1. Two important consequences of dispersal in metacommunities
2. The four paradigms of metacommunity ecology
4. Application of metacommunity thinking to food webs and ecosystems
5. A critique of metacommunity thinking
Spatial dynamics presents some of the biggest challenges in modern ecology. These occur when the movement of organisms in space affects their populations and consequently affects how they interact with other species. It has long been known that spatial dynamics can be very important in regulating species interactions. For example, Huffaker (1958) found that spatial structure in the form of patchy resources with limited dispersal was important in allowing coexistence of the predatory mite Tylodromus occidentalis with its prey, the six-spotted mite Eote-tranychus sexmaculatus. In a different context, Watt (1947) recognized that a spatial “mosaic” of patches was key in regulating the process of succession in communities because patches at different stages of succession were key sources of colonists during the process as patches underwent successional cycles. Despite this long recognition that spatial effects were important in community ecology, however, a satisfying conceptual, theoretical, and experimental understanding of spatial dynamics is still in development (Tilman and Kareiva, 1997; Hanski, 1999; Chesson et al., 2005).
mass effects. Variation in community composition determined by source-sink relations among patches
metacommunity. A set of local communities connected by the dispersal of at least one component species
neutral dynamic. Variation in community composition determined by stochastic effects of dispersal and demography among species with equivalent niches
patch dynamics. Variation in community composition determined by extinctions of species in patches and colonization among patches
species sorting. Variation in community composition determined by the optimization of fitness among species across patches
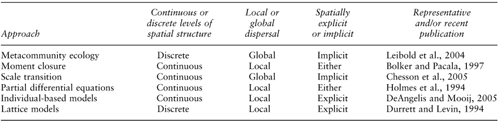
Spatial dynamics is intimately linked with the principle of dispersal. Much of the work has examined passive dispersal in which organisms do not have much control over where they go (cases of dispersal where there is such control are mostly studied in behavioral ecology, where they often involve habitat selection behavior). There are numerous approaches to understanding how dispersal affects community interactions, and some of these are outlined in table 1. These approaches vary (Durrett and Levin, 1994; Bolker and Pacala, 1997) in whether they view space as consisting of discrete patches or a continuous landscape, whether they view dispersal as a local process or a global one, and whether they account for space explicitly (having a “map” of locations) or implicitly (just taking into account that there are distinct areas but not keeping track of where they are) as well as whether they account for the discrete nature of individuals. Generally, the simpler approaches are easier to understand but are more likely to oversimplify the situations than the more complex ones. These approaches also differ in their goals, with some of them focused on accounting for how population density varies in space and time, some focused on understanding coexistence, and some focused on understanding diversity or other questions. Although different approaches often give somewhat different answers, there are many common insights that can result (Durrett and Levin, 1994).
Table 1. Some spatial approaches to community ecology

A useful and simple organizing framework for thinking about the some of the basic elements of these approaches is the metacommunity. A metacommunity is defined as a set of local communities that are linked by dispersal of at least one component species (plate 5). It thus views spatial structure in a simple hierarchical way with local communities existing at a distinct and lower level than the metacommunity itself. The advantage of “metacommunity thinking” is that it captures many of the salient features of spatial ecology in a way that is reasonably accessible for verbal modeling, for guiding our intuition, and for generating more precise theoretical models. And although there are a number of important challenges for future work (some of these discussed below) and limitations, it also serves as a useful way to explore more complex spatial dynamics that does not match the strict hierarchy of spatial organization assumed in the metacommunity concept.
Current work on metacommunity thinking has focused on two effects that dispersal plays in such a simple hierarchy. First, dispersal is key in allowing new species to colonize local communities from which they were previously absent (Hanski, 1999). Thus, in a closed community (no dispersal), changes in community composition are limited to extinction (and possibly sympatric speciation), but this will be very different in communities that can receive colonizing immigrants from other communities. Thus, dispersal within a metacommunity is a key process affecting local community assembly (the process of colonizations and extinctions that determines which species are present in a community such as occurs during succession), and this is one way that the composition of a regional biota can influence local communities. If dispersal among local communities is very slow, the process of community assembly will also be slower and likely to be more stochastic and less predictable than if dispersal is high, and this may have many consequences to patterns of biodiversity and community composition.
The second effect of dispersal among local communities in a metacommunity is to homogenize differences among the local communities. This effect is particularly true when the dispersal rates are sufficient to maintain “sink” populations in some local communities that are supported by immigration from “source” populations in other local communities (these are also sometimes termed mass effects). As dispersal gets higher and higher, any intrinsic local differences in the fitness (per capita production) of local populations will affect these local population densities less and less because they are increasingly overwhelmed by the composition of the migrants. If such homogenization simultaneously affects many species in the metacommunity, then community composition will be homogenized among the local patches. Taken to an extreme, if the dispersal rate is extremely high, such homogenization will mean that the spatial patchiness that might be identified at the lower level (the local community) is actually irrelevant to the organisms involved. Instead, these organisms view such an assemblage of patches as a single patch with properties that are some sort of weighted average of the component patch attributes. At this point, what we call the metacommunity is effectively just a local community as far as the organisms involved are concerned.
These two consequences of dispersal can interact with each other if different species have very different dispersal rates. It is possible, for example, that one set of species will be strongly subjected to the homogenization effect, whereas another is more strongly affected by the dispersal-limited community assembly. Work is only now beginning to understand the consequences of such variability in dispersal.
Metacommunity thinking is still in the early stages of development. Historically, a number of approaches to thinking about metacommunity dynamics have been developed in relative isolation from one another (Leibold et al., 2004; Holyoak et al., 2005). These approaches differ in the assumptions they make about dispersal rates and about the amount of trait environmental heterogeneity (figure 1). Current work is trying to synthesize these paradigms into a common framework, but they still illustrate the dominant views that guide much of the thinking about metacommunities.
Figure 1. A conceptual overview of metacommunity paradigms in relation to the amount of dispersal and the amount of environmental heterogeneity that affect local traits of component species. SS refers to species sorting, ME refers to mass effects, PD refers to patch dynamics, and NM refers to neutral models. The arrows that point from NM indicate that the neutral models can account for any of the time scales involved. One important distinction not shown in this figure is that NM assumes all species have similar dispersal rates, whereas PD does not.
The patch dynamics paradigm is closely related to metapopulation models in population biology (Hanski, 1999) and has mostly focused on patch occupancy (whether a species is present in a patch rather than its density). Much of the work done in this area has not adequately evaluated how environmental heterogeneity among patches affects results. Instead, the focus has been on colonization-extinction dynamics, often under a possible trade-off among species between their colonizing ability and their competitive ability. A unique feature of these models is that extinctions within patches occur for stochastic reasons (either demographic stochasticity in small populations or disturbance/environmental change; Lande, 1993). If colonization events are on time scales that are similar to or slower than these extinctions, patch dynamics can explain some of the variation in community assembly that results. One sometimes confusing issue is that a number of authors have used the same mathematical formalism to address sessile organisms by assuming that patches (which might be better called “microsites”) consist of single individuals (e.g., Tilman, 1994; Hubbell, 2001; Mouquet and Loreau, 2003) so that the death of individuals is equivalent to the extinction rate, and the establishment of an individual is equivalent to the rate of colonization and/or competitive exclusion. These microsite patch dynamic models have in some cases then served to model theories about the patches (containing many such individuals that occur in a metacommunity at a yet higher spatial scale subject to mass effects dispersal; see below). Thus, the full model is really addressing mass effects even though the way individuals are modeled corresponds to the patch dynamics approach.
The species sorting paradigm is perhaps the most intuitively obvious (see Chase and Leibold, 2003). Here, dispersal is seen as the fuel for community assembly, and local interactions determine how this assembly proceeds. Most of the work has focused on the assembly of either competitive assemblages (e.g., Tilman, 1982) or of food webs (e.g., Holt et al., 1994; Leibold, 1996), and much of this work has studied how environmental context (i.e., environmental heterogeneity among patches) alters expected patterns of community structure. Relatively little work in this area has considered how regional communities are regulated by the cumulative effects of these processes. This framework usually ignores any effects of dispersal on local population sizes or their dynamics and is thus more appropriate when dispersal is small relative to demographic rates but still large relative to local population extinction rates. The bulk of equilibrium population ecology theory can easily be related to this approach (especially relevant are mechanistic approaches such as those described by MacArthur, 1972, and Tilman, 1982, reviewed by Chase and Leibold, 2003), but only limited work has examined how regional community structure is regulated under this view (Leibold, 1998; Shurin et al., 2004).
The mass effects paradigm (Shmida and Wilson, 1985) has probably received the most attention even if it is probably the most complicated case. Here, dispersal is sufficient to have consequences on local population persistence, size, and dynamics. Numerous approaches fall into this general framework including much of what is also considered spatial ecology (see Tilman and Kareiva, 1997; table 1). The most important phenomena in this perspective occur when dispersal can allow populations to persist in local communities as sink populations that are supported by immigration from other populations that are source populations for them (Holt, 1985; Pulliam, 1988). Obviously, this may allow more species to coexist in local populations than might be predicted by nichebased models of local community structure (i.e., species sorting), but the results can also be more complex if the mass effects are sufficient to alter the likelihood of persistence of local populations (e.g., Amarasekare and Nisbet, 2001; Amarasekare et al., 2004). Thus, immigration of organisms from elsewhere can overwhelm local populations even if these are otherwise better suited to local conditions and drive down diversity instead.
Figure 2. Species richness as a function of the proportion of dispersal between communities. Black circles, local (α) scales; diamonds, between-community (β) scales; gray circles, regional (γ) scales. amax is the dispersal value at which species diversity is maximal. (From Mouquet and Loreau, 2003)
The neutral dynamics paradigm is the most recently developed one (Bell, 2001; Hubbell, 2001). It is based on the premise that differences among species in their ecological traits are negligible and that stochastic forces of demography and migration among local communities are more important in regulating some aspects of community ecology. This premise is somewhat controversial, and ongoing theoretical and empirical debates are still unresolved (see the special feature on this topic in Ecology, volume 87, issue 6, 2006). At a minimum, however, this approach does two things. First, it can serve as a null hypothesis for conclusions made by the other approaches (Bell, 2001). Second, and perhaps more importantly, it is also important in drawing attention to the stochastic demographic processes that tend to be ignored by other approaches but likely interact with the more deterministic ones described by the other paradigms.
Although these four paradigms have been developed in reasonably independent ways, they can be viewed as a continuum depending on time scales and on the degree of environmental and trait heterogeneity (figure 1). Several studies have explored this continuum. Law and Leibold (2005) explore the relationship between species sorting and patch dynamics in a simple model of nontransitive competition among three species and show that the assumption of stochastic extinctions plays a critical role in regulating metacommunity structure at both the regional and local scales. Shurin et al. (2004) examine how patch dynamics and species sorting interact to affect the likelihood of alternate stable states in competitive metacommunities. They show that local priority effects do not always lead to likely existence of alternate stable states because coexistence among the species at the regional level can be strongly constrained (i.e., species that would produce local alternate stable states at a local scale do not always coexist easily at the metacommunity scale).
More work has been done at the interface of species-sorting and mass effects (e.g., Amarasekare and Nisbet, 2001; Mouquet and Loreau, 2003). The study by Mouquet and Loreau (2003) is illustrative of this continuum and shows how dispersal affects local (a) and regional (y) diversity as well as among-community composition turnover (b diversity) as one goes from a situation that more closely matches species sorting to one that ranges through the various effects that mass effects have on diversity (figure 2). At very low dispersal, each distinct patch is inhabited by a species that is a specialist on that patch type (so that local diversity is minimal and b diversity is maximal). As dispersal increases, there is a point at which local diversity is increasingly enhanced by mass effects (immigrants from other patches survive long enough to maintain increasingly large populations). Thus, a diversity increases, and b diversity decreases. However, at yet higher dispersal rates, some species become extinct in the metacommunity as a whole because their average fitness (over all the patches they inhabit) is less than that of surviving species given the increasing homogenization of the metacommunity. This then means that local diversity also declines. Finally, when the dispersal rate is so high that the metacommunity is effectively one homogeneous patch (from the point of view of the interacting species), there is only one species present in the metacommunity (and in any local patch as well).
Predictions of this model for biodiversity have now begun to be successfully tested in microcosms (Cadotte, 2006a; Matthiesen and Hillebrand, 2006; figure 3), in mesocosms (Forbes and Chase, 2002), and even in some convenient natural systems such as the biota that inhabit pitcher plants (Kneitel and Miller, 2003), but the issue is still somewhat unresolved in broader-scale meta-analyses (Cadotte, 2006b).
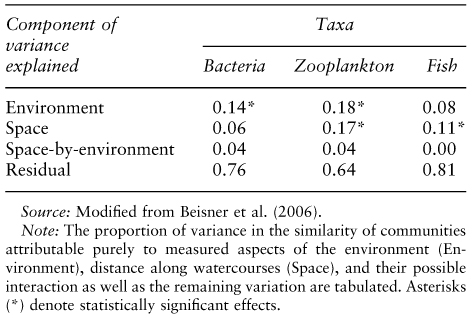
On the empirical side, synthetic studies have also begun to seek ways to identify which of these paradigms is more apparent based on census data from metacommunities. The most common approach has been to recognize that species sorting predicts that differences in composition among local communities reflect environmental differences. And conversely, it predicts that there should be no purely spatial effects (those that are unrelated to how environmental conditions change in space; of course, it is always difficult to establish that all relevant environmental variables are understood, and it is known that environmental variation covaries with space, but some of this can be accounted for in statistical analyses). Alternatively, both mass effects and patch dynamics would predict that there should be some resemblance between communities if they are near each other regardless of whether they resemble each other environmentally. Thus, similarity of community composition can be related to environmental similarity and/or spatial proximity using Mantel tests (Manly, 1986) or similar methods (e.g., Borcard et al., 2004). To date, such studies have almost universally shown that there is substantial similarity in composition that can be explained by similarity of environments (consistent with species sorting), but they also show that there is often some additional degree of similarity related to proximity (see Cottenie, 2005), which could be consistent with any of the other paradigms. There is also often statistical interaction between the two, although these studies have not adequately described this interaction or interpreted it. Beisner et al. (2006; table 2) show that variation in such patterns can be related to the general dispersiveness of different taxa with slowly dispersing taxa showing more effects of proximity and less effect of environment than more rapidly dispersing taxa. This would suggest that, in that study at least, the proximity effects result from patch dynamic processes rather than mass effects. Cottenie et al. (2003) also show that there can be proximity effects in the composition of zooplankton species in ponds that are interconnected by stream flow (table 3), but this effect seems most important when the flow rates in the streams are high (Michels et al., 2001). In this case, the proximity effects would thus be more likely to depend on mass effects rather than patch dynamics.
Figure 3. Effects of dispersal rate on local (α in figure 2) and total (γ in figure 2) diversity as well as on β diversity (i.e., community differentiation, β in figure 2) in experimental protist metacommunities in the experiments of Cadotte (2006). In addition to manipulating dispersal, the initial β diversity was manipulated to either 0 (all initial local communities were identical with 13 species present) or 6 (each local community had 7 of the 13 species present, whereas the entire metacommunity had 13 species present). Points identified with different letters (A-D) in each panel identify points that do not differ significantly from each other.
The ideas described above focus on how metacommunity dynamics (involving any or all the paradigms described above) affect coexistence and diversity of competing species. However, metacommunity thinking is also being used to better understand other aspects of communities involving not just competition but other aspects of community-ecosystems ecology including food-web structure and features of ecosystems.
The implications of metacommunity dynamics for understanding food webs are only beginning to be investigated, but an intriguing set of phenomena have begun to emerge. First, following on the initial work on predator-prey interactions (Huffaker, 1958), spatial structure can stabilize otherwise unstable interactions between predators and their prey in more complex food webs (Holyoak, 2000; Holt, 2002). Second, community assembly of complex food webs that involve cyclical recurrent patterns of community composition are facilitated by dispersal and may produce different patterns of biodiversity at local versus regional scales (Steiner and Leibold, 2004). Finally, there are complex, novel, and sometimes counterintuitive mechanisms of interactions in food webs subject to mass effects in metacommunities (e.g., Holt, 2002; Callaway and Hastings, 2002; Brose et al., 2004). And although it has been suggested that food web architecture may vary consistently with spatial scale (e.g., Brose et al., 2004), it is not yet clear if the links between theory and data are conclusive in helping us to understand these processes.
Table 2. Variance partitioning of community composition for bacteria (fastest dispersal), zooplankton (intermediate dispersal), and fish (slowest dispersal) in lakes
Perhaps more intriguing are the ways that metacommunity thinking is changing the interpretation of ecosystem attributes in ecology. Several recent studies illustrate this. First, Mouquet et al. (2002) show that the effect of biodiversity on emergent aggregate properties of ecosystems (e.g., their productivity or standing crop of plants) depends on how the initial biodiversity was maintained. If many of the species are maintained as relatively poorly locally adapted sink populations, then the effects of changes in diversity on ecosystem attributes may be different than if all the species are self-maintained at the local scale. Additional work by Thebault and Loreau (2006) shows that this may additionally depend on food web dynamics. Second, Leibold et al. (1997) have argued that the scaling of plant and herbivore abundances with productivity results from the ways that different plant species with different defense versus exploitation traits are selected at different levels of productivity and that this occurs only when communities are interconnected by dispersal.
Table 3. Variance partitioning of community composition for zooplankton
Component of variance explained |
Zooplankton |
Environment |
0.20* |
Space |
0.17* |
Space-by-environment |
0.02 |
Residual |
0.62 |
Source: Modified from Cottenie et al. (2003).
Note: The proportion of variance in the similarity of communities attributable purely to measured aspects of the environment (Environment), distance along watercourses (Space), and their possible interaction as well as the remaining variation are tabulated. Asterisks (*) denote statistically significant effects.
The idea that metacommunity dynamics has important implications for ecosystems is even more developed in the concept of “meta-ecosystems” (Loreau et al., 2003), where the movement of materials in space (either passively via diffusion or flow, or actively, via the movement of individuals through dispersal) is also considered (Polis et al., 1997). Work in this area is just beginning.
The above discussions illustrate a rich array of ways that taking into account the dispersal of organisms influences community and ecosystem thinking. Many of these insights are based on the simplest version of the concept of metacommunity and ignore numerous details that might matter a lot. Thus, for example, it may not be enough to consider the simple hierarchy of local-regional community that is implied by the discussion above; it may matter that some local communities are more isolated than others; it may matter that some species disperse more than others; the particular arrangement of patches may matter; and what about spatial dynamics in more continuous (less discrete) situations such as landscapes and gradients? What about organisms that disperse actively and selectively rather than passively? What about organisms that evolve in response to their environments? These and a number of other complications are barely addressed by the simple metacommunity concept outlined above. They indicate that metacommunity thinking can open new ways of thinking about community ecology.
Addressing these issues more satisfyingly, however, will require more sophisticated approaches (Chesson et al., 2005). Some of these are already ongoing as outlined in table 1, but many are not. Work to date shows that many of these issues do modify our expectations to some degree. However, this work also demonstrates that the broad insights provided by simple metacommunity thinking described above can be quite general. Just how they resolve themselves and how important these issues are present an exciting current direction in ecology both on the theoretical and empirical fronts. Overall, these studies show that many aspects of ecology are strongly modified by dispersal so that previous ecological work that is strongly limited to closed communities is likely to be of limited use in understanding larger-scale patterns in biodiversity and other aspects of community ecology.
Amarasekare, P., and R. M. Nisbet. 2001. Spatial heterogeneity, source-sink dynamics, and the local coexistence of competing species. American Naturalist 158: 572–584.
Beisner, B. E., P. R. Peres, E. S. Lindstrom, A. Barnett, and M. L. Longhi. 2006. The role of environmental and spatial processes in structuring lake communities from bacteria to fish. Ecology 87: 2985–2991
Bell, G. 2001. Neutral macroecology. Science 293: 2413–2418.
Bolker, B., and S. W. Pacala. 1997. Using moment equations to understand stochastically driven spatial pattern formation in ecological systems. Theoretical Population Biology 52: 179–197.
Borcard, D., P. Legendre, C. Avois-Jacquet, and H. Tuomisto. 2004. Dissecting the spatial structure of ecological data at multiple scales. Ecology 85: 1826–1832.
Brose, U., A. Ostling, K. Harrison, and N. D. Martinez. 2004. Unified spatial scaling of species and their trophic interactions. Nature 428: 167–171.
Cadotte, M. W. 2006a. Metacommunity influences on community richness at multiple spatial scales: A microcosm experiment. Ecology 87: 1008–1016.
Cadotte, M. W. 2006b. Dispersal and species diversity: A metaanalysis. American Naturalist 167: 913–924.
Callaway, D. S., and A. Hastings. Consumer movement through differentially subsidized habitats creates a spatial food web with unexpected results. Ecology Letters 5: 329332.
Chase, J. M., and M. A. Leibold. 2003. Ecological Niches. Chicago: University of Chicago Press.
Chesson, P., M. J. Donahue, B. A. Melbourne, and A.L.W. Sears. 2005. Scale transition theory for understanding mechanisms in metacommunities. In M. Holyoak, M. A. Leibold, and R. D. Holt, eds., Metacommunities: Spatial Dynamics and Ecological Communities. Chicago: University of Chicago Press, 279–306.
Cottenie, K. 2005. Integrating environmental and spatial processes in ecological community dynamics. Ecology Letters 8: 1175–1182.
Cottenie, K., E. Michels, N. Nuytten, and L. De Meester. 2003. Zooplankton metacommunity structure: Regional vs. local processes in highly interconnected ponds. Ecology 84: 991–1000.
DeAngelis, D. L., and W. M. Mooij. 2005. Individual-based modeling of ecological and evolutionary processes. Annual Review of Ecology and Systematics 36: 147–168.
Durrett, R., and S. A. Levin. 1994. The importance of being discrete and spatial. Theoretical Population Biology 46: 363–395.
Forbes, A. E., and J. N. Chase. The role of habitat connectivity and landscape geometry in experimental zooplankton metacommunities. Oikos 96: 433–440.
Hanski, I. 1999. Metapopulation Ecology. Oxford: Oxford University Press.
Holmes, E. E., M. A. Lewis, J. E. Banks, and R. R. Veitt. 1994. Partial-differential equations in ecology—spatial interactions and population dynamics. Ecology 75: 17–29.
Holt, R. D. 1985. Population dynamics in two-patch environments: Some anomalous consequences of an optimal habitat distribution. Theoretical Population Biology 28: 181–208.
Holt, R. D. 2002. Food webs in space: On the interplay of dynamic instability and spatial processes. Ecological Research 17: 261–273.
Holt, R. D., J. Grover, and D. Tilman. 1994. Simple rules for interspecific dominance in systems with exploitative and apparent competition. American Naturalist 144: 741777.
Holyoak, M. 2000. Habitat subdivision causes changes in food web structure. Ecology Letters 3: 509–515.
Holyoak, M., M. A. Leibold, and R. D. Holt, eds. 2005. Metacommunities: Spatial Dynamics and Ecological Communities. Chicago: University of Chicago Press.
Hoopes, M. F., N. Mouquet, and M. Holyoak. 2004. Mechanisms of coexistence in competitive metacommunities. American Naturalist 164: 310–326.
Hubbell, S. 2001. The Unified Neutral Theory of Biodiversity and Biogeography. Princeton, NJ: Princeton University Press.
Huffaker, C. B. 1958. Experimental studies on predation: Dispersion factors and predator-prey oscillations. Hilgardia 27: 343–383.
Kneitel, J. M., and T. E. Miller. 2003. Dispersal rates affect species composition in metacommunities of Sarracenia purpurea inquilines. American Naturalist 162: 165–171.
Lande, R. 1993. Risks of population extinction from demographic and environmental stochasticity and random catastrophes. American Naturalist 142: 911–927.
Law, R., and M. A. Leibold. 2005. Assembly dynamic in metacommunities. In M. Holyoak, M. A. Leibold, and R. D. Holt, eds. Metacommunities: Spatial Dynamics and Ecological Communities. Chicago: University of Chicago Press, 263–278.
Leibold, M. A. 1996. A graphical model of keystone predators in food webs: Trophic regulation and the abundance, incidence, and diversity patterns in communities. American Naturalist 147: 784–812.
Leibold, M. A. 1998. Similarity and local coexistence of species in regional biotas. Evolutionary Ecology 12: 95–110.
Leibold, M. A., J. M. Chase, J. B. Shurin, and A. L. Downing. 1997. Species turnover and the regulation of trophic structure. Annual Review of Ecology and Systematics. 28: 467–494.
Leibold, M. A., M. Holyoak, N. Mouquet, P. Amarasekare, J. M. Chase, M. F. Hoopes, R. D. Holt, J. B. Shurin, R. Law, D. Tilman, M. Loreau, and A. Gonzalez. 2004. The metacommunity concept: A framework for large scale community ecology? Ecology Letters 7: 601–613.
Loreau, M., N. Mouquet, and R. D. Holt. 2003. Meta-ecosystems: A theoretical framework for a spatial ecosystem ecology. Ecology Letters 6: 673–679.
Manly, B.F.J. 1986. Randomization and regression methods for testing for associations with geographical, environmental and biological distances between populations. Researches in Population Ecology 28: 201–218.
Matthiessen, B., and H. Hillebrand. 2006. Dispersal frequency affects local biomass production by controlling local diversity. Ecology Letters 9: 652–662.
McArthur, R. H. 1972. Geographical Ecology: Patterns in the Distribution of Species. New York: Harper & Row.
Michels, E., K. Cottenie, L. Neys, and L. De Meester. 2001. Zooplankton on the move: First results on the quantification of dispersal of zooplankton in a set of interconnected ponds. Hydrobiologia 442: 117–126.
Mouquet, N., and M. Loreau. 2003. Community patterns in source-sink metacommunities. American Naturalist 162: 544–557.
Mouquet, N., J. L. Moore, and M. Loreau. 2002. Plant species richness and community productivity: Why the mechanism that promotes coexistence matters. Ecology Letters 5: 56–65.
Polis, G. A., W. B. Anderson, and R. D. Holt. 1997. Toward an integration of landscape and food web ecology: The dynamics of spatially subsidized food webs. Annual Review of Ecology and Systematics 28: 289–316.
Pulliam, H. R. 1988. Sources, sinks, and population regulation. American Naturalist 132: 652–661.
Shmida, A., and M. V. Wilson. 1985. Biological determinants of species diversity. Journal of Biogeography 12: 1–20.
Shurin, J. B., P. Amarasekare, J. M. Chase, R. D. Holt, M. F. Hoopes, and M. A. Leibold. 2004. Alternative stable states and regional community structure. Journal of Theoretical Biology 227: 359–368.
Steiner, C. F., and M. A. Leibold. 2004. Cyclic assembly trajectories and scale-dependent productivity-diversity relationships. Ecology 85: 107–113.
The bault, E., and M. Loreau. 2006. The relationship between biodiversity and ecosystem functioning in food webs. Ecological Research 21: 17–25.
Tilman, D. 1982. Resource Competition and Community Structure. Princeton, NJ: Princeton University Press.
Tilman, D. 1994. Competition and biodiversity in spatially structure habitats. Ecology 75: 2–16.
Tilman, D., and P. Kareiva. 1997. Spatial Ecology: The Role of Space in Populations Dynamics and Interspecific Interactions. Princeton, NJ: Princeton University Press.
Watt, A. S. 1947. Pattern and process in the plant community. Journal of Ecology 35: 1–22.