EIGHT
Form and Function in the Feeding of Fishes
CONTENTS
Evolutionary Trends in Trophic Morphology
THE PREVIOUS CHAPTER FOCUSED on the overall body shape of fishes, functional aspects of swimming or position holding, and the ways in which body form and function are related to habitat. This chapter looks at some basic elements of the head skeleton of North American freshwater fishes and how bone and muscle shapes and positions relate to modes of feeding. Ray-finned fishes, the Actinopterygii (see Figure 7.5), display tremendous diversity in structural and behavioral aspects of prey capture—a not surprising finding given the great diversity of the group. In fact, diversity of trophic morphology (i.e., teeth, jaws, suspensorium, etc.) is a hallmark of actinopterygian radiation. What fishes eat is most closely (morphologically) related to their dentition, but jaw construction and jaw movements strongly influence how they eat (Gosline 1987). Although the size of prey consumed by fishes is generally related to body size, with the general exception of suspension feeders, the prey size–body size relationship is affected by morphological and energetic constraints (the cost-to-benefit ratio of a prey item). Gape limitation generally refers to prey dimensions relative to the size of the oral jaw opening, and predators that are constrained in the size of prey they consume by the size of their mouths are said to be gape limited. However, the opening of the oral jaws is only the first of three “filters” encountered by prey on their way into the esophagus of a predator. For instance, in Largemouth Bass (Micropterus salmoides) the oral jaw opening is larger than the passage through the pharyngeal jaws at the back of the mouth. (In a 200 mm SL fish the pharyngeal gape is only 55% of the oral gape.) Prey size can be further limited by the throat diameter, which is constrained by the cleithral bones of the pectoral girdle. To some extent the size limitations of the pharyngeal gape and throat gape are offset by flexibility and deformability of the prey and/or by crushing or tearing actions of the pharyngeal teeth (Wainwright and Richard 1995). The study of functional morphology of fishes, both in terms of locomotion (Chapter 7) and feeding, has benefitted extensively from technological developments (Box 8.1).
BOX 8.1 • Technology and Functional Morphology
Studies of functional morphology in fishes have benefitted greatly from ongoing developments in technology. Historically, morphological functions were inferred from examination of prepared skeletons, by detailed studies of serial transverse sections of the skull or other elements, by manipulating structures in cleared and stained or freshly killed specimens, and by still photographs of feeding or other behaviors (Alexander 1967b; Anker 1974). These approaches can still be valuable and continue to be used where appropriate.
However, functional morphologists have been quick to take advantage of new developments, including X-ray and high-speed film and video, such as Nyberg’s (1971) study of Largemouth Bass feeding. Being able to relate muscle firing using electromyography in conjunction with high speed videography has been a major breakthrough in the understanding of form and function (Sibbing 1991b; Ferry-Graham and Lauder 2001). Another major advance was the use of fluid-filled pressure transducers, first introduced by Alexander (1969) to analyze pressure gradients in the buccal and opercular cavities of feeding fishes; later advances resulted in reducing the size and increasing the sensitivity of pressure transducers (Lauder 1983d). The ability to see food particle movement within the mouth cavity of feeding fishes was made possible by using fiberoptic endoscopes, which can be used in conjunction with miniaturized thermistor flow meters to simultaneously measure flow velocities (Sanderson et al. 1991). Water flow around the heads of freely swimming feeding fishes, or along the body and fins of freely swimming fish, can now be accurately described using digital particle image velocimetry (DPIV) (Lauder and Drucker 2000; Ferry-Graham and Lauder 2001). DPIV provides an instantaneous measure of water velocity by measuring movements of microparticles placed in the water and illuminated by a laser beam (Ferry-Graham et al. 2003; Day et al. 2005). The ability to integrate extensive digital data on pressure, water velocity, muscle firing, and so on has, of course, been made possible by the accessibility of powerful personal computers.
EVOLUTIONARY TRENDS IN TROPHIC MORPHOLOGY
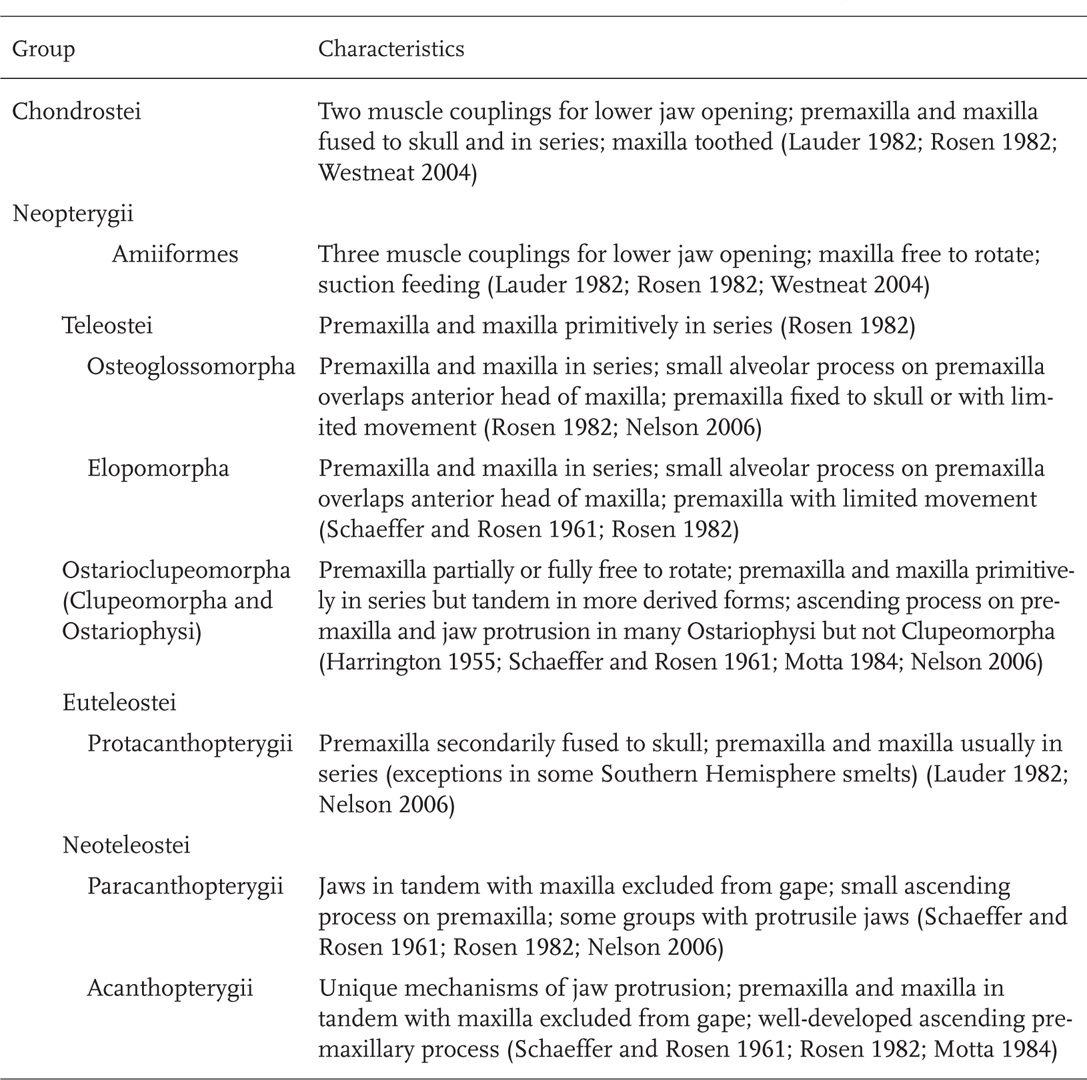
The structures involved in the feeding of fishes (upper and lower jaws, jaw suspension, mouth and throat cavities, and associated musculature) show a progression in complexity and degrees of freedom of movement over evolutionary time (Schaeffer and Rosen 1961; Lauder 1982). These changes are associated with greatly expanded modes of foraging, especially as shown by teleosts. Bony fishes have a highly kinetic skull containing some 60 skeletal units that are controlled by approximately 80 groups of muscles (Winter-bottom 1974; Sanford and Wainwright 2002). Tracing the patterns of evolution in jaw function is challenging because of the tremendous diversity of ray-finned fishes, and because more derived groups in each major lineage frequently differ in jaw function and morphology from more ancestral groups in the same lineage and may independently converge on the same or similar patterns. Ancestral patterns for each group are presented first in Table 8.1, followed by patterns in more derived forms. In early Paleozoic ray-finned fishes (subclass Chondrostei), the bones of the upper jaws (the premaxilla and maxilla) were fused to other dermal bones of the skull and only the lower jaws (mandibles) moved (Figure 8.1A, B; Table 8.1). The bones of the cheek and throat (opercles, preopercles, subopercles, and branchiostegal rays) also had limited mobility so that lateral expansion of the buccal cavity was minimal (Schaeffer and Rosen 1961; Lauder 1982). Mouth opening was achieved by two pathways: (1) elevation of the head caused by contraction of the dorsal (epaxial) muscles—the epaxial-neurocranial pathway, and (2) depression of the lower jaw from contraction of throat musculature (sternohyoideus muscle) and contraction of hypaxial musculature connecting to the lower jaw via the pectoral girdle to the ventro-medial hyoid series (the hypaxial musculoskeletal pathway) (Figure 8.1B). Although most fossils of early ray-finned fishes occur in marine habitats, several genera are recorded from fresh water, including Limnomis and Cuneognathus (Friedman and Blom 2006). Limnomis, one of the earliest of North American bony fishes (Figure 8.1A), is known from the Late Devonian Catskill Formation in Pennsylvania. It apparently lived in shallow, low-velocity habitats, such as floodplains and oxbow lakes, where it fed on soft-bodied invertebrates. It is considered a pioneer in the exploitation of North American freshwater ecosystems (Daeschler 2000).
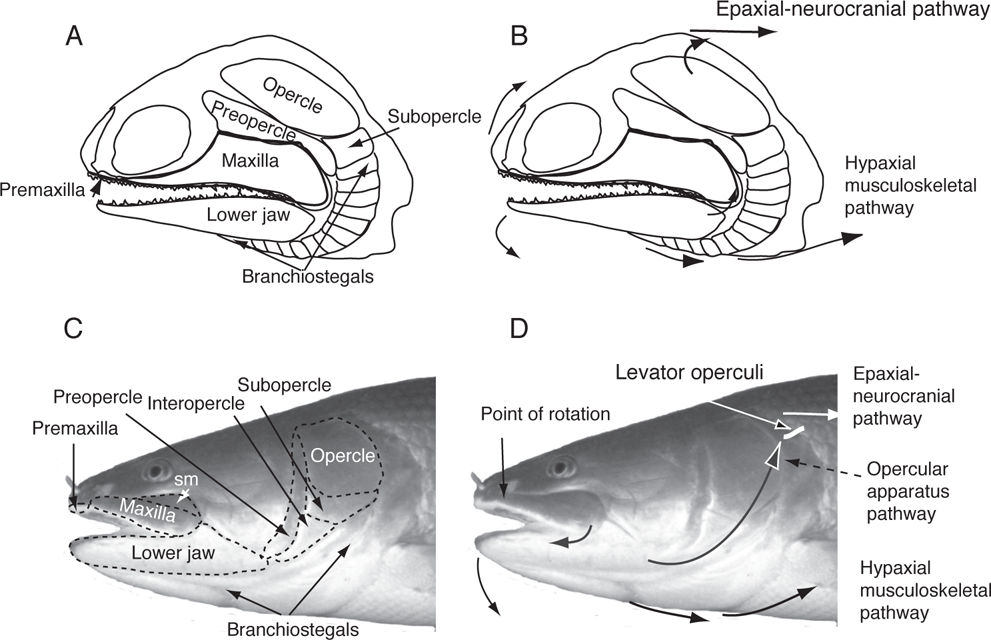
FIGURE 8.1. Trends in the evolution of jaw mobility in preteleostean, ray-finned fishes (Actinopterygii).
A. Jaw elements of early ray-finned fishes, illustrated by the late Devonian chondrostean, Limnomis. Drawing based on Daeschler (2000).
B. Muscle forces and rotational movements involved in the two pathways of jaw opening in Limnomis.
C. Jaw elements of Bowfin (Amia calva), an early neopterygian; dotted lines highlight outlines of some of the bones; sm = supramaxilla.
D. Muscle forces and rotational movements involved in the three pathways of jaw opening in Bowfin. Rotation of the maxilla was the first level of upper jaw mobility. Muscle forces and rotational elements are based on Lauder (1980, 1982).
The jaw mobility of living species of the subclass Chondrostei (Sturgeons and Paddlefishes, order Acipenseriformes) differs greatly from Paleozoic and early Mesozoic forms. With the exception of the highly specialized Paddlefish (Polyodon spathula), all sturgeons and the primitive Chinese Paddlefish (Psephurus gladius) have the upper jaw free from the braincase (neurocranium) (Figure 8.2). The resultant mobility allows jaw projection and is related to active foraging on invertebrates and fishes (Bemis et al. 1997; Findeis 1997). In addition, the role of head lift in opening the mouth, important in ancestral Chondrostei (Figure 8.1A,B), is lost in modern acipenseriform fishes. Instead, modern acipenseriforms are unique in having an enlarged mandibular arch muscle involved with jaw opening and projection (Bemis et al. 1997).
TABLE 8.1 Trends in Jaw Form and Function in Ray-Finned Fishes, the Actinopterygii
At the early neopterygian level (see Chapter 7; Figure 7.5), represented by Bowfin (Amia calva), the upper jaw shows the first level of mobility (with the exception of the more derived chondrosteans mentioned previously). The maxilla is detached posteriorly from the cheek bones and has a pivot point anteriorly where it abuts the premaxilla so that the posterior part of the bone swings forward during jaw opening and acts to prevent fluid inflow (therefore, loss of suction) through the sides of the mouth (Figure 8.1C) (Lauder 1980, 1982; Rosen 1982). The epaxialneurocranial pathway is retained for elevation of the head. In addition, opening of the lower jaw is accomplished through two independent biomechanical pathways (Figure 8.1D). The hypaxial musculoskeletal pathway is essentially the same as shown in early actinopterygians through the hypaxial muscle contraction via the pectoral girdle, sternohyoideus, and hyoid bones. A newer opercular apparatus pathway involves a muscle (levator operculi) causing dorsal rotation of the opercular bones (opercle, subopercle, and interopercle) via a ligament connecting the interopercle to the posterodorsal edge of the lower jaw (Figure 8.1D). Two independent pathways for depression of the lower jaw allow for much greater flexibility in the timing of depression of jaw elements and in fluid movement through the oral cavity; these two pathways are retained in most all Neopterygii (Lauder 1982).
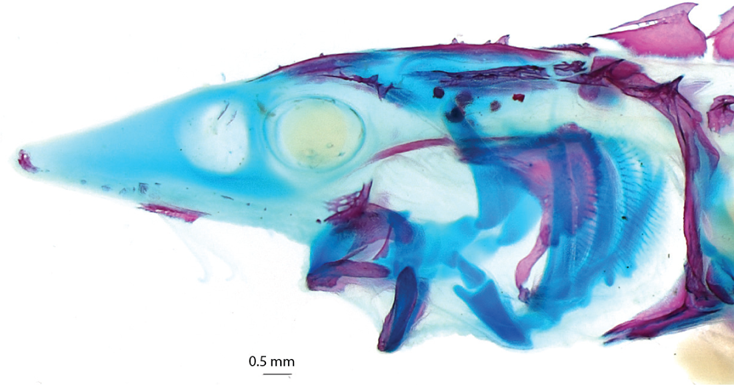
FIGURE 8.2. Elements of jaw protrusion in derived chondrosteans, the Acipenseriformes, showing the freedom of the upper jaw from the braincase. Illustrated by a 21-day posthatch White Sturgeon (Acipenser transmontanus). Specimen preparation and photograph courtesy of Katie May Laumann.
Teleosts (Table 8.1; Chapter 7; Figure 7.5) show the next stage of jaw kineticism with the development of a posterior process on the premaxilla, the alveolar process (Figure 8.3B). Although present, this process remains weakly developed in the most basal teleostean lineages such as the Bony Tongues (Hiodontidae) (Figure 8.3A). The alveolar process overlaps with the anterior arm of the maxilla, and the maxilla and premaxilla swing forward as a unit when the lower jaw is opened. The premaxilla is hinged anteriorly with the ethmoid region of the skull (Rosen 1982). Exceptions occur in generalized predators where the force of the strike on the premaxilla and powerful closing of the lower jaw dictate little or no mobility of the premaxillary bone (Schaeffer and Rosen 1961; Lauder 1982; Westneat 2004). In the Osteoglossomorpha, represented in North American freshwater by Mooneye and Goldeye (genus Hiodon), the ancestral condition is largely maintained, with bones of the upper jaw in series and with limited premaxillary movement (Figure 8.3A). The Elopomorpha, represented in freshwater by American Eel (Anguilla rostrata), show a similar pattern (Table 8.1). Basal members of the Ostarioclupeomorpha lineage, which include herrings and shads (Clupeomorpha) and minnows, suckers, and catfishes (Ostariophysi), maintain the ancestral teleostean plan, as do more derived clupeomorphs such as Threadfin Shad (Dorosoma petenense) (Figure 8.3B). However, several groups of ostariophysans, including Cypriniformes (minnows and suckers), have developed highly protrusile jaws, have the premaxilla and maxilla in tandem, and have the maxilla excluded from the gape (Motta 1984; Table 8.1; Figure 8.3C). Trouts, salmons, pikes, and pickerels (Protacanthopterygii) retain the basic teleostean pattern of a premaxilla with limited movement, maxillary rotation during jaw opening, and the premaxilla and maxilla in series, although fusion of the premaxilla to the skull is considered a secondary change (Lauder 1982; Table 8.1). The two lineages of advanced teleosts, the Paracanthopterygii and the Acanthopterygii, both have the premaxilla and maxilla in tandem and the maxilla excluded from the gape (Figure 8.3D). The Paracanthopterygii, represented in North American freshwater by Trout-Perches (Percopsidae), Pirate Perches (Aphredoderidae), Cavefishes (Amblyopsidae), and Burbot (Gadidae), only have a small ascending process on the premaxillae (Figure 8.3E), and only some groups have protrusile jaws (Table 8.1). Acanthopterygians, which are represented by close to 15,000 species worldwide, have a toothless maxilla that is excluded from the gape, a premaxilla with well-developed ascending and alveolar processes, highly protrusile jaws, and show a variety of different mechanisms for jaw protrusion (Table 8.1; Figure 8.3E) (Lauder 1982).
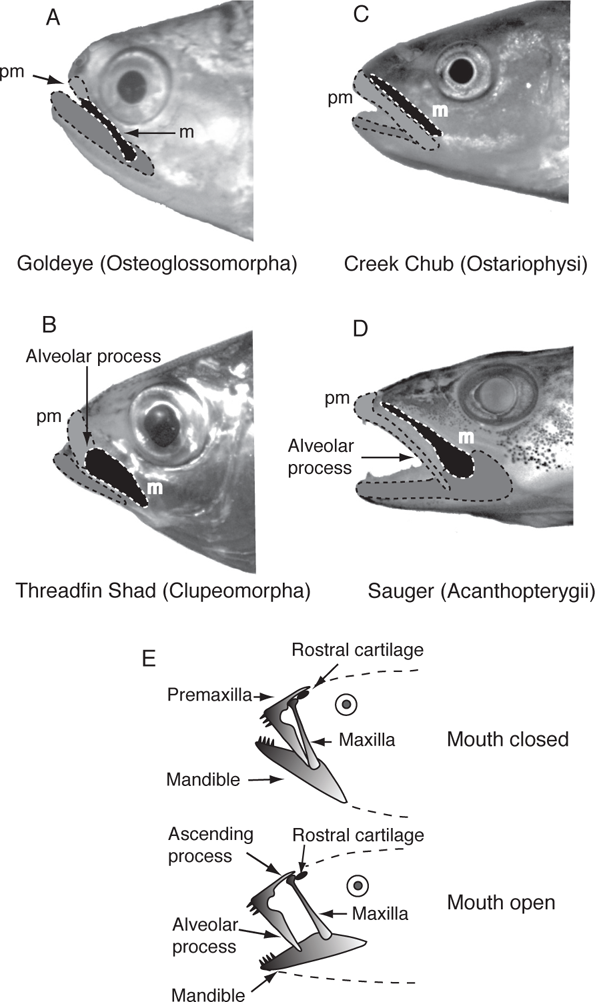
FIGURE 8.3. Trends in mobility of upper jaw elements in teleosts.
A. Goldeye (Hiodon alosoides), a lower teleost.
B. Threadfin Shad (Dorosoma petenense), a lower teleost.
C. Creek Chub (Semotilus atromaculatus), a lower teleost.
D. Sauger (Sander canadensis), an advanced teleost. Names in parentheses indicate higherlevel groups referred to in the text; pm = premaxillary bone; m = maxillary bone.
E. A schematic diagram of upper jaw bones of an advanced percomorph showing the configuration of jaw bones during mouth opening. Photo of Goldeye by J. Baker; the drawing is based on Alexander (1967b) and Ferry-Graham et al. (2008).
Jaw Protrusion
Approximately 50% of bony fishes (the Teleostei) have protrusile jaws, meaning that components of the upper and sometimes lower jaws move forward during the feeding strike, and the evolution of jaw protrusion is associated with the wide radiation of teleostean feeding mechanisms (Motta 1984; Gosline 1987; Holzman et al. 2008a). Jaw protrusion also occurs in some living chondrosteans (sturgeons and Chinese Paddlefish). Mechanisms of jaw protrusion have arisen independently in different groups of fishes and may incorporate various approaches (Table 8.1) (Motta 1984; Ferry-Graham et al. 2008; Holzman et al. 2008a).
An initial development in the evolution of protrusile jaws was the freeing of the rostral cartilage from the premaxilla and the presence of a moderate ascending process on the premaxilla; development of a long premaxillary ascending process was a later adaptation. In teleosts with a long ascending process of the premaxilla (Figure 8.3E), jaws are protruded as the ascending process slides along the rostral cartilage cushion at the front of the skull; however, a long ascending process does not always indicate extreme jaw protrusion—some fishes (primarily marine groups such as wrasses) with a long ascending process have only limited protrusion and instead the ascending process is involved in stabilizing the jaw (Gosline 1987). As mobility increased, the rostral cartilage became free from the rostrum so that it could also move forward. Jaws with a short ascending process on the premaxilla tend to rotate about the front of the skull as the mouth is opened and have limited protrusion. Protrusion can be independent of opening and closing of the mouth, at least to a certain degree.
Even though they evolved independently, jaw protrusion in the ostariophysans and in the percomorphs have many similarities. In both groups, the upper jaw, formed from the left and right premaxillary bones, is projected forward and generally upward during feeding as the ascending processes of the premaxillae slide along the rostral cartilage and the ventral ends of the alveolar processes lap over the lower jaw and close off the sides of the gape, creating a more rounded mouth opening (Figure 8.3E) (Alexander 1966, 1967b; Ferry-Graham et al. 2008). A rounded mouth is advantageous in terms of sucking in prey, although the reduced mouth opening limits the size of prey that can be consumed (Carroll et al. 2004). In contrast, the killifishes and their allies (Cyprinodontiformes; Atherinomorpha) have the alveolar process of the premaxilla tightly connected by strong ligaments to the lower jaw so that it cannot swing forward during mouth opening and occlude the jaw opening (Ferry-Graham et al. 2008). Because of this connection, the mouth tends to move forward and downward during protrusion. Some occlusion along the sides of the gape can occur by the lip membrane, but in most cases the open jaw has more of a “beak-like” rather than a rounded appearance.
Jaw protrusion in North American freshwater fishes seems to occur by at least four primary means (Table 8.2): (1) protrusion caused by depression of the lower jaw through various ligamentous connections, with the maxilla often serving as a supporting strut (Type A); (2) protrusion directly as a result of maxillary twisting (Type B); (3) a combination of mandibular depression and maxillary twisting (Types A + B); and (4) protrusion primarily by neurocranial elevation (Type C) (Alexander 1967a, b, c; Gosline 1981; Motta 1984; Hernandez et al. 2008). The most widespread mechanism, represented by five orders and seven families, is protrusion of the premaxillae caused by mandibular depression (Table 8.2). The number of taxa achieving protrusion of the premaxillae via twisting of the maxillae, although once considered to be high (Alexander 1967b), has apparently been overestimated (Motta 1984). Among families represented in North American fresh water, this mechanism is only documented in mullet (Mugilidae) (Table 8.2). Basal members of the order Cyprinodontiformes, such as the genera Kryptolebias (formerly the genus Rivulus) and Fundulus, employ both maxillary twisting and mandibular depression (Hernandez et al. 2008, 2009). Finally, jaw protrusion that involves the tilting back of the head (neurocranial elevation) is perhaps more common than previously thought (Motta 1984). Examples occur among advanced percomorphs such as the Centrarchidae and African cichlids (jaw protrusion in New World cichlids has apparently not been studied) (Table 8.2). In addition, secondary loss of jaw protrusion has occurred repeatedly through loss or reduction of premaxillary movement. Examples of North American genera that show loss of jaw protrusion include the darter genus Percina and the cavefish genus Amblyopsis (Gosline 1981, 1987).
Advantages of Jaw Protrusion
Jaw protrusion potentially provides a variety of advantages, as suggested by Alexander (1967b), Lauder and Liem (1981), and others. Several of the suggested advantages of jaw protrusion are explored further in the following section titled “Modes of Prey Capture.” One of the most commonly cited advantages is that the jaws accelerate ahead of the body as a fish moves toward a potential prey. For instance, in Largemouth Bass jaw protrusion equals about 10% of head length, and a 10-cm Largemouth Bass gains 27 cms−1, equivalent to 87% of average attack velocity, by protruding the mouth; a 25-cm Largemouth Bass has a higher protrusion velocity of 39 cms−1, but because of the overall faster approach speed related to larger body size, this is equivalent to 50% of average attack velocity (Nyberg 1971). Other things being equal, the benefit of protrusion for a gain in approach velocity is greater in small versus large fishes.
A second suggestion involves increased hydrodynamic efficiency for suction feeding obtained by having a rounder mouth opening. Protrusion may also help suction efficiency by further expanding the buccal cavity. However, a round mouth opening is not limited to fishes with protrusible jaws because many primitive teleosts and even a preteleost such as Bowfin, all of which lack jaw protrusion, also have rather circular mouth openings as a result of the forward rotational movements of the lower part of the maxillae (Lauder 1979, 1980).
TABLE 8.2 Basic Mechanisms of Jaw Protrusion in North American Freshwater Fishes
Families are listed if protrusion mechanism has been described in any member genus
A third suggested advantage is that of increasing the volume of the mouth and pharyngeal cavities—most fishes with protrusible jaws can keep the premaxillae protruded while the mouth is partially closed. The advantage results from the greater speed of closing the mouth and the greater volume of water that can be sucked into the mouth without being blown out again (and thus potentially expelling the prey) as the mouth closes (Alexander 1967b)
A fourth suggested advantage of jaw protrusion is that fishes feeding on benthic prey can remain closer to horizontal when feeding. When the mouth of a typical percomorph is closed, the lower jaw slopes upward from the point of articulation (Figure 8.3E). If the upper jaw is not protruded, the open mouth will point somewhat upwards, so that the fish would have to approach prey at a steeper angle. In addition to providing a better body orientation for predator avoidance, jaw protrusion could also increase the ability to grasp prey (Alexander 1967b).
MODES OF PREY CAPTURE
There are two major stages in the acquisition of food by fishes, namely prey capture (often involving the jaws) and prey processing (often involving the teeth in the back of the mouth—the pharyngeal teeth) (Liem 1980a; Wainwright and Bellwood 2002). Fishes employ a range of jaw and throat musculature and bones to capture prey, and in many cases a single species might use several different modes depending on the size and location of a prey item. The three primary approaches of prey capture are manipulation, ram, and suction (Liem 1980a, b; Wainwright et al. 2007). Prey manipulation involves a diversity of jaw movements designed to clip off parts of prey; rasp tissue from prey (as in lamprey feeding); bite prey with strong jaws; scrape material from the substratum; or grip prey, or parts thereof, and pull them from hiding. Ram feeding involves forward velocity to overtake mobile prey and bring them into the jaws. Suction feeding is based on the creation of a negative pressure gradient so that a discrete water mass containing a prey item is pulled into the mouth (Liem 1980a, b; Wainwright et al. 2007). Often these approaches are used in concert, such as manipulation and suction, or ram and suction.
Suction feeding, which likely originated with jawed vertebrates, represents the primitive yet dominant feeding mode in bony fishes and is used by all bony fishes at some stage in their lives (Liem 1980a; Lauder and Shaffer 1993; Wainwright et al. 2007). Suction is maximized by a relatively small mouth opening (primarily as a result of lateral occlusion of the gape by the ventral arms of the premaxillae and maxillae), well-developed hyoid muscles to rapidly lower the floor of the mouth, and development of other cranial elements to quickly expand the mouth and throat cavities so that a rapid pressure gradient develops (Wainwright and Day 2007; Hernandez et al. 2008). In contrast, effective ram feeding occurs in fishes with large mouths and strong adductor muscles to forcibly close the jaws, and also strong bones in the jaws and head to effectively deal with the stresses of jaw closing (Hernandez et al. 2008). Manipulation, especially exemplified in various cichlid species, involves mobile jaws, modified tooth shapes, and changes in development of jaw musculature.
Although it is convenient to categorize fishes into various feeding modes, doing so obscures the often high level of phenotypic or genetic variability in feeding modes within a single taxon. Some species show developmental plasticity in feeding behaviors and feeding morphology in response to the type of food or its location. For instance, Western Mosquitofish (Gambusia affinis) that fed on attached or free prey developed differences in head shape. Those raised on attached food developed shorter, wider heads; anteriorly shifted eyes; lower snout positions; and a sloping caudal peduncle compared to fish raised on free prey. Differences were largely due to developmental plasticity rather than differential mortality. Free prey were attacked using suction whereas attached prey were obtained by biting and scraping (Ruehl and DeWitt 2005).
Suction Feeding
Suction feeding normally involves four phases: preparation, expansion, compression, and recovery (Gibb and Ferry-Graham 2005). During preparation, the buccal cavity is compressed, followed by a rapid expansion, which brings water and prey into the mouth with little or no mastication, and prey are swallowed by action of the pharyngeal jaws (modified gill-arch elements) (Wainwright 2006). Expansion is followed by a slow compression phase where water is expelled through the gill openings (and rarely through the mouth), and a recovery phase where jaw elements return to a relaxed, prefeeding condition. Suction feeding has been studied in various groups, including the Cyprinidae (Alexander 1969), but especially in the Centrarchidae.
There are functional trade-offs in suction feeding between fishes that have relatively slow ram speeds, such as Sunfishes, and those that have high ram speeds such as trout and salmon, Black Basses, and Bowfin. Although both groups employ suction and ram feeding, in the latter group suction is considerably reduced relative to ram (Carroll et al. 2004). Fishes with slow attack speeds should have larger pressure differentials between buccal and opercular cavities and greater forces of suction compared to fishes with generally higher attack speeds (Lauder 1986). This has been supported by recent studies comparing Bluegill (Lepomis macrochirus) (low attack speed) and Largemouth Bass (high attack speed) that show higher fluid speeds toward the mouth and greater pressure gradients in Bluegill (Higham et al. 2006a, b).
In Bluegill (size 14–18 cm SL) feeding on earthworms or tethered ghost shrimp, the initial strike takes place close to the prey at 0.6–2.3 cm or 0.04–0.15 body lengths (Gillis and Lauder 1995; Holzman et al. 2008b). The actual strike may involve a ram component (forward velocity), jaw protrusion, cranial elevation, and suction (Ferry-Graham et al. 2003). Fluid speeds vary with the rapidity of mouth opening, which is faster for mobile prey, and average approximately 30 cms−1 with maximum values of over 250 cms−1 measured at one-half of the peak gape size in front of the mouth (Day et al. 2005; Higham et al. 2005, 2006b). Absolute fluid speeds, and thus overall ability of suction feeding, also increase with body size, although induced flows scale with measures of body size so there is no overall change in how buccal expansion translates to water motion (Holzman et al. 2008c). Because larger fish also have higher swimming speeds, the combined effect of higher speed and suction results in increased closing speed. The mushroom-shaped suction plume is highly localized, with the highest suction velocity at the mouth opening and declining to only 5% of the maximum suction velocity at a distance of only one mouth diameter away (Day et al. 2005).
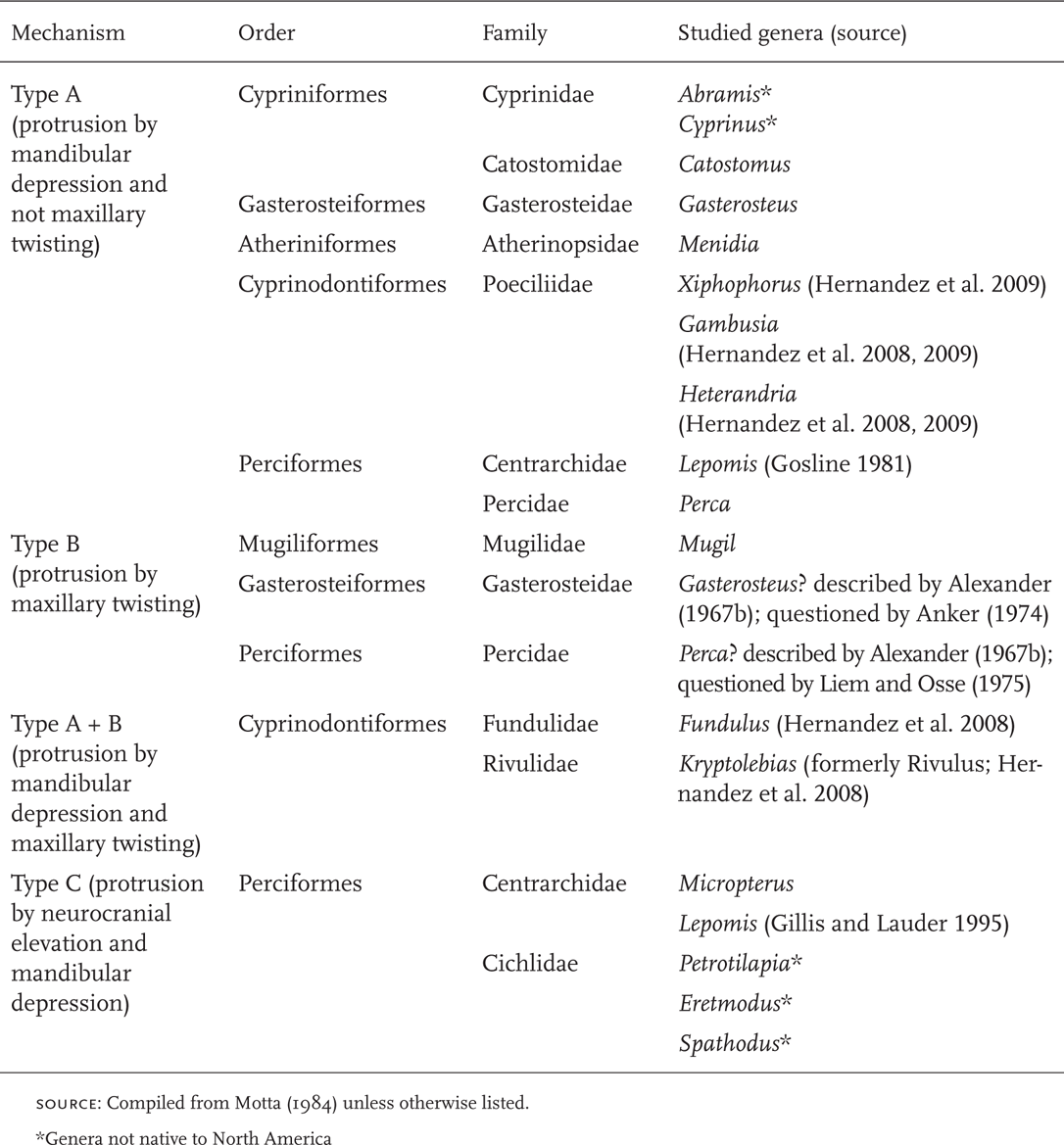
Bluegill often combine ram and suction feeding. As ram speeds increase, the water being drawn into the mouth becomes more focused in front of the mouth (drawn in from all directions in stationary fish) and the shape of the ingested water volume becomes more elongate (Figure 8.4A, B) (Higham et al. 2005). From the perspective of the prey, the combination of suction and ram results in a faster closing time between predator and prey because the water mass containing the prey is moving toward the fish at the same time the fish is moving toward the prey. As long as the ratio of ram speed to fluid speed caused by suction stays within 0–20%, there is apparently no trade-off between ram speed and fluid speed. Bluegills usually decelerate during prey capture, perhaps to reduce ram speeds to ≤ 20% of fluid speed so that no hydrodynamic trade-offs occur, and also to allow finer control of movements, which is required because of the increased degree of focusing at moderate ram speeds compared to no ram speed (Higham et al. 2005).
Successful suction feeding requires timing the maximum pressure gradient so that it centers on the volume of water with the prey item and has sufficient pressure generation to overcome prey escape behaviors of swimming or attaching to the substratum (Holzman et al. 2007; Wainwright and Day 2007). A critical aspect of suction feeding is that the flow of water into the mouth is essentially unidirectional so that the prey item is carried into the mouth and then into the throat while the water exits through the opercular opening or gill slits. Unidirectional flow is achieved by a wave of muscle activity that moves from anterior to posterior, resulting in a sequence of closely timed events. First, the lower jaw is depressed, followed by the dorsal rotation of the neurocranium. This is followed by the floor of the mouth (hyoid region) being depressed and finally by the maximal expansion of the opercular region. This pattern of movement for suction feeding on nonelusive prey is conserved across a wide range of teleosts, having been observed in fishes in the orders Cypriniformes, Cyprinodontiformes, Perciformes, and Pleuronectiformes (Gibb and Ferry-Graham 2005).
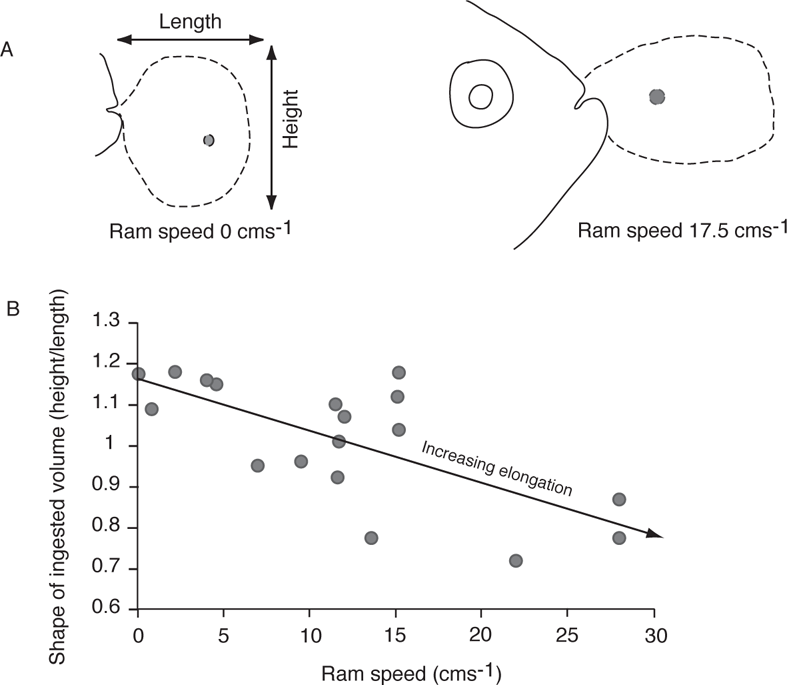
FIGURE 8.4. Shape of the ingested water volume relative to ram speed in suction-feeding Bluegill.
A. The outline (dashed line) of the ingested water volume in stationary and swimming fish. Closed circle indicates the midpoint of the prey.
B. The height to length ratio of the ingested water volume versus ram speed. Based on Higham et al. (2005).
In the case of elusive behavior by the prey, ram speed allows a predator to approach closer to the prey and thus overcome the escape force as well as increase the overall closing speed between predator and prey (Higham et al. 2005; Wainwright and Day 2007). An additional aspect for suction feeders is to minimize clues to mobile prey so that any avoidance behavior is delayed. Bluegills are able to reduce hydrodynamic cues to prey, such as bow waves or other swimming disturbances, by coordinating aspects of the strike. The strike is initiated prior to the bow wave reaching the prey so that by the time a prey has hydrodynamic cues, forward jaw protrusion and strong suction already have the prey moving into the mouth. The result is that Bluegill, at least in terms of hydrodynamic cues, are essentially “ambush predators” (Holzman and Wainwright 2009). In general, low ram speeds would be expected where prey perceive their predators by hydrodynamic clues, whereas high ram speeds would be expected for predators feeding on visually oriented prey (Holzman and Wainwright 2009).
The relative ability for suction feeding varies among other sunfishes, other centrarchids, and, of course, among other kinds of fishes. At least within the Centrarchidae, morphological features related to the force of suction feeding can be expressed in a simple model (Figure 8.5) (Carroll et al. 2004). Because suction involves, in part, the dorsal rotation of the skull, suction increases relative to the cross-sectional area of the epaxial muscles and as L1 (the distance between the centroid of force generated by epaxial muscles and the rotational fulcrum, located approximately at a point between the posttemporal bone of the skull and the supracleithrum of the pectoral girdle) increases. Suction decreases as the projected buccal area increases and as L2 (the distance from the fulcrum and the center of force of the buccal area) increases. Other things being equal, the force of suction in feeding should be greater in deep-bodied fishes with correspondingly large epaxial muscle areas and small buccal areas and be lower in more streamlined fishes with reduced cross-sectional areas of epaxial muscles and large buccal areas. Within the genus Lepomis, Spotted Sunfish (L. punctatus), which also have a small buccal area, short L2, large epaxial muscles, and large L1, are similar to Bluegill in generating high degrees of suction. In contrast, Redear Sunfish (L. microlophus), which are specialized to feed on benthic, hard-bodied prey such as mollusks, have relatively smaller epaxial muscle area, smaller L1, greater buccal area, and larger L2 compared to Spotted Sunfish and Bluegill (Carroll et al. 2004). Other species with deep bodies and large epaxial muscle area, such as flatfishes, also are suction specialists (Gibb and Ferry-Graham 2005).
There are trade-offs in morphological specializations required for suction. Greater suction requires smaller mouths, smaller buccal areas, and deeper bodies (to accommodate the epaxial muscles), and so reduces the maximum size of prey and, because of reduced streamlining (see Chapter 7), the maximum approach velocities (Carroll et al. 2004); however, deeper bodies increase maneuverability. The trade-offs in body form relative to suction ability, as predicted from the model (Carroll et al. 2004; Wainwright et al. 2007), are illustrated by five species of southeastern centrarchids (Figure 8.6). The deeper-bodied species of sunfish show greater reliance on suction compared to ram feeding (high approach velocity), in contrast to the more streamlined Largemouth Bass; however, ram feeders may experience a lower overall strike accuracy (measured as how centered the prey is in the ingested water mass) (Higham et al. 2006a). Largemouth Bass, and other species of Black Basses (genus Micropterus) are specialized piscivores, even though the specialization for piscivory limits morphological diversity that might include other feeding modes (Collar et al. 2009). Successful feeding on fish prey is related to a larger body and mouth size, greater buccal volume, reduced jaw protrusion, and reduced size of the pharyngeal jaws so as not to inhibit passage of prey into the esophagus (Carroll and Wainwright 2009; Collar et al. 2009). Despite differences in the role of suction versus ram feeding in centrarchids, the pattern of muscle activity involved in creating suction has been conserved across the group (Wainwright and Lauder 1992).
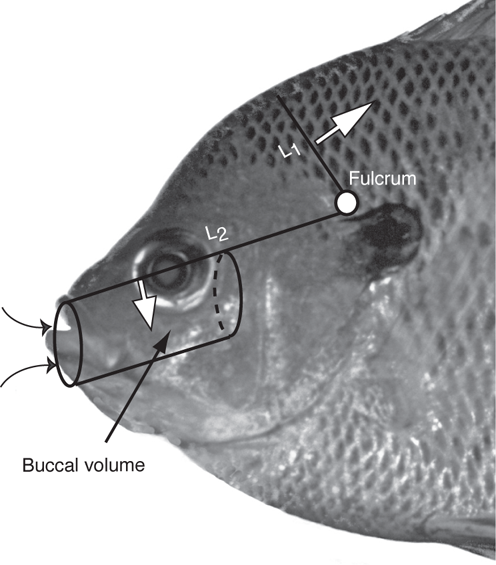
FIGURE 8.5. A schematic of relative forces generated during suction feeding. The schematic model of suction feeding (based on Carroll et al. 2004 and Wainwright et al. 2007) is overlain on the head of a Bluegill. Locations are approximate and are only to show relative locations of forces. The fulcrum is located at a point between the posttemporal bone of the skull and the supracleithral bone of the pectoral girdle. L1 is the distance from the fulcrum to the centroid of force generated by epaxial musculature; L2 is the distance from the fulcrum to the centroid of force exerted on the buccal area. The force generated by suction increases as the epaxial muscle area and L1 increase and decreases as the projected buccal area and L2 increase.
Largemouth Bass, fed on Goldfish (Carassius auratus), initiated the strike sequence at one body length removed from the Goldfish by a rapid approach and an explosive expansion of the mouth and throat (Svanbäck et al. 2002). However, strike initiation distances vary with prey kind and mobility. For Largemouth Bass feeding on tethered Ghost Shrimp (Palaemonetes spp.), strike distances were much smaller (1.25 cm or 0.07 body length) compared to fish feeding on Goldfish (Holzman et al. 2008b). Approach velocities increase proportionately with fish size, but times required to open and close the mouth (the gape cycle time) are fastest for small compared to large bass and can range from 37 ms in a 33 mm SL bass to 80 ms in a 201 mm SL Largemouth Bass (Richard and Wainwright 1995). Strikes that are the fastest show higher synchrony of the initiation of muscle firing, the fastest time to peak gape and forward rotation of the jaws, and result in the highest pressure gradient (Svanbäck et al. 2002). A typical strike requires the simultaneous elevation of the head, depression of the hyoid apparatus (the bones supporting the jaw joint), and depression of the lower jaw. Lower jaw depression causes the anterior rotation of the maxillae and protrusion of the premaxillae (Figure 8.3E). Jaw closing can occur with the mouth cavity still expanded and typically happens when the prey is between the upper and lower jaws by adduction of the lower jaw and lowering of the head (Richard and Wainwright 1995).
FIGURE 8.6. Relative suction ability in five species of southeastern centrarchids. Based on model predictions from Carroll et al. (2004) and Wainwright et al. (2007). The Redspotted Sunfish is shown instead of its close relative the Spotted Sunfish, which was used in the two studies.
Suspension Feeding
Various freshwater fishes, including some in the families Cyprinidae and Clupeidae, employ suction to move water containing organic detritus or small food items such as phytoplankton or zooplankton into their mouths. Although initially it was thought that most fishes removed particles from the water by direct sieving action of their gill rakers, removal methods turn out to be much more varied (Figure 8.7) (Rubenstein and Koehl 1977). When fluid flow is primarily perpendicular to the gill rakers, the rakers can function as dead-end filters or sticky filters; when flow is essentially parallel to the gill rakers, they can function as crossflow filters, which act to concentrate prey at the back of the throat (Rubenstein and Koehl 1977; Brainerd 2001; Sanderson et al. 2001). Although suspension feeding occurs in at least 60 species of marine and freshwater fishes, actual mechanisms of particle retention are known for only a few (Goodrich et al. 2000).
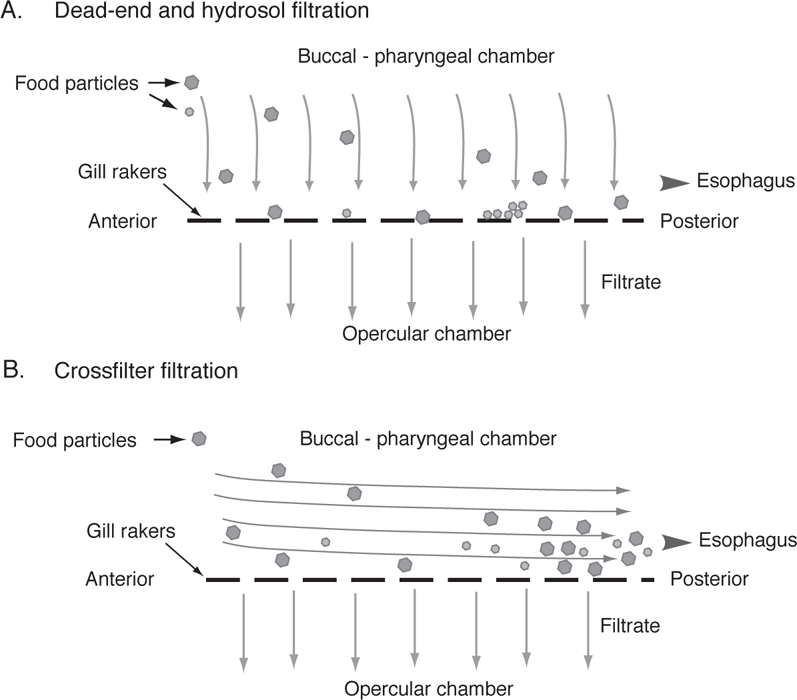
FIGURE 8.7. Modes of filtering in suspension-feeding fishes.
A. Dead-end and hydrosol filtration. In dead-end filtration (shown by large particles), food particles are retained that are larger than the pore spaces of the gill-raker sieve. In hydrosol filtration (shown by small particles) food particles are retained by sticking to mucus on the gill rakers or over the pore spaces.
B. Crossflow filtration, showing the increasing concentration of food in the posterior part of the oral chamber as water progressively exits past the gill rakers. Based on Brainerd (2001) and Sanderson et al. (1996, 2001).
DEAD-END FILTERS DEAD-end filtration involves fluid flow perpendicular to the filter, with fluid (the filtrate) passing through the gill-raker filter while leaving a build-up of food particles on the surface of the gill rakers (Figure 8.7A). All particles that exceed the pore size of the sieve are retained and smaller particles pass through with the fluid (Rubenstein and Koehl 1977). However, as food particles are trapped on the filter, they act to change properties of the filter (pores become clogged and pore sizes decrease) and to increase flow resistance, consequently requiring some mechanism to transfer the food from the gill rakers to the esophagus. A European minnow, the Common Bream (Abramis brama) uses gill rakers as a type of dead-end filter to capture zooplankton. However, rather than trapping prey in the pore spaces between adjacent gill rakers, prey are collected in channels formed by adjacent transverse ridges on the gill arches and by gill rakers on adjacent arches (Hoogenboezem et al. 1991). Using muscles that originate on the gill arch and insert on the expanded bases of the gill rakers, Common Bream can also reduce channel widths, and thus effective pore sizes, relative to the size of prey being consumed (Hoogenboezem et al. 1993). Common Carp (Cyprinus carpio), a nonindigenous minnow of European origin that is widespread in North America, also possesses muscles that would allow reduced channel widths; whether similar mechanisms are used among native North American minnows to increase suspension-feeding ability is unknown (Van den Berg et al. 1994).
HYDROSOL (STICKY) FILTERS Hydrosol filters act similarly to dead-end filters, except that they possess a sticky mucus that serves to trap particles smaller than the actual pore sizes of the filter (Figure 8.7A). Suspension feeding using hydrosol filtration has not been documented among native North American freshwater fishes but is known for the Nile Tilapia (Oreochromis niloticus), a fish widely used in North America for aquaculture. Nile Tilapia apparently adjust the level of mucus secretion from the gill rakers relative to the particle sizes being ingested. For large particles, mucus secretion is minimal so that gill rakers function as a deadend filter. However, for small particles such as bacteria, phytoplankton, and diatoms, there is active mucus secretion, often forming strands of mucus extending off of the gill rakers, and thus functioning as a hydrosol filter (Sanderson et al. 1996).
CROSSFLOW FILTERS Crossflow filtration involves the fluid moving parallel to the gill-raker surface and is the same technique used by industry to clarify beverages such as beer or wine (Brainerd 2001). During crossflow filtration, the food particles flow parallel to the surface of the gill rakers while water gradually exits through the gill rakers, resulting in a progressively concentrated slurry of food as it moves to the back of the throat or to the roof of the oral chamber (Figure 8.7B). Unlike a dead-end sieve, the gap widths between the gill rakers do not act as thresholds to preysize retention. Also, unlike hydrosol or dead-end sieves, flow of liquid along the gills can be relatively fast, with measured values of approximately 55 cms−1 as determined for Gizzard Shad (Dorosoma cepedianum) and Goldfish (Sanderson et al. 2001).
Gizzard Shad and two nonnative minnows, Goldfish and Common Carp (Cyprinus carpio), use suction to pass suspensions of food and water rapidly from anterior to posterior. Gill rakers are not mucus covered and particles rarely stick to the surface of the gill rakers, indicating that these fishes use crossflow filtration to concentrate food particles and directly pass the concentrated slurry of food particles into the esophagus (Drenner et al. 1982b; Sanderson et al. 2001; Callan and Sanderson 2003).
Sacramento Blackfish (Orthodon microlepidotus), a minnow native to the San Joaquin-Sacramento River system in California, is an example of a species concentrating food particles on the roof of the mouth via crossflow filtration. During feeding, Sacramento Blackfish move water containing planktonic algae and zooplankton into the mouth using pulses of suction (Johnson and Vinyard 1987). Nonselective feeding occurs in individuals larger than 200 mm SL, but smaller fish use sight to direct feeding actions at individual prey or groups of prey (Johnson and Vinyard 1987). As the suspension of water and food items moves through channels between the rows of gill rakers, water gradually exits and the concentrated slurry of food particles is carried dorsally to the roof of the pharynx where food particles become trapped in the mucus-covered palatal organ (Sanderson et al. 1991, 1998). Suspension feeding involves movements similar, albeit exaggerated, to normal respiration. As such, it is no surprise that crossflow filtration and particle retention occur at a reduced level in Sacramento Blackfish undergoing normal respiration (Sanderson and Cech 1995).
Ram Feeding
Ram and suction feeding can be seen as two ends of a continuum of feeding tactics, as shown previously for Largemouth Bass and Bluegill. In ram feeding, fishes primarily close the distance to their prey by forward movement rather than using muscles to pull the water mass containing the prey toward them. Fishes that employ ram often have elongate jaws and feed on active prey such as fishes, either by stalking the prey or by capturing prey from ambush such as gars, pike and pickerel. Ram feeding also occurs in large planktivores, such as Paddlefish (Polyodon spathula), that entrain plankton in their gill rakers as they swim through the water with their mouths open (Porter and Motta 2004).
Ambush Predators
Fishes such as gars (genera Lepisosteus and Atractosteus) and pike and pickerel (family Esocidae) frequently employ ambush feeding. In a typical feeding sequence, based on a study of Florida Gar (Lepisosteus platyrhincus), feeding actions take place from anterior to posterior and involve expansive, compressive, and recovery phases (Porter and Motta 2004). Initially, Florida Gar slowly move alongside their prey, and the actual strike is sideways and initiated by strong lateral bending. Strike distances are small, and in the case of Florida Gar, average only 11.7% of the gar’s total body length. Strikes occur quickly and rapidly, averaging only 32 ms in duration and speeds of 3.6 body lengths per second. The sequence of events involves the mouth opening beginning with the strike initiation, depression of the hyoid area, achievement of maximum gape, jaw closing, and hyoid elevation. Once prey are held by the jaws there are short, quick lateral and forward movements to manipulate the prey before it is moved to the throat and swallowed (Porter and Motta 2004).
The attack approach in pike and pickerel, based on Tiger Muskellunge (a hybrid between Northern Pike, Esox lucius, and Muskellunge, E. masquinongy), Chain Pickerel (E. niger), and Northern Pike, differ from that in Florida Gar in that Esox attack moving forward and not sideways to their prey (Webb and Skadsen 1980; Harper and Blake 1991). However, other aspects are similar. The attack generally begins with a slow approach toward prey using the median and paired fins (MPF) (Webb and Skadsen 1980), and the actual strike starts either with the body straight and then quickly bending into an S-shaped posture and then bending into an opposite S-shape as propulsion continues (termed pattern A) or starting with the body already in an S-shape (termed pattern B) (Webb and Skadsen 1980). The S-start provides for maximum straight-line acceleration toward the prey. In both Tiger Muskellunge and Chain Pickerel, type A starts occurred at a greater distance (22% and 37% body length, respectively) compared to type B starts (7.8% and 14% body length, respectively) (Webb and Skadsen 1980; Rand and Lauder 1981). Strikes occurred in well less than a second, but compared to Florida Gar, the strike duration was longer and generally less than 200 ms for Tiger Muskellunge (Webb and Skadsen 1980), between 84 and 189 ms for Northern Pike (Harper and Blake 1991), and between 51 and 98 ms for Chain Pickerel (Rand and Lauder 1981). Acceleration rates, from first movement to reaching the prey, were 2.4–4.0 body lengths per second in Chain Pickerel (Rand and Lauder 1981) and 3.7–4.0 body lengths per second in Northern Pike (Harper and Blake 1991), and thus essentially the same as for Florida Gar.
Suspension Feeding
Suspension feeding occurs both by active suction, discussed in the section on suction feeding, and by ram. During ram suspension feeding, fishes move through the water with their mouths open and the trunk musculature basically takes the place of buccal and opercular muscles in terms of gill ventilation and suspension feeding (Burggren and Bemis 1992). Although ram suspension feeders are relatively common in the marine environment, with such examples as herrings, anchovies, mackerels, Manta Rays (family Mobulidae), Basking Sharks (Cetorhinus maximus), and Whale Sharks (Rhincodon typus) (Sanderson and Wassersug 1993; Helfman et al. 2009), this approach is infrequent in freshwater fishes. Paddlefish, which are adapted to large rivers and their associated floodplain lakes with their often abundant plankton densities, swim constantly soon after hatching (Burggren and Bemis 1992). Both juvenile and adult Paddlefish (Polyodon spathula) rely on ram ventilation of the gills and use ram suspension feeding (Figure 8.8). During feeding, Paddlefish increase their swimming speed 1.6 times above that used for gill ventilation. Flow in the mouth cavity can be 19 cms−1 or about 60% of the swimming velocity during ram suspension feeding. Although initial prey capture is based on ram and not suction, the second phase of suspension feeding requires suction to increase the flow velocity in the mouth above the swimming speed. Referred to as a “hydrodynamic tongue,” this stream of water helps to move prey from the gill rakers into the esophagus (Sanderson et al. 1994). In adult Paddlefish, the gill rakers function as a dead-end filter. The sieve formed by the gill rakers has an average “mesh size” of approximately 0.06–0.09 mm and prey can be retained down to an average width of 0.10 mm (Rosen and Hales 1981). Although the gill rakers are well developed in adult Paddlefish, juvenile Paddlefish lack well-developed gill rakers until they reach approximately 100 mm TL. Juveniles also show increased feeding selectivity compared to adults, leading to the suggestion that juvenile Paddlefish do not filter feed (Rosen and Hales 1981). However, behavioral studies show that Paddlefish between 60 and 130 mm TL will use ram filter feeding (Burggren and Bemis 1992), indicating that gill rakers might function in some other way, as discussed previously in the section titled “Suction and Suspension Feeding.”

FIGURE 8.8. Orientation of the gill arches and gill rakers of Paddlefish (Polyodon spathula), a ram suspensionfeeding fish. Photo by W. T. Slack and S. G. George.
Manipulation
Manipulation constitutes the third main category of prey capture, as defined by Liem (1980a). It involves use of teeth of the upper and lower jaws and includes a range of approaches including biting, rotational feeding, picking, and scraping.
Biting
Most simply, biting refers to the forceful contact of the oral jaws with the prey and can include feeding on entire prey items, as demonstrated by many piscivorous and insectivorous fishes, removing pieces from a prey item, such as scale eating in various topminnows and pupfish (Able 1976), or tearing off chunks of a prey, as shown by shaking and rotational feeding of American Eels (Helfman and Clark 1986). Biting fishes generally possess well-developed oral jaws and have strong adductor muscles to allow forceful jaw closing (Alfaro et al. 2001). As emphasized earlier, major categories of feeding modes are not necessarily exclusive. Consequently, fishes that employ biting might also use ram feeding to close the distance to their prey. Compared to suction feeding, biting is an evolutionarily derived behavior in bony fishes, and muscle activity sequences differ between fishes that use suction, such as Bluegill and Largemouth Bass, and fishes that use little or no suction. Suction requires a short onset time of muscle activity to generate the highest force, whereas in biting there is less constraint on the timing of muscle firing, and increased jaw strength and muscle mass tend to result in longer closing times. In addition, the muscles, such as the sternohyoideus, that pull down and back on the hyoid region to help expand the oral cavity in suction feeding are able to take on other roles in biting (Alfaro et al. 2001; Horn and Ferry-Graham 2006).
Fishes that consume parts of other fishes, such as chunks of flesh, scales, or ectoparasites, although more common in tropical freshwater or marine habitats, are also represented by North American freshwater fishes (see also Chapter 12). Species showing cleaning behavior actively remove ectoparasites (such as trematodes and leeches), mucus, diseased tissue, or unwanted food particles from other fishes, either of the same or different species (Losey 1972). Sheepshead Minnow (Cyprinodon variegatus), Striped Killifish (Fundulus majalis), Rainwater Killifish (Lucania parva), and Diamond Killifish (Adinia xenica) all either show posturing that would invite cleaning activity from other individuals or exhibit actual cleaning activity themselves (Hastings and Yerger 1971; Able 1976).
Predators that bite or tear pieces out of their prey overcome the limitations of gape size encountered by those that swallow their prey whole. American Eels employ two behaviors, shaking and rotational feeding, to tear pieces from prey that are too large to consume whole by suction feeding (Helfman and Winkelman 1991). Jaw teeth in American Eels are numerous, small, and sharp (setiform teeth) and, while not providing the ability to directly bite off pieces of prey, they are effective at grasping a prey so that other behaviors can be effective in tearing off pieces. Small pieces are removed by grasping the prey and shaking it—an approach common in a number of different fish groups. Rotational (spinning) behavior is employed to remove larger chunks but this behavior is uncommon among fishes (Helfman and Clark 1986). During rotational feeding, the body is extended and spins rapidly along its longitudinal axis. In fact, American Eels can achieve up to 14 rotations per second, approximately three times faster than achieved by human figure skaters (Helfman and Clark 1986). Compared to suction feeding on entire prey items or using shaking to remove pieces of prey, rotational feeding requires more energy and is likely employed in the wild only when American Eels come across large prey that cannot be consumed by other behaviors (Helfman and Winkelman 1991).
Picking and Scraping
Fishes with jaws specialized for picking and scraping employ fine motor control of the jaws to obtain individual food items from the substratum or water column, while being able to avoid or reduce the intake of nonnutritive items (Horn and Ferry-Graham 2006). The effectiveness of a picking-based feeding mechanism requires finely controlled, “forceps-like” movements of the upper and lower jaws (Motta 1988). Pickers are also characterized by jaws in which the force application has been shifted anteriorly and a biomechanical coupling that allows upper and lower jaw movements to be synchronized. This morphological transformation appears to be associated with functional specialization for algal scraping and grazing.
Fishes in the order Cyprinodontiformes, which includes North American freshwater representatives of the families Aplocheilidae (rivulines), Fundulidae (topminnows), Poeciliidae (livebearers), Goodeidae (goodeids), and Cyprinodontidae (pupfishes), often employ a picking type of feeding mechanism (Ferry-Graham et al. 2008; Hernandez et al. 2008). Jaw protrusion in derived compared to basal cyprinodontiform fishes (Table 8.2) or to the atheriniform fishes (the sister group to the Cyprinodontiformes) is more specialized. It is in part controlled by the increasing complexity and greater amounts of cellular tissue of a ligament connecting the premaxilla and the mandible (the premaxillomandibular ligament) and by changes in insertion of a branch of the major jaw-closing musculature, the adductor mandibulae, so that it inserts on the premaxilla in addition to the mandible (Figure 8.9) (Hernandez et al. 2009). These changes allow greater coordination of the upper and lower jaws and increase the extent of jaw protrusion in derived versus basal cyprinodontiform fishes. For example, the derived Mexican Molly (Poecilia sphenops) has a divided premaxillomandibular ligament and a more complex branching of the adductor mandibulae, which inserts on the premaxilla in addition to the maxilla and mandible. This is in contrast to the more basal livebearer, the Western Mosquitofish, and the basal killifish, the Redface Topminnow (Fundulus rubrifrons; formerly F. cingulatus), whose branches of the adductor mandibulae complex only insert on the maxilla and the mandible (Figs. 8.9 and 8.10) (Hernandez et al. 2008, 2009). The increased independence of control over the premaxilla in the Mexican Molly may provide for greater dexterity that is needed for grazing on epiphytic algae through repeated scraping motions (Hernandez et al. 2008; Gibb et al. 2008). In addition, the lineage including Heterandria, Xiphophorus, and Poecilia is characterized by increased mobility of lower jaw shape as a result of mandibular bending allowed by a joint between the dentary and the angular/articular bones (Figure 8.9) (Gibb et al. 2008). Consequently, evolutionary changes in jaw morphology of cyprinodontiform fishes have been in the direction of greater jaw dexterity during feeding events. During the early evolution of cyprinodontiforms, increased dexterity and precision, granted by the ligament that directly connects the upper jaw to the lower jaw, may have enhanced the ability of basal species to select individual prey items from the substratum or water column using picking-based prey capture behavior. During the later evolution of this clade, the direct control of upper jaw movements during retraction, granted by the insertion of a branch of the adductor mandibulae complex on the premaxilla, enhanced the ability of derived species to remove encrusting material using a nipping or scraping-based feeding behavior; lower jaw bending apparently further increased the efficiency of algal scraping (Gibb et al. 2008; Hernandez et al. 2008, 2009). Of the species included in Figure 8.10, all microcarnivores except for Floridichthys have basic conical teeth, whereas tooth morphologies of omnivores include weakly spatulate, spatulate, or tricuspid shapes, and tooth shapes of grazers include recurved spatulate or tricuspid teeth.
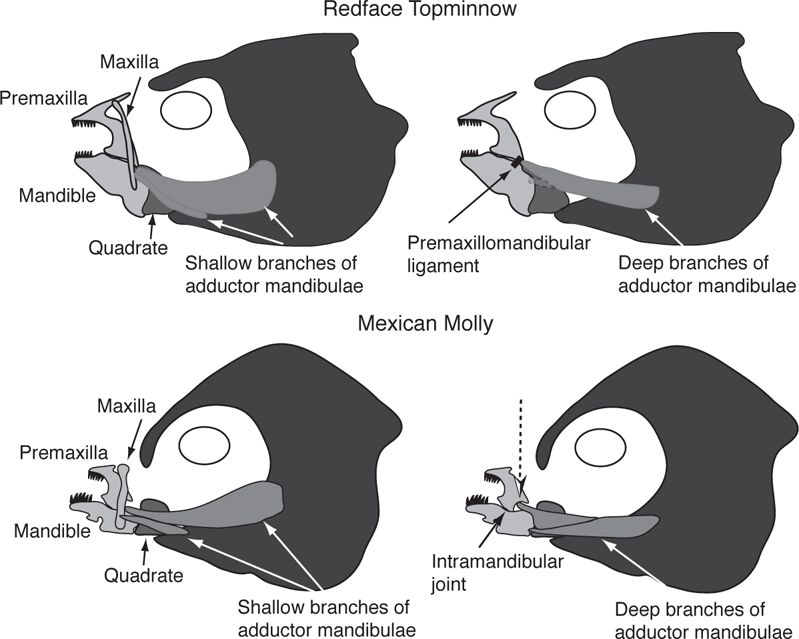
FIGURE 8.9. Upper and lower jaw bones and insertions of the adductor mandibulae complex in the Redface Topminnow (Fundulus rubrifrons) and the Mexican Molly (Poecilia sphenops). The left panels show the shallow branches of the adductor mandibulae complex; the right panels show the deep branches of the adductor mandibulae complex. For the Redface Topminnow, the heavy black line shows the location of the premaxillomandibular ligament (present but not shown in the Mexican Molly). For the Mexican Molly, the dashed arrow shows the branch of the deep adductor mandibulae inserting on the premaxilla and the black arrow shows the location of the intramandibular joint. Based on Gibb et al. (2008) and Hernandez et al. (2008, 2009).

FIGURE 8.10. Morphological, feeding, and phylogenetic relationships of selected fishes in the order Cyprinodontiformes and the sister group Atheriniformes. Lineages possessing modified adductor mandibulae muscles are also shown in bold. Drawings on the far right show general premaxillary or dentary tooth shapes; rectangles show trophic levels. Based on Hernandez et al. (2008, 2009), Gibb et al. (2008), with additional ecological and morphological information from Parenti (1981) and Ross (2001).
Prey Processing
Manipulation, postcapture processing such as mastication, and swallowing of prey involve the pharyngeal jaws, which are formed from upper and lower elements of the pharyngeal arches, associated bones, and ligaments, and from processes of other bones (Gosline 1973; Lauder 1982, 1983a, 1983b; Lauder and Wainwright 1992). The level of prey processing varies greatly among fishes. In suction-feeding fishes, prey tend to be swallowed with minimal postcapture processing. In contrast, fishes such as the Cyprinidae totally lack true teeth in the oral jaws, and all food processing is achieved by the toothed pharyngeal jaws. (Danionella dracula, a recently described minnow from northern Myanmar [Burma], does have tooth-like extensions from the upper and lower jaw bones [Britz et al. 2009].) In more derived fishes, teeth in the oral jaws and the pharyngeal teeth can be well developed (Sibbing 1991a). The pharyngeal jaws are used to remove hard outer structures of prey or otherwise reduce the size of the prey so that they can pass into the esophagus, as in molluscivores like Pumpkinseed (Lepomis gibbosus) or the molariform morph of the Cuatro Ciénegas Cichlid (Herichthys minckleyi) (Lauder 1983c; Hulsey et al. 2005; Helfman et al. 2009).
In teleosts, the lower pharyngeal jaw elements are formed from the hypertrophied fifth gill arches—the first four arches support the gill filaments and gill rakers (Evans and Deubler 1955). The upper pharyngeal jaw elements in teleosts consist of 1–5 paired tooth plates that may or may not be fused to the gillarch elements; the upper tooth plates are often reduced to 1–2 pairs in more derived groups (Lauder 1982). In addition to elements associated with gill arches, the upper biting surface can be formed from processes originating on neurocranial bones, such as the masticatory process of the basioccipital bone in cyprinids (Harrington 1955). Ventrally, lower teleosts have paired tooth plates that are aligned but not necessarily fused with the ceratobranchial bones of the fifth gill arch; in more derived teleosts, these elements become fused to the fifth ceratobranchials (Lauder 1982; Helfman et al. 2009). Pharyngeal jaws are particularly well developed in the cyprinids, catostomids, certain sunfishes, and cichlids.
Cypriniform Pharyngeal Jaws
Fishes in the order Cypriniformes, a group of ostariophysan fishes containing the families Cyprinidae and Catostomidae, are the first group of the teleosts (Chapter 7; Figure 7.5) to have well-developed pharyngeal dentition (Nelson 2006; Helfman et al. 2009). The pharyngeal teeth, which make up the ventral part of the pharyngeal jaws, are in one to three rows in cyprinids and in a single row with more than 16 teeth in catostomids (Berra 2001). In native North American minnows there are no more than two rows of teeth, and the major row (innermost row) has no more than six teeth and usually four to five teeth (Evans and Deubler 1955; Page and Burr 1991). Shapes of pharyngeal teeth vary from sharp, hooked teeth or conical teeth in piscivores and insectivores, to flat crushing tooth plates in detritivores and omnivores, to molariform teeth in certain herbivores and molluscivores, although some species such as carp have different tooth shapes in the same arch (i.e., a heterodont condition) (Evans and Deubler 1955; Sibbing 1988, 1991a). Pharyngeal teeth are replaced continually throughout life (Evans and Deubler 1955). The upper dentition consists of the enlarged masticatory process, which is covered with a horny pad (i.e., the chewing pad) in minnows and a soft pad in suckers (Sibbing 1982; Nelson 2006).
Pharyngeal teeth work against the dorsal chewing pad and are capable of complex grinding, crushing, and shredding movements, depending on the species (Sibbing 1982). In Common Carp, the pharyngeal arch is moved by modified gill-arch muscles; by epaxial muscles, which indirectly act to raise the pharyngeal bones; and by hypaxial muscles inserting on the pectoral girdle, which act to pull the teeth downward and backward, resulting in a sliding motion of the teeth against the chewing pad. Even though cypriniform fishes lack a retractor dorsalis muscle that acts to retract the upper pharyngeal jaws, as in more derived teleosts, the chewing pad is also mobile relative to the lower pharyngeal teeth, moving with the base of the skull and being powered by the epaxial body musculature (Sibbing 1982, 1988; Lauder and Wainwright 1992). However, the lower pharyngeal jaw is most important in prey transport and swallowing in minnows as well as in other lower teleosts such as esocids and salmonids (Lauder 1983b). (As an aside, the powerful shearing and tearing motions of the pharyngeal jaws explain why food habit studies of minnows are made challenging by the general lack of intact prey items.)
Sunfish Pharyngeal Jaws
In contrast to minnows, both the lower and upper elements of the fifth branchial arches support pharyngeal jaws in centrarchids and other neoteleostean fishes (Chapter 7; Figure 7.5), and there is a large muscle, the retractor dorsalis, that originates on the anterior vertebra and inserts on the upper pharyngeal jaws—in fact, this muscle is one of the defining characters of the Neoteleostei (Lauder 1983b; Lauder and Wainwright 1992). The retractor dorsalis acts to move the upper pharyngeal jaws backward and is in part responsible for the upper pharyngeal jaws taking over the major role in food transport from the lower pharyngeal jaws in the neoteleosts (Lauder 1983b). Among the neoteleosts, the degree of development of the pharyngeal jaws, teeth size and shape, and muscle development differs widely depending on the species and the kind of food eaten.
Among sunfish species, in addition to backward movement of the upper pharyngeal jaws by the retractor dorsalis, the jaws are moved ventrally against the lower pharyngeal jaws by rotation of upper gill-arch bones (the epibranchials). A general pattern of pharyngeal jaw muscle activity during prey mastication and movement into the esophagus, identified in insectivorous and piscivorous centrarchids and Yellow Perch (Percidae, Perca flavescens), is for a rhythmic back-and-forth movement of the lower pharyngeal jaws and strong retraction of the upper pharyngeal jaws that starts halfway through the backward movement of the lower jaws and continues into the forward lower pharyngeal jaw movement—this is referred to as the transport pattern (Lauder 1983a, c; Wainwright and Lauder 1992). Two sister species of Sunfish, Pumpkinseed and Redear Sunfish, have hypertrophied pharyngeal jaws, dentition, and musculature allowing them to crush snail shells and consume snails, a diet that is uncommon among other species of Lepomis (Lauder 1983a, c). The two species have a generally nonoverlapping natural range, with Pumpkinseed restricted to the northern half of the eastern United States and southern Canada, and Redear Sunfish restricted to the southeastern United States (Lee 1980a, b). The Pumpkinseed retains the generalized rhythmic pattern when feeding on nonmollusk prey; however, when fed snails it is able to alter the pattern of muscle activity so that the upper and lower pharyngeal jaw muscles contract synchronously—referred to as the crushing pattern. Redear Sunfish have lost the ancestral rhythmic transport pattern of muscles firing and only show the synchronous crushing pattern—to a Redear Sunfish, all prey are processed as though they were snails (Lauder 1983a, c). Although both Pumpkinseed and Redear Sunfish are specifically adapted for feeding on mollusks, the more specialized Redear Sunfish is capable of generating 50–100% more crushing force and switches at a smaller size, compared to Pumpkinseed, to a diet composed largely of snails (Wainwright 1996; Huckins 1997). Pumpkinseed also show polymorphism in the size and strength of the pharyngeal muscles and bones that seem related to the presence of snail prey as the fish develop. In a lake with few snails, Pumpkinseed had smaller pharyngeal muscles and less robust jaws compared to fish in a lake with abundant snail prey, although tooth morphology, other than increased wear in the snail-eating morph, did not differ (Wainwright et al. 1991).
Cichlid Pharyngeal Jaws
In contrast to minnows, both the lower and upper elements of the fifth branchial arches hypertrophy in cichlids and, in contrast to both minnows and sunfishes, the lower pharyngeal jaws are fused, resulting in greater versatility of movements and lesser individual muscle loadings (Liem 1973; Sibbing 1991a). The presence of a complex pharyngeal jaw structure has freed the oral jaws from the role of food manipulation and processing, leaving oral jaws with a single role of prey capture. This has led to a high diversity of form and function in the oral jaws (Liem 1973).
The Cuatro Ciénegas Cichlid, endemic to the clear spring pools of the Cuatro Ciénegas basin in Coahuila, Mexico, displays at least three morphs: a piscivorous morph involving body shape and cranial structure, and two morphs involving the size and shape of the pharyngeal teeth (Swanson et al. 2003). Of the latter two morphs, the papilliform morph has a more delicate lower pharyngeal jaw; reduced size of pharyngeal jaw musculature; and narrow, needlelike teeth in contrast to the molariform morph, which is characterized by a more robust lower pharyngeal jaw; hypertrophied pharyngeal jaw musculature; and broad, crushing molariform teeth. The papilliform morph feeds primarily on detritus, algae, and soft-bodied invertebrates; the molariform morph also consumes soft-bodied invertebrates, algae, and detritus when food is abundant but polarizes on snails when food becomes limiting and uses the molariform teeth and hypertrophied musculature to crush the shells (Liem and Kaufman 1984; Swanson et al. 2003; Hulsey et al. 2006). In fact, the molariform morph of Cuatro Ciénegas Cichlids produces the highest size-specific crushing force known among freshwater or marine fishes that use their pharyngeal jaws to crush mollusks (Hulsey et al. 2005). In some pools the papilliform and molariform morphs also differ in feeding behaviors and locations, with the former primarily scraping or scooping prey from the surface of travertine formations and the latter primarily diving into the soft substratum to engulf both hard and soft-bodied prey, although differences in behaviors are modulated by availability of hard and soft substrata. The occurrence of the two pharyngeal tooth morphs is related both to a genetic component and to phenotypic plasticity (Swanson et al. 2003, 2008).
SUMMARY
Fishes employ a high diversity of feeding modes, and jaw structures show a general trend toward increased complexity and degrees of freedom of movement over evolutionary time. One of the early changes in jaw morphology was the freeing of the maxillary bones from the skull so that the posterior ends could rotate forward during mouth opening and thus aid in the development of suction during feeding. Suction feeding represents the most basal, and most widespread, feeding mode among fishes. Other trends include the development of a posterior process on the premaxillary bones (toothed in more derived fishes) and the exclusion of the maxillary bone from the gape (and loss of teeth on the maxilla). The development of protrusile jaws, accompanied by the development of an ascending process on the premaxilla, has occurred in about half of all bony fishes as well as in some living chondrosteans. Jaw protrusion can aid in capture success by increasing the rate of approach to a prey item, creating a rounder mouth opening to aid in suction feeding, thus increasing the volume of the mouth and pharyngeal cavities and allowing faster jaw closing with less chance of prey expulsion. It also allows for a more horizontal body orientation in benthic feeding fishes. Fishes that use suction also employ various degrees of ram feeding.
Suspension feeding is less common among freshwater fishes than in marine fishes, but there are freshwater examples among North American species. Suspension feeders can use gill rakers as a dead-end sieve where water flow is largely perpendicular to the gill rakers and particles are collected in the spaces between the gill rakers, or by using the gill rakers and other structures as crossflow filters where water flow is largely parallel to the gill-raker surface and prey are concentrated toward the back of the throat while water exits through the gill rakers.
Several groups of more derived fishes have jaws that are specialized for picking and scraping—among North American freshwater fishes picking and scraping morphologies and behaviors are best developed among the Cyprinodontiformes. Pickers have the force application of the jaws shifted anteriorly and there is increased muscular control and flexibility of the jaws.
Prey processing is largely accomplished by a second set of jaws—the pharyngeal jaws. In some groups of fishes, such as those in the order Cypriniformes, pharyngeal jaws are highly developed and function to shred or macerate prey, in addition to transporting food into the esophagus. In the Cypriniformes, the lower pharyngeal jaws take the major role in prey transport. In more derived fishes such as centrarchids and cichlids, the upper pharyngeal jaws are primarily responsible for prey transport. In these groups, the jaws of some species are greatly hypertrophied and have enlarged molariform teeth, which allow them to feed on hard-bodied prey such as snails.
Although the literature on functional morphology of fishes is large and rapidly growing, information on the two largest families, Cyprinidae and Percidae, is surprisingly limited. Within the percids, only one genus (Perca) seems to have been studied. Within the cyprinids, most of the information is based on European taxa, and even then on only a few species. The one exception includes the landmark studies of suspension feeding in a North American cyprinid, the Sacramento Blackfish.
SUPPLEMENTAL READING
Higham, T. E., S. W. Day, and P. C. Wainwright. 2005. Sucking while swimming: Evaluating the effects of ram speed on suction generation in Bluegill sunfish Lepomis macrochirus using digital particle image velocimetry. The Journal of Experimental Biology 208:2653–60. An example of using Digital Particle Image Velocimetry and high-speed video to understand fluid movements during suction feeding.
Ferry-Graham, L. A., and G. V. Lauder. 2001. Aquatic prey capture in ray-finned fishes: A century of progress and new directions. Journal of Morphology 248:99–119. Highlights changes in the understanding of prey capture coincident with technological innovations.
Lauder, G. V. 1982. Patterns of evolution in the feeding mechanism of Actinopterygian fishes. American Zoologist 22:275–85. A classic paper on feeding mechanisms in ray-finned fishes.
Sanderson, S. L., A. Y. Cheer, J. S. Goodrich, J. D. Graziano, and W. T. Callan. 2001. Crossflow filtration in suspension-feeding fishes. Nature 412:439–41. Details the complexity of mechanisms in suspension-feeding fishes.