1. Human use and abuse of coral reefs at multiple scales
2. Biogeography, hot spots, and conservation priorities
3. Population dynamics and dispersal
4. Habitat fragmentation in the sea
5. No-take areas, dispersal, and seascape dynamics
Coral reef ecosystems exhibit complex dynamics driven by multiple, interacting processes that operate across a range of scales, from local to global and from days to millions of years. Many reefs have been degraded by human action in recent decades, reducing their capacity to absorb recurrent natural and unnatural disturbances. Rebuilding and sustaining the resilience of coral reefs will depend on interventions that are based on an improved understanding of multiscale processes. The current emphasis on conservation of biodiversity hot spots and on establishing networks of no-take areas does not adequately recognize the functional role of key species groups and the critical seascape connections between protected and unprotected reefs.
biodiversity hot spots. Regions with exceptionally high species richness, often selected as priority targets for the protection of marine ecosystems.
endemics. Species with small geographic ranges.
functional group. A group of species that share a common ecological function, regardless of their taxonomic affinities. An example is the herbivores found on coral reefs, a diverse assemblage that includes many species of fish, sea urchins, and threatened species such as green turtles and dugongs.
pandemics. Species with very large geographic ranges.
planula. The free-swimming larva of corals. Planulae are released directly by brooded corals following internal fertilization. Spawning corals release both eggs and sperm, and fertilization is external.
spatial refuge. A location where a species or local population is less likely to be affected by its predators, competitors, or pathogens or other processes impacting on its survival, growth, and reproduction.
Coral reefs are iconic high-diversity ecosystems that are important for coastal human societies, primarily in developing countries. They support the livelihoods of well over 250 million people, primarily through subsistence fisheries and international tourism. Despite their intrinsic aesthetic, cultural, and social value, many coral reefs worldwide have been degraded, especially in the past 20–30 years, reducing their capacity to regenerate from natural and human disturbances. The primary causes of these declines are coastal runoff resulting from land clearing and increased urbanization, overfishing, and climate change. Through time, the scale of human impacts has grown, with even the most remote reefs being increasingly vulnerable to global warming and ocean acidification. Coral reefs are structured by spatial processes that range in scale from global to local, and their capacity to regenerate following disturbance depends on sources of resilience that operate at multiple scales. However, the scales of management of marine ecosystems are usually mismatched to the scales of important processes and to a growing array of human impacts. Interventions are often fragmented and too small in scale to be effective. An emerging approach to management highlights the importance of key multiscale processes undertaken by critical functional groups of species (including the role of humans) that sustain ecosystem resilience across temporal and spatial scales ranging from global to local.
Many conservation groups and governments focus on the preservation of biodiversity hot spots as a priority. However, there are several new lines of evidence to suggest that regions with low species richness are more vulnerable and are of no less priority for intervention (Hughes et al., 2002). The primary coral reef biodiversity hot spot is located in the central Indo-Pacific, a large triangular region that straddles the equator, centered on the Philippines, Indonesia, Malaysia, and Papua New Guinea. The diversity of corals and other reef-associated species declines northward and southward away from the central Indo-Pacific hot spot as well to the east across the Pacific and westward across the Indian Ocean. Two secondary coral reef hot spots occur in the Red Sea and in the Caribbean. The similarity in regional-scale biodiversity patterns among major groups such as corals, reef fish, molluscs, and crustaceans reflects their shared history and a common set of mechanisms (e.g., barriers to dispersal) that exert a similar influence on many taxonomic groups.
On land, biodiversity hot spots generally contain large numbers of endemic species that are potentially vulnerable to extinction because of their restricted distribution, especially if they are also uncommon or highly specialized. However, the central Indo-Pacific hot spot is largely the result of overlapping pandemic species whose ranges include the hot spot but also extend westward across the Indian Ocean to East Africa, and/or eastward to the Central Pacific. Only 1% of Indo-Pacific corals are endemic to the central Indo-Pacific hot spot. Similarly, only 3% of reef fish have geographic ranges that lie entirely within the hot spot boundaries. Low-diversity peripheral regions (such as Hawaii, the eastern Pacific, and high-latitude subtropical reefs) have proportionately more endemics than the central Indo-Pacific hot spot. The loss of species from low-diversity locations is likely to be more important because such extinctions affect a greater proportion of an already impoverished fauna.
Low-diversity coral reefs (in the Caribbean, the Eastern Pacific, and at many high-latitude locations in the Indo-Pacific) have both fewer functional groups and lower functional redundancy within functional groups; i.e., functional groups there may be absent or represented by just a single species. For example, Caribbean reefs have about 15% of the number of coral species found on reefs throughout most of the tropical Indo-Pacific oceans. Fast-growing bushy corals with high rates of larval recruitment are diverse and abundant throughout most of the Pacific and Indian oceans and in the Red Sea, but this functional group of corals is absent entirely from the modern Caribbean fauna. An example of low functional redundancy is provided by Acropora palmata and A. cervicornis, the only two species of tall three-dimensional branching corals in the Caribbean today. These two species are now increasingly rare because of their failure to regenerate from recent mass mortalities caused by hurricanes, algal blooms, sedimentation and runoff, disease, and climate change. Their decline illustrates the vulnerability of depauperate regions that have little or no functional redundancy to compensate for the loss of one or two critically important species.
Another vulnerability of low-diversity regions is that they tend to have small populations that are mostly self-seeding and genetically isolated from elsewhere. In particular, long-distance dispersal by corals to and from geographically isolated, high-latitude reefs is very limited compared to the much higher levels of connectivity among adjacent parts of the central Indo-Pacific hot spot. Dispersal to isolated reefs or islands cannot be achieved incrementally from one generation to the next through a series of stepping-stones as it is, for example, along the 2000-km length of the Great Barrier Reef. Consequently, the depletion of isolated coral populations (e.g., because of escalating global warming) could have persistent impacts over very long periods because these distant populations cannot be rescued by larval recruitment from elsewhere once the local brood stock is lost. Furthermore, the limited genetic variation that is typically associated with isolated, inbreeding populations means that they are likely to have a reduced capacity to respond rapidly to environmental change. This triple vulnerability—a high proportion of endemics, low diversity within functional groups, and isolation by distance—makes coral reef “cold spots” much more vulnerable than hot spots.
Almost all marine species have a larval phase, and for many reef-associated species, it is the only phase of their life cycle when significant dispersal occurs. At a sufficiently local scale, most larvae come from elsewhere, and the reproductive output of a local population is dispersed. Consequently, local extinctions or depletions caused by human or natural disturbances are often quickly reversed by recruitment of larvae that come from robust populations somewhere else. Conversely, even when survivorship and fecundity are high, populations of site-attached adults such as corals and reef fish will nonetheless become locally extinct if recruitment fails to eventuate from elsewhere. Longer-lived species are buffered against fluctuations in recruitment because their populations persist through periods of low recruitment. In contrast, short-lived species are more vulnerable to recruitment failure because each new cohort of recruits represents a large proportion of the local population. Marine ecologists have traditionally assumed that the long-term supply of larvae, although often highly variable in the short term, is inexhaustible. However, there are a growing number of examples, particularly the collapse of fisheries, where widespread reductions in brood stocks have led to diminished levels of recruitment. Similarly, large-scale variation in the density of coral recruits along the Great Barrier Reef and from year to year is strongly associated with spatial and temporal changes in the fecundity of adults. Therefore, there is a two-way chicken-and-egg link between adults and recruits: more adults mean more recruits are produced; and more recruits lead (with a time lag for growth) to more adults.
The degree of connectivity between local populations varies among marine species, which has important implications for dispersal of larvae, pollutants, disease, and exotic species, for population and community dynamics, and for understanding larger, biogeographic-scale patterns of species distributions. For some species, the larval phase is very short, and local populations are largely self-seeded. More typically, larvae are dispersed varying distances among local populations, which collectively comprise a metapopulation. Two dramatic events on coral reefs have illustrated the importance of connectivity and metapopulation dynamics. One is the recurrent population explosion of the coral-eating crown-of-thorns starfish, Acanthaster planci, in many parts of the Indo-Pacific. On the Great Barrier Reef, for instance, there have been three cycles of outbreaks in the past 50 years, each taking several years to spread via the recruitment of starfish larvae along 10° of latitude, resulting in substantial reduction in coral cover on more than 200 reefs. The other example is the 1983–1984 population crash of the sea urchin, Diadema antillarum, which suffered 98–99% mortality caused by the dispersal of its pathogen from island to island throughout the Caribbean. The die-off caused persistent blooms of seaweed on many overfished reefs, where Diadema was the most dominant herbivore. Following the loss of most of the adult breeding population, recruitment of juvenile Diadema remains suppressed more than three decades later.
The answer to the question “how far do larvae go?” is complex and relates in part to the biology of larval development. Corals can be classified into two reproductive groups, broadcast spawners and brooders, which have markedly different traits that affect their dispersal. Spawners release both eggs and sperm, and fertilization occurs externally. The resulting larvae are capable of settling after 3–7 days, depending on species. In contrast, brooders release much larger, well-developed planulae that are fertilized internally. Planulae are capable of settling quickly, usually within a few hours to a day or two after release. This is somewhat surprising because planulae are much larger than the larvae of spawners and are potentially better provisioned for long-distance dispersal. However, the available evidence suggests that brooders often settle more locally than the larvae of spawners (see below). The offspring of both brooders and spawners can remain viable in the water column for weeks, but their numbers rapidly deplete with time through mortality from predation and starvation. These longer-distance larvae are few in number but are very important for maintaining gene flow, especially near biogeographic boundaries. However, there is no correlation between the breeding mode of corals (brooder versus spawner) and the size of a species’ biogeographic range—the proportion of endemics and pandemics is very similar in both spawners and brooders.
The relationship between larval dispersal and the genetic composition of populations at multiple scales provides a fundamental link between ecology and evolution. Where larvae come from, how far they go, and the genetic consequences of past and present dispersal remain poorly understood for the vast majority of species. Most of the genetic studies of coral reef species have been conducted on the Great Barrier Reef, which encompasses about 2500 distinct reefs, separated from each other usually by a few tens of kilometers, stretching north–south for nearly 2000 km. Some reefs fringe parts of the mainland or inshore islands, but most occur as a broad band on the mid- and outer continental shelf, generally 40–250 km offshore. This stepping-stone physical array is markedly different from many other Pacific Ocean and Indian Ocean reefs that are isolated individually or that occur in clusters comprising remote archipelagos. Fish, echinoderms, mollusks, and other taxonomic groups with long-lived larval stages (typically 4–6 weeks) have low levels of genetic differentiation and high levels of inferred gene flow along the Great Barrier Reef. For most corals, especially brooders, larval recruitment is local, within reefs or among close neighbors. Individual reefs depend primarily on self-seeding for the maintenance of local coral populations. Long-distance dispersal of coral larvae is important over evolutionary time scales for preventing the divergence of species and the accumulation of fixed genetic differences but makes a minimal contribution demographically to the maintenance and regulation of local populations.
The species composition of larval recruits on coral reefs varies at multiple scales. The replenishment of coral populations is most commonly measured using artificial panels that provide a standardized substrate that can be experimentally deployed and retrieved, onto which larvae can attach. At a biogeographic scale, recruitment in the Caribbean is dominated by brooders, whereas spawners are predominant in the tropical Indo-Pacific, reflecting major differences in species composition of adults. Regional-scale patterns also occur along latitudinal gradients to the south and north of the central Indo-Pacific hot spot, along the length of the Great Barrier Reef, and the Ryukyu Island chain. Recruitment of larvae on reefs closest to the hot spot is dominated by spawners, whereas depauperate subtropical locations have lower rates of recruitment, principally by brooders. The species composition of recruits also varies among habitats and depths, mainly because of different larval behaviors, physiological tolerances, and postsettlement mortality, and is a major contributor to patterns of abundance and diversity at small scales.
A common perception is that seascapes are more intact and less subject to fragmentation effects than landscapes, but is that really true? Fragmentation on land continues to subdivide large populations, creating metapopulations, where immigration and emigration now play a more significant role than before. In the sea, connectivity is a natural feature of open populations. Nonetheless, there are growing signs that habitat fragmentation and the loss of reproductive adults (e.g., through overfishing, disease, or climate change) are disrupting stock recruitment relationships, leading to lower rates of larval recruitment or recruitment failure. Coastal mangroves adjoining coral reefs have been reduced to small remnants in many countries, particularly in tropical Asia, to make way for coastal settlements, tourism developments, and prawn farms. Similarly, nearshore coral reefs, seagrass beds, and associated habitats have been degraded to varying extents in different geographic regions, especially because of pollution, climate change, and disease. At least 40% of the world’s coral reefs have been damaged by bleaching caused by thermal stress in the past two to three decades (Wilkinson, 2004). In most of the Caribbean, coral cover has declined by 80% or more since the 1970s (Gardener et al., 2003), with flow-on effects for many reef species that rely on the three-dimensional structure provided by branching corals. Similarly, fish stocks have been depleted by subsistence and commercial fishing almost everywhere.
In most parts of the Caribbean, the number of juvenile corals detected in reef surveys has declined sharply over the past 25 years, providing today only a very small fraction of the number of new colonies needed to maintain population sizes. Thick stands of fleshy seaweed continue to inhibit settlement of coral larvae, and new recruits are often overgrown and killed by the algae. The size of the larval pool is also likely to have decreased sharply because adult corals are fewer in number, smaller, and are often physiologically stressed. Importantly, these different mechanisms of recruitment failure offer contrasting prospects for the future. If the decline in replenishment is caused primarily by competition with seaweed, a reversal of the algal blooms (e.g., through better management of herbivorous fish) would quickly enhance coral recruitment. This would favor fast-growing species of corals that have high rates of larval recruitment, fast growth, and early reproduction. Slower-growing corals that tend to have naturally low rates of recruitment, such as the important reef frame builder Montastrea annularis, will take much longer, a century or more, to recover to pre-1980 levels even if their recruitment resumes. Conversely, if the recruitment failure is also caused by reduced production of larvae, there will be a much longer period of recovery and recolonization by coral recruits, even if the algal blooms were reversed. Weedy species, such as some soft corals, zooanthids, gorgonians, and sponges, are likely to rebound before most corals. For brooding corals with limited dispersal, such as Agaricia, recruitment rates may remain depressed until local breeding stocks can recover. Colonization by other corals that have greater long-distance dispersal may be less affected, with potentially far-reaching consequences for the long-term species composition of Caribbean reefs.
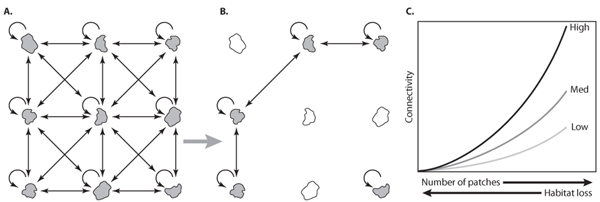
The relative susceptibility to habitat fragmentation of species with different dispersal capabilities is shown graphically in figure 1. Each patch of habitat (e.g., an island in the Caribbean or an individual reef on the Great Barrier Reef) can self-seed, receive, or export larvae. Species with long-distance dispersal should be more resistant to habitat fragmentation because the loss of nearby patches does not preclude dispersal to and from more distant locations. Therefore, habitat loss and fragmentation cause a filtering effect that impacts most on species with limited dispersal (figure 1). On the Great Barrier Reef, approximately 600 reefs out of 2500 have been significantly damaged in the past 45 years by runoff, outbreaks of crown-of-thorns starfish, and two bouts of coral bleaching in 1998 and 2002 (Bellwood et al., 2004). A much higher proportion of reefs have been degraded in other regions, particularly in the Caribbean, the Indian Ocean, and in densely populated parts of Southeast Asia. A major concern is that local degradation could trigger larger-scale collapses, causing the remaining “healthy” reefs to collapse once a critical threshold is reached. Importantly, because systemwide collapse is an emergent property of small-scale dynamics, even the most rigorous management of remnant areas could be too little, too late. The important lesson for management is that small-scale interventions may not be enough to prevent systemwide collapse arising from the accumulating impacts of fragmentation on metapopulation and community dynamics.
Figure 1. A graphic model showing dispersal of larvae among patches of habitat, a key process for maintaining marine populations and ecosystems. Arrows depict potential dispersal pathways among patches and self-seeding within patches (for clarity, longer-distance arrows are omitted). (A) An intact system with high connectivity. (B) A damaged ecosystem, showing reduced larval connectivity caused by habitat fragmentation and loss of brood stock. (C) The nonlinear relationship between habitat loss and the strength of larval connections for species with high, medium, and low dispersal abilities. Species with limited dispersal are more vulnerable to recruitment failure. (Modified with permission from Hughes, Terence P., David R. Bellwood, Carl Folke, Robert S. Steneck, and James Wilson. 2005. New paradigms for supporting the resilience of marine ecosystems. Trends in Ecology and Evolution 20: 380–386)
The history of fisheries is dominated by the steady expansion of fishing effort to deeper and more remote locations and the elimination of spatial refuges that have helped to sustain heavily harvested areas in the past through immigration of larvae or adults. Subsistence and artisanal fisheries on coral reefs target a wide range of species, which differ hugely in their response to fishing, depending in large part on their life histories and reliance on recruitment. Slow-growing, long-lived species such as turtles and sharks can only be harvested sustainably at low intensities and take a long time to recover from overexploitation. For example, the 95% or so loss of dugongs from the southern two-thirds of the Great Barrier Reef in the past few decades will take more than 150 years to reverse, assuming the remnant population can grow at its maximum capacity of 3–4% per annum. In contrast, short-lived species can generally be harvested at high intensities and recover quickly from population crashes, so long as pulses of recruitment continue to maintain the harvested stock. Consequently, multispecies fisheries often cause a predictable change in taxonomic composition (favoring short-lived species) even where there is relatively little targeting of individual species.
No-take areas, where fishing is prohibited, are important tools for reinstating spatial refuges and rebuilding depleted stocks. When fishing is reduced, more adults of harvested species attain a larger size, and their reproductive output increases disproportionately. Some larvae may be retained within the no-take area, but most are likely to be dispersed and may help to restock the fishery outside. Apart from their utility in managing targeted species, no-take areas can also help to restore the structure of food webs and build the resilience of ecosystems. Increasingly, herbivorous fish have become a prime target of many coral reef fisheries, replacing depleted stocks of predatory fishes such as sharks and groupers that now comprise a smaller proportion of the overall catch. Herbivorous fishes, such as parrotfish, surgeonfish, and rabbitfish, play several key roles in the dynamics of tropical reefs: they graze fleshy seaweeds that compete with juvenile and adult corals for space; some erode dead coral skeletons and generate reef sediments, and their position in the food chain means they are an important energetic link between plants and predators. The removal of herbivores, especially on reefs that are also polluted, can lead to abrupt shifts from dominance by corals to persistent blooms of fleshy seaweed. Increasing concern about the combined impacts of fishing, pollution, and climate change on the Great Barrier Reef Marine Park was a major factor in recently setting aside 33% (over 100,000 km2) as permanent no-take areas.
Most no-take areas on coral reefs are very small, often a few square kilometers or even less. Clearly, these are too small to protect highly mobile species such as dugongs, sharks, and turtles that are heavily targeted outside the no-take area. Similarly, the flow of larvae across the boundary of no-take areas is multidirectional—larvae arrive and larvae leave. Proponents of no-take areas often focus on their potential for reseeding adjoining regions. However, in many cases, the replenishment of local populations within protected areas relies on an influx of larvae from the surrounding reef matrix (including the “good” larvae of fishes and corals and the “bad” propagules of algae and diseases). Clearly, the success or failure of a network of no-take areas depends critically on areas outside that are part of the same highly connected reef system. As is the case on land, fragmented seascapes or networks of no-take areas are strongly dependent on the surrounding matrix, which typically dominates the overall dynamics. Consequently, a larger-scale approach to management is urgently required, recognizing the broader seascape as an interacting patchwork of both no-take and non-no-take areas.
Alcala, Angel C., and Garry R. Russ. 2006. No-take marine reserves and reef fisheries management in the Philippines: A new people power revolution. Ambio 35: 245–254. This is a fascinating account of the evolution of governance and management of coastal resources, illustrating the interplay between science and local and national societies.
Bellwood, David R., Terence P. Hughes, Carl Folke, and Magnus Nyström. 2004. Confronting the coral reef crisis. Nature 429: 827–833.
Bertness, Mark D., Steven D. Gaines, and Mark E. Hay, eds. 2001. Marine Community Ecology. Sunderland, MA: SinauerAssociates. This volume is a comprehensive and informative textbook in three parts: (1) Processes influencing patterns in marine communities; (2) an overview of the ecology of eight community types, including coral reefs; and (3) a section on conservation and management.
Birkeland, Charles, ed. 1996. Life and Death of Coral Reefs. London: Chapman & Hall. This edited volume provides a very comprehensive overview of the geology and history of coral reefs, their evolution and ecology, biogeography, and human impacts on them.
Gardner, Toby A., Isabelle M. Côté, Jennifer A. Gill, Alastair Grant, and Andrew R. Watkinson. 2003. Long-term region-wide declines in Caribbean corals. Science 301: 958–960.
Hughes, Terence P., Andrew H. Baird, David R. Bellwood, Margaret Card, Sean R. Connolly, Carl Folke, Richard Grosberg, Ove Hoegh-Guldberg, Jeremy B. C. Jackson, Joanie Kleypas, Janice M. Lough, Paul Marshall, Magnus Nyström, Steven R. Palumbi, John M. Pandolfi, Brian Rosen, and Joan Roughgarden. 2003. Climate change, human impacts, and the resilience of coral reefs. Science 301: 929–933.
Hughes, Terence P., David R. Bellwood, and Sean R. Connolly. 2002. Biodiversity hotspots, centers of endemicity, and the conservation of coral reefs. Ecology Letters 5.775–784.
Jones, Geoff P., Maya Srinivasan, and Glenn R. Almany. 2007. Population connectivity and conservation of marine biodiversity. Oceanography 20: 43–53. This article focuses on how knowledge of connectivity can help to improve strategies for conserving marine biodiversity.
Karlson, Ronald. 1999. Dynamics of Coral Communities. Dordrecht: Kluwer. This book focuses on the theory and field evidence for various processes that influence the dynamics of coral communities at multiple scales, including ecological succession, interspecific competition, predator-prey interactions, disturbance, assembly “rules,” and regional enrichment of coral communities at biogeographical scales.
Pandolfi, John M., Roger H. Bradbury, Enric Sala, Terence P. Hughes, Karen A. Bjorndal, Richard G. Cooke, Deborah McArdle, Loren McClenachan, Marah J. H. Newman, Gustavo Paredes, Robert R. Warner, and Jeremy B. C. Jackson. 2003. Global trajectories of the long-term decline of coral reef ecosystems. Science 301: 955–958.
Sobel, Jack, and Craig Dahlgren, eds. 2004. Marine Reserves: A Guide to Science, Design and Use. Washington, DC: Island Press. This book provides a useful overview of marine reserves, focusing mainly on potential fisheries outcomes. It includes detailed case studies from California, the Bahamas, and Belize as well as a brief global overview featuring New Zealand, the Philippines, the Mediterranean, Chile, and Australia.
Wilkinson, Clive R., ed. 2004. Status of the Coral Reefs of the World: 2004. Townsville, QLD: Global Coral Reef Monitoring Network and Australian Institute of Marine Science.