and evolutionary change. Indeed, adaptive phenotypic plasticity is thought to evolve because it enables organisms to produce the optimal phenotype for the various environments that they may experience during their lifetime. Thus, by favoring the evolution of phenotypic plasticity, agents of selection may indirectly change the phenotypes of individual organisms.
A common misunderstanding about phenotypic selection acting on behavior is that individual organisms will perform actions for the good of their species. However, if altruists survive and reproduce at lower rates than other individuals in the same population, then the tendency to behave altruistically should not evolve, unless the altruists receive some other benefit.
As it turns out, nearly every act of altruism that has been studied in detail increases the altruist’s fitness, either because beneficiaries reciprocate or because the beneficiaries are genetically related to the altruist. Helping nondescendant kin (relatives other than offspring) can increase an altruist’s fitness because relatives share genes. Moreover, fitness gained by personal reproduction (direct fitness) and fitness gained by helping nondescendant kin (indirect fitness) can both be expressed in identical genetic terms. We can sum up an individual’s total contribution of genes to the next generation, creating a quantitative measure called inclusive fitness. Thus, altruism may be adaptive if it ultimately results in more shared genes being transmitted to the next generation. In general, natural selection should always favor traits that maximize an individual’s inclusive fitness.
It is often assumed that the evolutionary response to selection is slow. We have already seen, however, that phenotypic selection is often sufficiently strong to cause substantial evolutionary change in a relatively short time. Moreover, phenotypic selection may even produce substantial evolutionary change in only one generation. Consider a population that contains abundant phenotypic variation. If this variation has high heritability, and if there is strong truncating selection, in which individuals with a trait value above a certain threshold value survive or reproduce while those below this value do not, then the population will evolve dramatically in only one generation.
For example, Peter and Rosemary Grant recently documented character displacement—evolution in resource-acquisition traits stemming from competition between species—in a species of Galápagos finch that recently (i.e., in the last 25 years) confronted a novel competitor (Grant and Grant, 2006). Remarkably, their data suggest that the focal species may have evolved away from its competitor in beak morphology in only one generation. Thus, paradoxically, evolution may happen so rapidly that we may actually fail to detect it.
As we have seen, numerous recent studies have measured phenotypic selection in the wild. Many interesting patterns have emerged from these studies. However, a number of questions remain unanswered. Here, we list four such questions.
First, does phenotypic selection vary over time and space? In particular, does the fact that environmental conditions change frequently cause the magnitude and even the direction of selection to change also? Such fluctuating selection could explain why most organisms appear to be experiencing at least some directional selection. If environments vary frequently, then the organisms living in these environments will tend to possess trait values that are suboptimal for their particular environment. Consequently, directional selection would always be acting to drive the trait value toward the current optimum. We need many more long-term field studies of selection in the wild to determine if the magnitude, direction, or mode of selection varies in time and space.
Second, is disruptive selection relatively common in nature, and, if so, what agents drive it? Specifically, is disruptive selection often mediated by density-or frequency-dependent processes, such as competition? Resolving this issue is vital for understanding the origins and maintenance of alternative phenotypes in populations (e.g., see figure 2), and, possibly, the origin of new species.
Third, what measure of fitness provides the most complete picture of selection? An operational definition of fitness is that it is the total number of offspring that an individual produces in its lifetime. Yet, for practical reasons, most studies consider only components of fitness, such as survival. We need more studies that determine how reliably individual fitness components predict true lifetime fitness in natural populations. We especially need more studies that compare the relative magnitude of selection on survival or fecundity (natural selection) with selection on mating success (sexual selection). As noted in section 4 above, the available data indicate that sexual selection is typically significantly stronger than natural selection. Does this result generally hold across diverse taxa? Moreover, to develop a truly comprehensive view of how phenotypic selection drives trait evolution, we need more selection studies that determine how trait expression influences an individual’s inclusive fitness.
Finally, what is the relative importance of evolution versus phenotypic plasticity in mediating rapid phenotypic responses to changing environments? Many organisms are currently undergoing rapid phenotypic change in response to ongoing human-mediated change in their environment. To what extent does such rapid phenotypic change reflect phenotypic plasticity as opposed to rapid evolution?
In sum, natural selection is the central organizing principle of evolutionary theory. This theory explains not only how living things diversify but also those features of living things that so wonderfully equip them for survival and reproduction. Although natural selection is a simple concept, modern research is only beginning to discover that it works in myriad and sometimes subtle ways.
Andersson, Malte. 1982. Female choice selects for extreme tail length in a widowbird. Nature 299: 818–820. A classic example of the use of manipulative experiments to document phenotypic selection in the wild.
Conner, Jeffrey K., and Daniel L. Hartl. 2004. A Primer of Ecological Genetics. Sunderland, MA: Sinauer Associates. An excellent overview of ecological genetics with a clear summary of how to measure selection.
Endler, John A. 1986. Natural Selection in the Wild. Princeton, NJ: Princeton University Press. A seminal monograph that describes advantages and disadvantages of various approaches for measuring phenotypic selection in natural populations.
Grant, Peter R., and B. Rosemary Grant. 2006. Evolution of character displacement in Darwin’s finches. Science 313: 224–226. An interesting example that describes rapid evolution in a classic system.
Kingsolver, Joel G., Hopi E. Hoekstra, Jon M. Hoekstra, David Berrigan, Sacha N. Vignieri, Chris H. Hill, Anhthu Hoang, Patricia Gilbert, and Peter Beerli. 2001. The strength of phenotypic selection in natural populations. American Naturalist 157: 245–261. Reviews numerous studies of selection in natural populations.
Kingsolver, Joel G., and David W. Pfennig. 2007. Patterns and power of phenotypic selection in nature. BioScience 57: 561–572. An overview of how phenotypic selection acts in natural populations. Portions of this chapter (especially parts of sections 3, 4, and 6) are adapted from this review.
Losos, Jonathan B., Thomas W. Schoener, R. Brian Langerhans, and David A. Spiller. 2006. Rapid temporal reversal in predator-driven natural selection. Science 314: 1111. Illustrates how directional selection can reverse direction rapidly.
Pfennig, David W., Amber M. Rice, and Ryan A. Martin. 2007. Field and experimental evidence for competition’s role in phenotypic divergence. Evolution 61: 257–271. Describes how different modes of selection may act on the same species when confronted with different environmental circumstances.