1. Ecological speciation: What it is and how to test for it
2. Forms of divergent selection
3. Forms of reproductive isolation
4. Genetic mechanisms linking selection and reproductive isolation
5. Geography of ecological speciation
6. Generality of ecological speciation
7. Remaining questions in the study of ecological speciation
Understanding how new species arise is a central goal of evolutionary biology. Recent years have seen renewed interest in the classic idea that adaptive evolution within species and the origin of new species are intimately linked. More specifically, barriers to genetic exchange between populations (termed reproductive isolation) are the hallmark of species, and evolutionary biologists have been asking whether ecologically based divergent natural selection, the process that is responsible for adaptive divergence between populations, may cause such reproductive barriers to evolve. Convincing examples of this process, termed ecological speciation, are accumulating in the literature, and comparative approaches suggest that it may be a widespread phenomenon taxonomically. Attention is now being given to understanding details of the process and uncovering generalities in its operation. Three main components of ecological speciation can be recognized: a source of ecologically based divergent selection, a form of reproductive isolation, and a genetic mechanism linking the two. Current research is focused on understanding these components during the various stages of ecological speciation from initiation to completion.
ecologically based divergent selection. Selection arising from environmental differences and/or ecological interactions (e.g., competition) that acts in contrasting directions on two populations (e.g., large body size confers high survival in one environment and low survival in the other) or favors opposite extremes of a trait within a single population (i.e., disruptive selection)
linkage disequilibrium. A statistical association between alleles at one locus and alleles at a different locus, the consequence of which is that selection on one locus (e.g., a locus affecting an ecological trait such as color pattern) causes a correlated evolutionary response at the other locus (e.g., a locus affecting mating preference)
pleiotropy. Multiple phenotypic effects of a gene (e.g., a gene affecting color pattern also affects mating preferences)
postmating isolation. Barriers to gene flow that act after mating (e.g., intermediate trait values of hybrids that make them poor competitors for resources, reducing their fitness)
premating isolation. Barriers to gene flow that act before mating (e.g., divergent mate preferences that prevent copulation between individuals from different populations)
reproductive isolation. A reduction or lack of genetic exchange (gene flow) between taxa
sympatric speciation. A geographic mode of speciation whereby a single population splits into two species in the absence of any geographic separation, often via disruptive selection
The idea that the macroevolutionary phenomenon of speciation is the result of the microevolutionary process of adaptation dates back at least to Charles Darwin. However, it was not until the popularization of the biological species concept in the middle of the last century, whereby speciation was defined as the process by which barriers to genetic exchange evolve between populations, that the study of Darwin’s “mystery of mysteries,” the origin of species, became empirically tractable. The past two decades have witnessed an explosion of speciation research, with much attention being given to understanding the role of divergent selection in speciation.
As defined by Dolph Schluter and others, ecological speciation is the process in which barriers to genetic exchange evolve between populations as a result of ecologically based divergent natural selection. Selection is ecological when it arises from differences in the environment or from interactions between populations over resource acquisition. Ecologically based selection can thus arise, for example, from an individual’s quest to obtain food and other nutrients, attract pollinators, or avoid predators. It can also arise from an individual’s interaction with other organisms in its attempt to achieve these goals (e.g., resource competition, predation). Selection is divergent when it acts in contrasting directions in the two populations. Included here is the special case of disruptive selection on a single population, in which selection favors opposite extremes of the same trait. During ecological speciation, populations experience divergent selection between environments or niches and thus differentiate in ecologically important traits. If these traits, or ones that are genetically correlated with them, affect reproductive isolation, then speciation occurs as a consequence.
Ecological speciation is distinguished from other models of speciation in which the evolution of reproductive isolation involves processes other than ecologically based divergent selection. These include models in which chance events play a central role, including speciation by polyploidization, hybridization, genetic drift, and population bottlenecks (i.e., drastic reductions in population size). Nonecological speciation also includes models in which selection is involved, but it is nonecological (e.g., sexual conflict, in which selection arises from an evolutionary conflict of interest between the sexes over traits related to reproduction), or it is not divergent between environments.
An alternative definition of ecological speciation would restrict it to situations in which the reproductive barriers themselves are ecological in nature, such as reduced hybrid fitness arising because intermediate hybrid phenotypes cause them to perform poorly in either parental environment (i.e., see the third point below). In contrast, incompatibilities between the parental genomes, expressed when they are brought together in hybrids, is an example of a nonecological barrier. However, when the goal is to understand mechanisms of speciation, it is of interest when both ecological and nonecological forms of reproductive isolation evolve through a specific evolutionary process (e.g., ecologically based divergent selection). Ecological speciation can therefore involve the evolution of any type of reproductive barrier, so long as ecologically based divergent selection is responsible. Ecological speciation can also occur under any spatial arrangement of populations, with population pairs being geographically separated (allopatry), contiguous (parapatry), or in complete contact (sympatry). Under any of these geographic scenarios, if divergent selection drives the evolution of reproductive isolation, then speciation is classified as ecological.
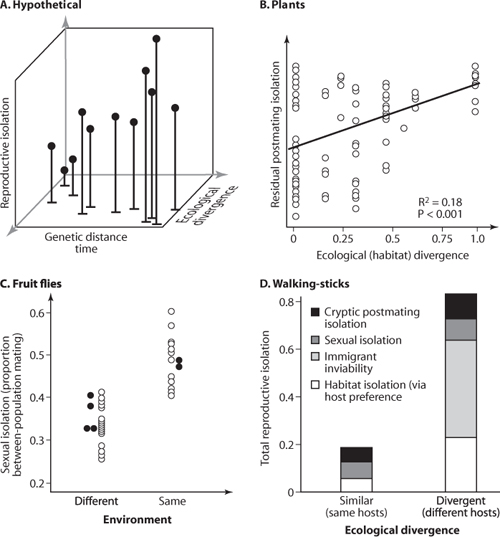
Laboratory evolution experiments using Drosophila fruit flies have shown that ecological speciation is feasible: when replicate populations are independently adapted to one of two environments, reproductive isolation tends to arise between populations from different environments, but not between populations evolved in similar environments. Classic examples of such experiments come from the work of Diane M. B. Dodd and of George Kilias and colleagues (figure 1). Convincing examples of ecological speciation in nature are also accumulating, with empirical tests tending to focus on three forms of evidence.
First, ecological speciation predicts that the strength of reproductive isolation between pairs of populations will be positively related to the magnitude of their ecological differentiation, independent of any correlation with divergence time (figure 1). This has been shown in Timema walking-stick insects studied by Patrik Nosil, Bernie J. Crespi, and Cristina P. Sandoval, in which pairs of populations adapted to different hostplant species exhibit stronger reproductive isolation than do pairs of populations adapted to the same hostplant species (figure 1). A special case of this scenario, termed parallel speciation, occurs when the same reproductive barriers evolve in independent populations experiencing similar environments. The Drosophila laboratory experiments described above demonstrate the initial stages of parallel speciation: independent populations adapted to one environment were reproductively isolated from populations adapted to the other environment but not from one another. A prime example of parallel speciation in nature comes from freshwater stickleback (Gasterosteus) fishes studied by Dolph Schluter, Howard D. Rundle, and colleagues. These fish come in two main forms, a slender limnetic that feeds primarily on plankton in the open water of a lake and a more robust benthic, which feeds on invertebrates in the shallows. Sympatric limnetic-benthic pairs occur in a number of lakes in western Canada, and molecular genetic evidence suggests that the pairs have arisen independently (i.e., the present-day phenotypic similarity of limnetics, and of benthics, from separate lakes is the result of parallel evolution and not shared ancestry). Mating trials demonstrate that reproductive isolation between limnetics and benthics has likewise evolved in parallel: premating isolation is strong between limnetics and benthics, even when they are taken from separate lakes, whereas premating isolation is absent within a form, even when they derive from different lakes (e.g., premating isolation is lacking between limnetic forms from different lakes). The repeated evolution of the same barriers to gene flow, in correlation with ecological divergence, is unlikely to occur via nonecological processes (e.g., genetic drift) and thus provides strong comparative evidence for ecological speciation.
Figure 1. Tests for ecological speciation, where the premise is to isolate an association between ecological divergence and levels of reproductive isolation, independent of the amount of time that population pairs have had to diverge from one another via non-ecological processes such as genetic drift. (A) A hypothetical scenario in which reproductive isolation increases with both genetic distance (a proxy for time) and ecological divergence. (B) The pattern predicted by ecological speciation, in this case a positive association between habitat divergence and residual postmating isolation (the effects of time have been statistically removed) between angiosperm species. The data come from a comparative study by Funk and colleagues, and the figure is reprinted with permission of the National Academy of Sciences U.S.A. (C) Evidence for ecological speciation from laboratory evolution studies. Shown is the proportion of matings occurring between independently evolved lines of Drosophila as a function of the similarity of their environments. Between-population mating is less common when populations have been adapted to different environments. Open circles are from work by Dodd, and closed circles are from work by Kilias and colleagues. (D) In Timema cristinae walking-stick insects, multiple forms of reproductive isolation are stronger between pairs of populations using different host-plant species (i.e., pairs with divergent ecologies) than between similar-aged pairs of populations using the same host-plant species (i.e., pairs with similar ecologies). The pattern was documented in a series of studies by Nosil, Crespi, and Sandoval, and the figure is reprinted with permission of the American Society for Naturalists.
Figure 2. A schematic illustration of the three components of ecological speciation. (A) A form of divergent selection is required, where selection is divergent when it acts in contrasting directions in two populations. (B) Forms of reproductive isolation are numerous and can act either before or after mating (premating and postmating isolation, respectively). Depicted are butterflies from populations adapted to different habitats (dark gray versus white-winged individuals) and hybrids between these parental forms (light gray). The form of premating isolation depicted is sexual isolation, where individuals prefer to mate with individuals that are the same color as themselves. The form of postmating isolation shown is one where hybrids suffer reduced fitness because their intermediate phenotype renders them unfit in either parental environment. Specifically, the hybrids do not match the substrate in either parental environment and suffer increased rates of visual predation as a result of this lack of crypsis. (C) A genetic mechanism is required to transmit selection on genes conferring local adaptation to genes causing reproductive isolation. Reproductive isolation can evolve because the genes under selection are the same as those conferring reproductive isolation (i.e., pleiotropy). Alternatively, reproductive isolation might evolve via the statistical association between genes under selection and those conferring reproductive isolation. This statistical association is termed linkage disequilibrium and is facilitated by proximity of the different genes on the same chromosome (i.e., physical linkage).
Second, ecological speciation is facilitated when the traits under divergent selection also cause reproductive isolation pleiotropically; such pleiotropy is therefore predicted to be common in cases of ecological speciation. A clear example comes from the work of Jim Mallet, Chris Jiggins, and colleagues on butterflies in the genus Heliconius. In this group of tropical butterflies, natural selection acts on mimetic coloration to reduce visual predation. Geographic variation in the phenotype of the comimic generates divergent selection among populations to match the local form. Because these color patterns are also used in mate choice, divergence in coloration generates premating isolation (i.e., sexual isolation) as a side effect.
Third, ecologically based divergent selection predicts that if hybrids can be formed between the parental populations, their fitness should be reduced for ecological reasons. This normally occurs because hybrid phenotypes are intermediate between the two parental forms, making them ill-suited for various tasks (e.g., acquiring resources, avoiding predation, finding a mate) in either parental environment. This type of hybrid fitness reduction is unlikely to arise via non-ecological mechanisms of speciation. Ecologically dependent reductions in growth rate have been shown in limnetic-benthic hybrids, and reduced survival of Heliconius hybrids is likely to arise from their intermediate coloration that fails to mimic either parental form.
As the above studies highlight, the case for ecological speciation is compelling: it clearly can, and does, occur. Attention is now being given to understanding the details of the process, including the three main components (a source of divergent selection, a form of reproductive isolation, and a genetic mechanism linking the two; figure 2), to testing for generalities, and to uncovering the geographic context of ecological speciation.
The first component of ecological speciation is a source of divergent selection (figure 2A). Three main sources have been recognized: differences between environments, ecological interactions, and sexual selection.
Divergent selection can arise because of differences between populations in their environments, including, for example, habitat structure, climate, and resource availability. As populations adapt to different environments, they may diverge from one another in many ways, evolving to look different, smell different, and behave differently. Such differences will contribute to speciation if, for example, they reduce the likelihood of between-population mating (perhaps because individuals from different populations no longer recognize one another as potential mates as in the Heliconius butterflies discussed above), or they reduce the fitness of any hybrids that are formed (perhaps, as mentioned earlier, because such hybrids are ill-suited to either parental niche, as in the stickleback example discussed above). It is highly unlikely that any two environments are identical, and it is not surprising, therefore, that environmental differences appear to be a common cause of divergent selection during ecological speciation.
Divergent selection may also arise between populations as a result of their ecological interactions with one another, most notably competition for shared resources. Divergent selection arising from such interactions is frequency dependent because individual fitness depends on the frequency of the various phenotypes within the population. Although interspecific competition appears common in nature and may play a key role in sympatric divergence between taxa, its consequences for the evolution of reproductive isolation are poorly understood. In the threespine sticklebacks discussed earlier, for example, resource competition has been strongly implicated in the morphological divergence of limnetics and benthics (i.e., ecological character displacement), as has adaptation to their different environments. Although the latter form of divergent selection has been implicated in the evolution of reproductive isolation, unambiguous evidence that the former promotes reproductive isolation is lacking.
Interbreeding (hybridization), another type of interaction between populations, can also contribute to ecological speciation via a process known as reinforcement. Reinforcement occurs when hybrids have reduced fitness such that selection favors parental individuals that are less likely to hybridize, thereby strengthening premating isolation. Although it features prominently in many models of speciation, reinforcement is difficult to categorize because it can complete a speciation process initiated by any mechanism, ecological or not. However, if hybrid fitness is reduced by ecological means, then reinforcement can be considered a component of ecological speciation. Reinforcement has been implicated in the ecological speciation of limnetic and benthic threespine sticklebacks: postmating isolation is ecological in nature, and premating isolation in sympatry appears to have been strengthened in response.
Sexual selection has long been hypothesized to be a powerful mechanism of speciation because it involves communication between a signaler and a receiver, thereby creating the potential for rapid coevolutionary diversification of mating signals and preferences that may generate reproductive isolation. Divergent sexual selection arises when mate preferences differ between populations. Such selection is considered a component of ecological speciation when it is initiated by divergent selection between environments. This can occur, for example, if two habitats vary in their signal transmission properties such that different signals are most detectable (i.e., favored by natural selection) within each. Different mating signals and preferences may then evolve in populations occupying either habitat. For example, Manuel Leal and Leo J. Fleishman studied populations of Anolis lizards that occupy different (mesic versus xeric) habitats. They found that light conditions differ between these habitats and that the dewlap spectral traits of the lizards, which are important for social and mating communication, have diverged between populations using different habitats in ways that increase signal detectability within the native habitat, potentially generating premating isolation between these populations. Divergence of mating signals can also occur if sensory or communication systems adapt to their specific environment, even outside of the mating context (e.g., to facilitate resource acquisition or predator avoidance). Several examples of divergence in display traits, sensory systems, or preferences in correlation with environment have now been reported.
The second component of ecological speciation is the form of reproductive isolation, of which many are possible, and speciation may involve one or more of them. Forms of reproductive isolation are commonly classified according to whether they occur before or after mating (premating and postmating isolation, respectively; figure 2B). The role of these reproductive barriers in speciation was thoroughly reviewed in a recent book on speciation by Jerry Coyne and H. Allen Orr (2004).
Premating isolation can arise when populations are separated in space (habitat) or time. Habitat isolation occurs when populations exhibit genetically based preferences for separate habitats, reducing the likelihood of between-population encounters and thus of interbreeding. For example, divergent host-plant preferences cause partial reproductive isolation between many herbivorous insect populations that mate on the plant on which they feed. Temporal isolation occurs when populations exhibit divergent developmental schedules such that mating happens at different times in each. A classic empirical example of such forms of reproductive isolation comes from the apple and hawthorne host races of Rhagoletis flies studied by Guy Bush, Jeffery L. Feder, and colleagues. Differences in host-plant preferences and developmental schedules cause substantial reproductive isolation between these host races. Additionally, if individuals immigrating into a foreign habitat are maladapted and die before mating, this immigrant inviability will also act to reduce interbreeding. All these forms of reproductive isolation are inherently ecological and thus are expected to commonly play a role in ecological speciation.
Another barrier that acts before mating is sexual isolation, arising from differences between populations in their mating signals and preferences. Sexual isolation is considered by many to be the main component of reproductive isolation between recently evolved taxa. Consistent with this, studies in cichlids and Drosophila have shown that sexual isolation appears necessary for species to coexist in nature. Likewise, the laboratory Drosophila experiments discussed above demonstrate that sexual isolation can evolve relatively rapidly when populations are subjected to divergent natural selection (figure 1). A number of examples from nature also exist in which adaptation to different environments has been implicated in the evolution of sexual isolation, including beetles, walking sticks, butterflies, and stickleback fish. For example, in the stickleback fish discussed previously, adaptation to their different habitats (open water versus shallows) causes divergence in body size, and because mate choice is assortative with respect to size, sexual isolation arises as a by-product.
Postmating isolation can arise when hybrid fitness is reduced because of an ecological mismatch between intermediate hybrid phenotypes and the environment, as was discussed earlier in the evidence for ecological speciation. An example of such ecologically dependent reductions in hybrid fitness stems from work on limnetic–benthic sticklebacks. Hybrids between the limnetic and benthic forms exhibit high fitness in the laboratory. In contrast, the fitness of hybrids in the wild is reduced relative to parental forms. Use of various types of hybrid crosses has shown that this reduction was a direct result of their intermediate phenotype and was not caused by genetic incompatibilities between the two forms (that could arise via any mechanism of speciation). Hybrids can also suffer reduced fitness because their sexual display traits and/or mate preferences reduce their mating success, in effect generating sexual selection against them. This has been shown in hybrid male sticklebacks in work by Steven Vamosi.
Postmating isolation can also result from genetic incompatibilities between divergent genomes, caused by negative interactions between genes that differ between populations, when these genes are brought together in hybrids. These incompatibilities reduce the fitness of hybrids and do not depend on an ecological interaction between phenotype and environment. However, it is still possible that such incompatibilities evolve as a byproduct of ecologically based divergent natural selection, for example, if alleles favored by selection within each population are incompatible with one another when brought together in the genome of a hybrid.
The final component of ecological speciation is the genetic mechanism by which selection on ecological traits is transmitted to the genes causing reproductive isolation, thereby driving the evolution of the latter. There are two ways this can occur, distinguished by the relationship between the genes under divergent selection (i.e., those affecting ecological traits) and those causing reproductive isolation (figure 2C). In the first, these genes are the same (e.g., a gene affecting color pattern pleiotropically affects mate preference). In this case, reproductive isolation is said to evolve by direct selection because the alleles responsible for reproductive isolation are themselves under selection, albeit for another reason. In the second, the genes under divergent selection are physically different from those causing reproductive isolation. In this case, reproductive isolation is said to evolve by indirect selection because selection acts on genes causing reproductive isolation only to the extent that they are nonrandomly associated (i.e., in linkage disequilibrium) with the genes directly under selection. When selection acts on genes affecting ecological traits, such nonrandom associations will cause a correlated evolutionary response in genes conferring reproductive isolation. The nature of these genetic associations is important because it affects the strength of selection transmitted to the genes affecting reproductive isolation.
Speciation is facilitated when genes under divergent selection cause reproductive isolation pleiotropically, and there are numerous ways this can occur. These include, for example, habitat isolation that evolves as a direct consequence of selection on genes affecting habitat choice. Selection might also act on ecological traits that incidentally affect mate preferences; the Drosophila lab experiments discussed previously suggest that this is not an unlikely occurrence, and the previously discussed mimetic color patterns in tropical butterflies of the genus Heliconius provide a classic example from nature. In plants, adaptation to different pollinators can cause premating isolation as a side effect. For example, work by Douglas Schemske and Toby Bradshaw has shown that divergent natural selection acts on a flower color gene in Mimulus monkeyflowers via the effects of color on attractiveness to pollinators. In Mimulus lewisii, pink-colored flowers are favored by bumblebees and discriminated against by hummingbirds. In contrast, M. cardinalis has red flowers, which are favored by hummingbirds and discriminated against by bumblebees. Adaptation to different pollinators via divergence in this flower color gene therefore directly affects the probability of cross-pollination (i.e., hybridization), a form of sexual isolation.
Other forms of reproductive isolation could also involve pleiotropy. For example, temporal isolation, caused by differences in flowering time could arise as a pleiotropic effect of adaptation to different environments, whereas postmating isolation can arise pleiotropically if alleles favored by selection within each population contribute to genetic incompatibilities in hybrids.
Indirect selection is less effective than direct selection in the evolution of reproductive isolation. This is because the genetic association between the two sets of genes (i.e., linkage disequilibrium) is not perfect, thereby reducing the strength of selection transmitted to the genes causing reproductive isolation (a phenomenon that has been likened to the slipping of a car’s clutch in that the wheels experience only a fraction of the power provided by the engine). The amount of linkage disequilibrium that exists can be affected by three factors. The first is the genetic basis of reproductive isolation, of which there is an important distinction between what are termed one-allele and two-allele mechanisms. In a one-allele mechanism, reproductive isolation arises from the fixation of the same allele in both populations (e.g., an allele causing individuals to prefer mates phenotypically similar to themselves, for example, individuals similar in body size). In a two-allele mechanism, different alleles fix in each population (e.g., a preference allele for large individuals in one population and a preference allele for small individuals in the other). This distinction is important because, in a two-allele mechanism, recombination will tend to break down linkage disequilibrium between the genes under divergent selection and those causing reproductive isolation. In contrast, recombination creates no such problem for a one-allele mechanism, and it is therefore a more powerful mechanism of speciation. The prevalence of these genetic mechanisms in nature is unknown.
The second factor is physical linkage. The maintenance of linkage disequilibrium is greatly facilitated by the physical linkage of genes on a chromosome because the likelihood of a recombination event declines with decreasing genetic map distance. Chromosomal inversions may thus play a role in ecological speciation by suppressing recombination, thus physically linking large regions of the genome.
The third factor is the strength and form of selection. Linkage disequilibrium can be maintained by strong selection that favors specific combinations of genes (i.e., correlational selection), and such selection may be important during sympatric speciation.
In general, data examining the relationship between genes under divergent selection and those causing reproductive isolation are sparse. In practice, separating pleiotropy from close physical linkage will be a difficult task, although their effects may ultimately be very similar. Important questions are how common pleiotropy and tight physical linkage are and how often they are of the form that would facilitate ecological speciation. Finally, we note that almost nothing is known about the types of genes involved in ecological speciation. Information on the genetics underlying ecological speciation may improve our mechanistic understanding of its operation in nature, including the type of genes involved and how they cause reproductive isolation.
Traditionally, speciation has been classified not by the mechanisms responsible (e.g., ecological speciation) but rather by the geographic context under which it occurs. These include allopatric, parapatric, and sympatric, with the latter being especially controversial and garnering much attention. Ecological speciation, however, can occur under any of these geographic contexts. The divergence of allopatric populations is unimpeded by the constraining effects of gene flow, so reproductive isolation is eventually expected to arise between them from chance events (e.g., genetic drift). Ecologically based divergent selection, however, can greatly accelerate this process and may commonly do so because allopatric pairs of populations often occupy different environments and are therefore subject to divergent selection. Sympatric speciation, in contrast, represents the opposite extreme in which speciation occurs in the absence of any geographic isolation. Strong disruptive selection is therefore required to overcome the homogenizing effects of gene flow, and such selection is expected to be ecological in origin. Ecological speciation is therefore a likely mechanism of sympatric speciation. Parapatric speciation represents an intermediate scenario in which gene flow is reduced but not eliminated by geographic barriers (including distance). Divergent selection is again required to overcome the effects of gene flow, making ecological speciation a likely mechanism.
Although attractive, the classification of speciation into these distinct geographic contexts may be overly simplistic and fail to capture the complexity of some speciation events in nature. Ecological speciation, for example, may often occur in stages that involve different geographic contexts (figure 3). The idea is that speciation begins when populations are allopatric, with reproductive isolation accumulating as a by-product of adaptation to their different environments. The second stage is initiated on secondary contact (parapatry or sympatry), with genetic exchange becoming possible at this point. Although the resulting gene flow is generally thought to constrain adaptive divergence and hamper speciation, ecological interactions are added as a source of divergent selection, and reinforcement also becomes possible. The amount of reproductive isolation that evolves during each stage indicates the primary geographic context of speciation, with the classic scenarios of allopatric and sympatric speciation representing the extremes in which, before secondary contact, reproductive isolation was either essentially complete or absent, respectively. Intermediate scenarios may be more common in nature, however, as suggested in the speciation of limnetic and benthic sticklebacks, which appears to have involved some reproductive isolation evolving during both phases. More complex scenarios are also possible, as suggested by recent molecular data from the apple and hawthorn races of Rhagoletis flies, traditionally put forward as a classic case of sympatric speciation. Feder and colleagues (2004) have shown that inversion polymorphisms, containing genetic variation affecting ecologically important diapause traits, trace their origins to allopatric populations in Mexico.
Figure 3. A scenario for ecological speciation under various geographic contexts. Reproductive isolation between two populations is absent at the beginning of the speciation process (at left) and evolves to completion (at right), as indicated by the solid line. The geographic context of speciation is indicated by the position of the dashed vertical line dividing the allopatric stage (left) from the sympatric/parapatric stage (right). This division can occur at any point in time, thus accommodating a range of geographic contexts and either a one-or two-stage process. For example, the extreme case of fully sympatric speciation occurs when the allopatric stage is absent (division line coincides with the y-axis). The opposite extreme of fully allopatric speciation occurs when reproductive isolation has evolved to completion before secondary contact occurs (division line falls at the right-hand extreme). Depicted is an intermediate, two-stage scenario in which partial reproductive isolation evolves in allopatry, but reproductive isolation does not evolve to completion until after secondary contact has occurred. The ecological causes of divergent selection by which reproductive isolation may evolve are listed within the panel for each stage. The figure is modified from a review of ecological speciation by Rundle and Nosil and reprinted with the permission of the British Ecological Society.
Comparative approaches can be used to investigate generalities of ecological speciation, including which, if any, forms of reproductive isolation are common, in what order they tend to arise, and the forms of divergent selection that drove their evolution. Likewise, the taxonomic generality of ecological speciation can also be explored using comparative approaches, as was done in a recent study by Daniel J. Funk, Patrik Nosil, and William B. Etges (2006). Using published data involving more than 500 species pairs of plants, invertebrates, and vertebrates, they found a positive association between ecological divergence and reproductive isolation for seven of eight groups. By controlling for divergence time using published genetic data, their results suggest that ecological speciation may be taxonomically widespread.
There is convincing evidence from the laboratory and nature that divergent natural selection can drive the evolution of reproductive isolation. Ecological speciation therefore most certainly occurs. Current research is aimed at determining the ecological sources of divergent selection, the types of reproductive barriers involved, and the genetic mechanisms linking them. Attention is also being given to the generality of the findings associated with these components.
There is much work remaining. Insufficient attention has been given to understanding the contribution of ecological interactions and sexual selection to the evolution of reproductive isolation. The relative importance of various forms of reproductive isolation and the likelihood that each evolves via divergent selection are also not well resolved. Direct tests of the genetic link between traits under selection and those conferring reproductive isolation are lacking too. Finally, the factors affecting the degree of progress toward the completion of ecological speciation are poorly understood. We hope that studies using a diversity of taxa and examining a wide range of divergence, from incipient to established species, will shed light on how ecological speciation unfolds from beginning to end.
Bradshaw, Toby, and Douglas Schemske. 2003. Allele substitution at a flower colour locus produces a pollinator shift in monkeyflowers. Nature 426: 176–178.
Coyne, Jerry, and H. Allen Orr. 2004. Speciation. Sunder-land, MA: Sinauer Associates.
Feder, Jeffery, Stewart Berlocher, Joe Roethele, Hattie Dambroski, James Smith, William Perry, Vesna Gavri-lovic, Kenneth Filchak, Juan Rull, and Martin Aluja. 2003. Allopatric genetic origins for sympatric host-plant shifts and race formation in Rhagoletis. Proceedings of the National Academy of Sciences U.S.A. 100: 10314–10319.
Funk, Daniel, Patrik Nosil, and William Etges. 2006. Ecological divergence exhibits consistently positive associations with reproductive isolation across disparate taxa. Proceedings of the National Academy of Sciences U.S.A. 103: 3209–3213.
Jiggins, Chris, Russell Naisbit, Rebecca Coe, and James Mallet. 2001. Reproductive isolation caused by colour pattern mimicry. Nature 411: 302–305.
Leal, M., and Leo J. Fleishman. 2004. Differences in visual signal design and detectability between allopatric populations of Anolis lizards. American Naturalist 163: 26–39.
Mayr, Ernst. 1963. Animal Species and Evolution. Cambridge, MA: Harvard University Press.
Nosil, Patrik. 2007. Divergent host-plant adaptation and reproductive isolation between ecotypes of Timema cristinae. American Naturalist 169: 151–162.
Rundle, Howard, Laura Nagel, Janette Boughman, and Dolph Schluter. 2000. Natural selection and parallel speciation in sympatric sticklebacks. Science 287: 306308.
Rundle, Howard, and Patrik Nosil. 2005. Ecological speciation. Ecology Letters 8: 336–352.
Schluter, Dolph. 2000. Ecology of Adaptive Radiation. Oxford: Oxford University Press.