TEN
Communication among Individuals
CONTENTS
Mate Attraction, Courtship, and Spawning
Social Status and Individual Recognition
AN INTEGRAL PART of the biology of fishes is their ability to communicate, both among individuals of the same population or species, as well as among different species. In fact, communication is a critical aspect, both in terms of helping to maintain reproductive isolation between species, and also to facilitate interactions among and between species. Communication, by definition, refers to the interaction between two or more individuals. Technically, communication is a phenomenon of one organism producing a signal that, when responded to by another organism or organisms, confers some advantage to the signaler or to its group. The signal can be visual, chemical, auditory, electrical, or a combination of modalities. Although fascinating, electrical communication is not known for North American freshwater fishes, being documented in relatively few fish groups in Africa, South America, and the marine environment (Moller 2006).
As with other life functions, communication evolves as a trade-off among conflicting demands. For instance, coloration in fishes is a balance between factors maximizing signal value for communication and those that make an individual inconspicuous and at less risk for predation. Communication also requires the integration of the signaling, receiving, and behavioral systems, all in the context of the physical and biotic components of the environment. All else being equal then, the signal mode with the highest rate of information transmission should prevail over modes transmitting less information. For example, visual signals contain temporal and spatial information compared to sound, which only has temporally varying information, but visual signals only work where there is sufficient water clarity or sufficient open water not obstructed by plants, debris, or other materials (Endler 1992).
The term sensory drive refers to all processes that might cause biases in the direction of evolution of sensory systems. For example, signal characteristics of the sender should evolve to best exploit the signal-reception characteristics of the receiver. However, the signal is also affected by predation risk, so the direction of evolution should favor a balance between selection for communication and selection against discovery by a predator. In addition, the transmission and quality of the signal are strongly affected by the environment, such as water chemistry and turbidity, ambient light penetration, and ambient sound. Consequently, natural selection should favor systems that have high information content, maximize the signal relative to background noise, minimize the degradation of the signal, and have signals that stimulate the sensory system of the receiver most effectively, while also minimizing threats of predation (Endler 1992). Because of strong environmental constraints on all forms of communication, signaling systems often vary greatly, even within the same species in different habitats. In addition, anthropogenic changes to aquatic systems can have major impacts on the expression, transmission, and receipt of signals, a potentially very important conservation concern that is often overlooked (Sluijs et al. 2011).
Compared to the terrestrial environment, the aquatic environment offers challenges and advantages among the various forms of communication. Because water is quite similar to living tissue in terms of optical and acoustic density, signal detection can be more difficult. Signals can also be distorted or attenuated by the physical properties of water and the nature of aquatic habitats. Although much less an issue in most freshwater habitats compared to marine systems, water acts as a color filter, alters light quality, and causes greater light attenuation per unit distance compared to air. Turbidity also can reduce or eliminate visual signals. Water also selectively filters sound waves—higher frequencies, with lower wave amplitudes, are attenuated faster than lower frequencies, which have higher wave amplitudes. In addition, signal disturbances caused by sound reflection and reverberation are problems in shallow water (Kasumyan 2008).
On the positive side, low-frequency sound waves are transmitted almost five times faster in water than air and equivalent acoustic oscillations require less energy to produce in water compared to air (Kasumyan 2008; Garrison 2009). Water is also a better medium for the transfer of chemicals that are characteristically associated with living organisms, although depending on current flow, the transmission can be unidirectional and chemical signals may persist in the environment after the stimulus for their release has long since passed.
CHEMORECEPTION
Compared to terrestrial organisms, fishes rely heavily on chemical signals, something that is not surprising considering that water is an excellent solvent and they are living in a milieu of waterborne compounds. Among North American freshwater fishes, chemoreception is used in a number of ways, including food location, sexual recognition, identification of individuals or species, predator recognition and avoidance, recognition of young, orientation to habitat, and cues in migration. In some cases, the use of chemosensory cues does not fit within the definition of communication in the strict sense, although the responses of the receivers can be relevant and adaptive.
Morphologically, chemoreception can be divided into three general categories of olfaction, taste, and a general chemosense, with the latter residing in free epidermal nerve endings of trigeminal (fifth cranial nerve) or spinal origin (Kotrschal 2000). Olfactory receptors are innervated by the paired first cranial nerves. These nerve bundles also include branches going to the retina, which allow olfactory stimuli to alter visual responsiveness of retinal cells (Stell et al. 1984; Kotrschal 1991). Taste receptors are innervated by cranial nerves 7, 9, and 10, as well as spinal nerves, and include taste buds, which are compound structures that also include tactile receptors, and solitary chemosensory cells (SCC) (Kotrschal 1991, 2000). In contrast to terrestrial vertebrates, taste receptors are not confined to the inside of the mouth and are also on the fins and the body, especially around the mouth and head but also laterally (Kotrschal 1991; Gomahr et al. 1992; Sorensen and Caprio 1998). Densities of SCCs, which can account for 60–90% of all epidermal sensory cells, can be as high as 4,000 per mm2 in cyprinids and 1,000–2,000 per mm2 in Bullhead Catfish (Ameiurus) (Kotrschal 1991). Taste buds are also widespread on the surface of the body and fins. Based on a study of European cyprinids, taste bud density is greatest around the head and decreases posteriorly. In addition, benthic fishes and fishes living in turbid water generally have higher taste bud densities than pelagic fishes or fishes living in clear water. The highest densities of taste buds, 200–300 per mm2, are along the throat area; in fins, the high densities are 100–150 per mm2. Especially for benthic species, taste bud density is greater along the ventral surface of the body and fins compared to the dorsal body surface and fins (Gomahr et al. 1992). The Sicklefin Chub (Macrhybopsis meeki), which inhabits large, turbid rivers of the Mississippi River drainage, also has high densities of taste buds on the body and fins (Davis and Miller 1967). As yet, little is known about the kinds of olfactory signals perceived by free nerve endings. Although chemosensory receptors can be distinguished morphologically, they are highly synergistic in function and functions often overlap among the three types (Kotrschal 2000).
Chemosensory Communication
Pheromones are external chemical stimuli (odors) that are secreted to the outside by an individual and received by a second individual or individuals of the same species in which they elicit a specific adaptive response that is not dependent on prior learning or experience (Liley 1982; Sorensen and Stacey 2004; Burnard et al. 2008). Allomones are chemical signals that carry information between different species. Communication via pheromones or allomones is advantageous because they can transmit through darkness or turbid water and around obstacles, metabolically they require little energy to produce, they have a range from close contact to several km, and they can be relatively persistent in the water column. On the negative side, the transmission of pheromones or allomones is dependent on diffusion or currents, so communication can be unidirectional or relatively slow. The slow fade-out limits possibilities for switching messages, and the message may remain long past the time of response and have unintended consequences, such as attracting predators (Liley 1982; Stacey and Sorensen 2005). Environments with different flow characteristics consequently have varying qualities of chemosensory signals (Sherman and Moore 2001). Although pheromones are involved in various behaviors, most work has been based on those involved in reproduction (Burnard et al. 2008).
The evolution of pheromones is thought to have involved three discrete steps, starting with the ancestral stage—the release of chemical substances from a donor fish without eliciting a response in another conspecific (the putative receiver; Figure 10.1) (Stacey and Sorensen 2005). In the second, or spying stage, the selection favors a response to the olfactory cue by the receiver, along with the possibility of increased sensitivity to the cue. At this point the donor remains unchanged, although the spying stage is a prerequisite for the third stage. Communication can evolve if there is positive selection on the donor for the production of a chemical cue responded to by conspecifics. In the final stage, both the donor and receiver are respectively specialized for the production and detection of the chemical signal. Historically, fish alarm substances have been considered pheromones. However, more recent studies suggest that alarm substances are primarily involved in immune functions. As such, alarm substances are treated in the “Predator Avoidance” section of Chapter 12.
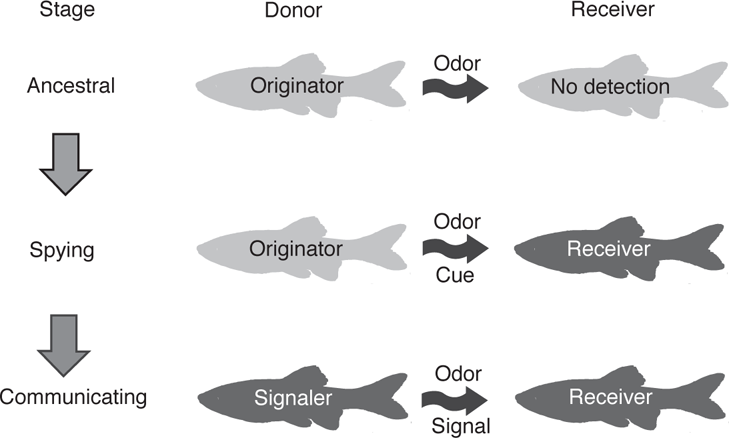
FIGURE 10.1. Three stages in the evolution of pheromonal communication in fishes. In the ancestral stage, the donor releases various odors into the water, but both the donor and receiver are unspecialized (indicated by light gray shading) and the receiver does not respond to the released odors. In the spying stage, the donor remains unspecialized, but the receiver has become specialized (indicated by dark gray shading) for detection and response to the chemical cue. In the communication stage, the donor is specialized for the production of the chemical cue and the receiver is specialized for the detection and response to the cue. Based on Stacey and Sorensen (2005).
Mate Attraction, Courtship, and Spawning
Pheromones are involved, or at least implicated, in mate attraction, courtship, and spawning among various fish groups. Olfactory responses to pheromones likely evolved relatively few times and have diversified slowly (Stacey and Cardwell 1995). Among North American freshwater fishes, examples of males responding to pheromones released by females include species in the families Petromyzontidae, Clupeidae, Cyprinidae, Characidae, Ictaluridae, Salmonidae, and Poeciliidae (Liley 1982; Burnard et al. 2008). In at least some cases, the response is due to gonadal hormones (i.e., prostaglandins and steroids) or their metabolites that are released into the environment and are thus active as hormonal pheromones (Stacey and Cardwell 1995; Stacey and Sorensen 2005; Burnard et al. 2008). This discovery of hormonal pheromones shows that the importance of hormones and their metabolites is not limited to intraindividual “communication” but is extended as well to nearby conspecifics. In fact, although hormones might act slowly within an individual in the development of secondary sex characteristics and sexual behavior, hormonal pheromones can very rapidly communicate hormonal status of an individual to its conspecifics.
Mature male Goldfish (Carassius auratus) display increased reproductive behavior when exposed to water occupied by female Goldfish that have ovulated eggs in their ovaries but do not respond in such a way to mature females that have not ovulated (Partridge et al. 1976; Liley 1982). Later work has shown that males are responding to prostaglandins (F prostaglandins), indicative of ovulation, and to various hormonal steroids (Stacey and Cardwell 1995). In addition to stimulating or affecting ovulation in fishes, F prostaglandins are involved in eliciting female sexual behavior and as a pheromone serving to attract males (Sorensen and Goetz 1993). Male Fathead Minnows (Pimephales promelas) show increased courtship behavior when exposed to water occupied by females injected with F prostaglandins, but do not respond directly to water with F prostaglandins but lacking in female Fathead Minnows, suggesting that it is not the presence of the prostaglandin alone, but that the prostaglandin causes females to release some other chemical substance that is attractive to males (Cole and Smith 1987). Female Sailfin and Dwarf mollies (Poecilia latipinna and P. chica), as well as females of other molly species, release a pheromone from the ovary during or subsequent to ovulation. The pheromone increases male swimming activity and social interactions among males, as well as increasing the frequency of mating attempts (Thiessen and Sturdivant 1977; Brett and Grosse 1982; Liley 1982). Similar responses have been shown in Sea Lampreys (Petromyzon marinus), Atlantic Salmon (Salmo salar), and Rainbow Trout (Oncorhynchus mykiss) (Liley 1982; Stacey and Cardwell 1995).
Female attraction to male chemical cues also occurs in a number of North American freshwater fish families, including Petromyzontidae, Salmonidae, Cyprinidae, Ictaluridae, and Cyprinodontidae (Stacey and Cardwell 1995; Kodric-Brown and Strecker 2001; Burnard et al. 2008). Male Sea Lampreys build nests in streams and then use a pheromone based on a specific bile acid to communicate their reproductive status and their location to ovulated female lampreys down current from them. This seems to be a case of active signaling by the males rather than the females responding to a metabolic by-product (i.e., spying). Male Sea Lampreys do not feed as mature adults, and thus the bile acids, produced in the liver, are not needed for digestion; in fact, the adult lampreys lack bile ducts and gall bladders. The bile acids are released by diffusion across the gill membranes and can attract females from distances of at least 65 meters in a stream with a discharge of 2.3 m3s−1 (Li et al. 2002).The mature female olfactory system is highly sensitive to the specific bile acid released by the males and can discriminate bile acid from mature males from other bile acids produced by conspecifics (Siefkes and Li 2004).
Male Fathead Minnows also produce a chemical substance (or substances) that is attractive to females during the reproductive season as well as to females with regressed gonads. Females show greater responsiveness to the male chemical stimulus in the morning, the typical spawning time for Fathead Minnows, compared to the afternoon when they become more responsive to chemical signals from other females. The male pheromone likely serves to guide reproductively ready females to nest sites of defending males (Cole and Smith 1992). There is also some evidence that male Channel Catfish (Ictalurus punctatus) use chemicals to identify the nest site and to attract females, as do Rainbow Trout and Lake Trout (Salvelinus namaycush) (Liley 1982; Zhang and Hara 2009). Although not demonstrated by field experiments, female Lake Trout are highly sensitive to bile acids that are produced by mature male conspecifics, strongly suggesting a sexual attraction system analogous to that of lampreys (Zhang et al. 2001; Zhang and Hara 2009). In addition to communication between sexes, pheromones also are used for same-sex communication. For instance, male Goldfish respond to the gonadal condition of other males so that they are synchronized in terms of milt production (Stacey and Sorensen 2005).
On a cautionary note, however, with the exception of Sea Lamprey, all the results mentioned previously were based on experiments done in static tank systems with consequently high concentrations of the pheromones. Evidence from the tropical Guppy (Poecilia reticulata) is cautionary in how olfactory signals might operate in nature. In both a natural stream and in a flowing-water laboratory stream, female Guppies did not show any evidence of attraction to chemical cues released by male or female conspecifics, although they did respond when tested in a static system. These results emphasize the importance of concentration and perhaps the importance of short-range effects in chemical signals (Archard et al. 2008). They also emphasize the need for testing the role of pheromones under more natural conditions.
Social Status and Individual Recognition
In addition to being involved in reproductive behavior, pheromones are also used to communicate individual identity (including kin recognition), social status, or other social signals. In a pioneering study, blinded individual Yellow Bullhead (Ameiurus natalis) were positively and negatively conditioned to respond to water from two different conspecifics held in separate aquaria. For the positive conditioning, fish were fed when water from one conspecific’s tank was introduced. For negative conditioning, fish were given a mild shock when water from the other conspecific’s tank was introduced. Once conditioned, the test fish responded to the positive stimulus by moving to the surface and showing feeding behavior; test fish responded to the negative stimulus by moving to the bottom of the aquarium and hiding. Overall, the test fish discriminated between the two conspecifics in 95.5% of the trials but were unable to discriminate between the two individuals when their sense of smell was blocked (Todd et al. 1967). In addition, Yellow Bullhead seemed to be able to recognize changes in social status through olfactory cues. In a similar study, Brown Bullhead (A. nebulosus) were able to correctly identify a conspecific 90% of the time as shown by positive responses to water taken from conspecifics’ aquaria (Carr and Carr 1985).
An aspect of social behavior, the level of aggression between individuals, is controlled by a protein-based pheromone in juvenile Brown Bullhead. When held at high population densities, juvenile Brown Bullhead show reduced aggressive behavior; however, juveniles held at low population densities are highly aggressive toward one another. When aggressive fish from low-density populations received water from high-density populations, they showed a significant decline in aggressive behavior (Carr and Carr 1986).
Chemosensory cues are used by fishes to identify kin, with North American examples from Cyprinidae, Ictaluridae, Salmonidae, and Poeciliidae (Brown and Brown 1996; Griffiths 2003; Ward and Hart 2003). Because the releaser apparently does not modulate the signal relative to the receiver, these cases better fit the case of spying rather than communication (Figure 10.1).
Work on European Threespine Sticklebacks (Gasterosteus aculeatus) indicates that odor is involved in kin recognition but that it is insufficient for kin recognition in the absence of visual cues. Kin recognition may be important in mate selection and the avoidance of inbreeding, in the avoidance of cannibalism, in school or shoal formation, and in the reduction of stress (Ward and Hart 2003). Olfactory determination of kin is particularly well demonstrated in salmonids, including the genera Oncorhynchus, Salmo, and Salvelinus (Griffiths 2003; Ward and Hart 2003).
Kin recognition appears to be based to a large extent on waterborne chemosensory cues associated with major histocompatability complex (MHC) genes (Olsén et al. 1998; Griffiths 2003). MHC genes are important in the immune system of vertebrates, including fishes, and are highly polymorphic such that individuals often differ in their MHC genotypes. The odor of individuals is related to their MHC genes, making kin recognition and recognition of individuals possible. Various hypotheses linking the MHC complex to specific odors have been proposed, although the exact linkage remains uncertain (Rajakaruna et al. 2006). High relatedness increases similarity among MHC genes and allows kin recognition. Among Atlantic Salmon, Arctic Char (Salvelinus alpinus), and Brook Trout (S. fontinalis), siblings show a preference for those that carry more similar MHC genes compared to siblings with more dissimilar MHC genes (Olsén et al. 2002; Rajakaruna et al. 2006). Overall, there is conflicting evidence on whether kin recognition is an innate or learned behavior, but at least in Arctic Char, some early association with kin is required for kin recognition using MHC to occur (Brown and Brown 1996; Olsén et al. 1998, 2002). The period of association likely occurs after hatching but prior to emergence from the redd (Brown and Brown 1996).
Given the known survivorship value of schooling, a fish could increase its inclusive fitness by joining a school made up of its siblings (Box 10.1) (Quinn and Busack 1985). A pioneering study on Coho Salmon (Oncorhynchus kisutch) showed that juvenile fish preferred water from siblings over water from nonsiblings or from other sources. In addition, there was only a slight decrease in preference for water from unfamiliar siblings compared to familiar siblings, indicating that the waterborne cue was not based solely on familiarity (Quinn and Busack 1985). Other studies, including work on Atlantic Salmon parr, have shown a reduction in aggression toward kin compared to nonrelated conspecifics and that the reduced aggression corresponded with better growth under laboratory conditions (Brown and Brown 1996; Griffiths 2003). In addition, kin recognition could play a role in the avoidance of inbreeding (Ward and Hart 2003). Although fishes have the ability to recognize kin and tend to associate with them in laboratory situations where the concentration of chemical cues is relatively great, initial evidence from natural systems generally did not indicate that fish tend to associate more with kin than nonkin. Evidence for the lack of relatedness within fish associations or schools comes from studies on European minnows (Phoxinus), a widespread North American minnow, the Common Shiner (Luxilus cornutus), and European populations of Threespine Sticklebacks (Dowling and Moore 1986; Griffiths 2003). Because Threespine Sticklebacks remain in the nest for several days after hatching, the potential for schools to consist of closely related fish seems likely. However, in nature, individuals in schools show low or no relatedness (Peuhkuri and Seppä 1998). These earlier studies of kin associations were necessarily limited in power by the techniques available, such as using protein (allozyme) electrophoresis. A more recent study on Brook Trout using microsatellite DNA suggests that fish schools often do show a greater degree of relatedness than that based on chance. At least in Brook Trout, schools seem to be hierarchically grouped, including familiar but unrelated fish, fish from the same population, and groups of kin, although more school members were unrelated than were kin. In addition, based on age-size relationships, groups of kin or population members remain together for up to four years (Fraser et al. 2005).
BOX 10.1 • Welfare of Relatives and Inclusive Fitness
The fitness of an individual is measured by the proportion of the individual’s genes present in the subsequent generation and is the basis for the operation of Darwinian natural selection. However, the genetic contribution of an individual is not fully dependent on its own survival to reproductive age and successful breeding and survival of offspring to adulthood. The reason for this is that individuals share some portion of their genotype with siblings, cousins, second cousins, and so on, with the proportion of shared genes decreasing in more distantly related relatives (Hamilton 1964a). This led early population geneticists such as Fisher and Haldane to realize the importance of relatedness in the understanding of the evolution of social behaviors, especially altruism. Hamilton (1964a) proposed a quantity, called inclusive fitness, that “allows for interactions between relatives on one another’s fitness.” Inclusive fitness provides a genetic answer for the evolution of limited sacrifices for kin by an individual based on the degree of relatedness shown by the kin. In the genetically modeled world of Hamilton (1964a), the benefit to a sibling must minimally average twice the loss to the altruistic individual, the benefit to a half-sib must average at least four times the loss, and the benefit to a cousin must minimally average eight times the loss to the individual. Put even more succinctly, from the standpoint of a genetically modeled world where behaviors are solely controlled genetically, no individual will sacrifice its life for any single individual but will sacrifice it when it can save more than two brothers, four half-brothers, or eight first cousins. Thus, given the ability to recognize kin, an individual fish should modulate its behavior toward an individual relative to the degree of relatedness. For instance, an individual could raise its inclusive fitness by being less aggressive to kin than nonkin or by performing behaviors such as alerting kin to the presence of a predator (Hamilton 1964b; Greenberg et al. 2002).
Given the few populations and species that have been studied using modern approaches, the degree that fish schools include groups of kin is still largely unknown and provides an exciting prospect for further work. However, in terms of expectations for the occurrence of highly related fish within a single school, there are trade-offs between the advantages of kin selection and the advantages of associating with less related individuals or, in reproductively active fishes, the avoidance of inbreeding (Griffiths and Armstrong 2001; Ward and Hart 2003). In general, the intensity of resource competition is thought to increase as relatedness among individuals increases, so that negative selection for kin-structured schools might also be occurring and that schools comprised of mixed genotypes would be favored (Griffiths and Armstrong 2001).
Migration
Migratory fishes employ chemical cues to locate streams, spawning sites, or feeding areas. Chemical cues can emanate from physical or biological aspects of the environment, including odors released by conspecifics. Although the use of these chemicals in migration generally does not appear to constitute communication, there are instances where true communication and/or spying could be occurring.
Extensive studies of Pacific and Atlantic salmon species clearly demonstrate that these fishes rely primarily on olfaction to identify their natal rivers and to return to natal spawning sites. In fact Buckland (1880), in reference to Atlantic Salmon homing to streams of the British Isles, said, “When the salmon is coming in from the sea he smells about till he scents the water of his own river.” Seventy-one years later, Arthur Hasler and his graduate student Warren Wisby provided the first experimental evidence for what became known as the olfactory hypothesis when they showed that fishes (in this case Bluntnose Minnow, Pimephales notatus) could differentiate between odors of two different streams (Hasler and Wisby 1951; Magnuson and Quinn 2005). This was followed by similar conditioning on Coho Salmon, showing that they could also discriminate between water from the same two streams used in the Bluntnose Minnow study (Hasler 1956). The olfactory hypothesis proposed that (1) streams differ in their chemical characteristics and that these characteristics remain consistent over time, (2) fishes are able to detect these differences, (3) the home-stream odors are learned by young salmon before they migrate to sea and young fishes do not respond to odors of nonnatal streams, and (4) the memory of the odors elicits upstream migration in adults. In a follow-up paper, Wisby and Hasler captured sexually mature Coho Salmon during their upstream migration from two connected streams. They temporarily blocked the nares of approximately one-half of the fish to stop the flow of water over the sensory tissue, and transported both the treated and untreated fish back downstream where they were released below the juncture of the two streams (Figure 10.2). Fish with blocked nares were unable to consistently enter the tributary where they were initially captured, whereas those with functioning nares generally made the correct choice (Wisby and Hasler 1954).
The olfactory hypothesis comprises two principal, but not mutually exclusive, hypotheses regarding the origin of the chemical signal: the imprinting hypothesis and the pheromone hypothesis (Yamamoto and Ueda 2009). The imprinting hypothesis states that fishes learn the chemical odors of their natal stream during a critical period (or periods) in their life cycle. The period when fishes imprint on the home-stream odor seems to be associated with elevated levels of thyroxine. Early studies of hatchery-reared Coho Salmon showed that imprinting occurred during the parr-to-smolt transformation (PST). However, in wild fish, which experience natural environmental cues and which, depending on individual populations and species, may leave the redd much earlier than the time of PST, imprinting generally occurs much earlier, often at the end of the embryonic development period (Dittman and Quinn 1996). In addition, because peaks in thyroxine occur several other times during the freshwater stage, such as during periods of rapid growth and during the PST, young salmon likely use multiple imprinting on stream odors to recognize sequential waypoints on their return migrations. Once olfactory cues have guided adult salmon back to their natal stream and the approximate section of the stream where they emerged from the redd, they use ecological and behavioral cues such as substratum size, current speed, intrasexual competition, and mate availability to determine the exact spot for the initiation of redd construction (Dittman and Quinn 1996). The chemical cue is contained in the organic fraction and likely produced from a mix of vegetation and soil types in the stream’s drainage basin.
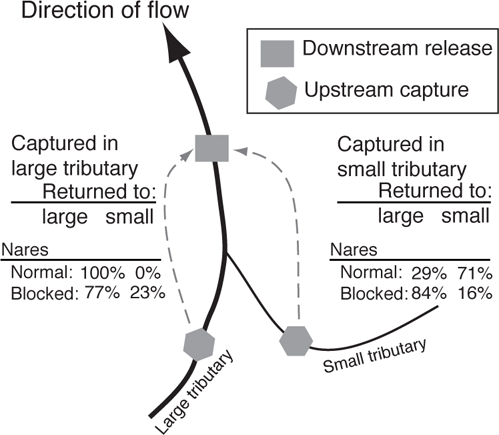
FIGURE 10.2. The role of olfaction in salmon homing. Coho Salmon with blocked nares (olfactory pits) were less successful in repeating their original choice of streams. Fish were initially captured during their upstream migrations at weirs in the large and small tributaries and then taken back downstream below the confluence and released. Based on Wisby and Hasler (1954).
Recent studies indicate that Pacific salmon species strongly respond to naturally occurring amino acids in streams (Yamamoto and Ueda 2009). Mature Chum Salmon (O. keta), in fact, are attracted to test water that has received an amino acid complement that matches their home-stream water. Most likely, the amino acid complement of streams is influenced by complex biological interactions occurring within the watershed such as microbial biofilms, soil and vegetation types, and litter (Yamamoto and Ueda 2009). Studies have also shown that salmon can be imprinted on artificial organic substances such as morpholine (a heterocyclic amine) so that they can be induced to return to whatever stream is receiving the artificial imprinting substance (Hasler and Scholz 1980).
The pheromone hypothesis proposes that adult migratory fishes are attracted to their home stream by pheromones released by juvenile conspecifics from the same population (Nordeng 1977). In addition to releasing pheromones from the natal stream by juvenile fish, the downstream seaward migration of smolt would, in most species of salmonids, overlap with part of the upstream migration of adults, providing an olfactory trail from coastal waters to the spawning grounds. If true, the pheromone hypothesis would constitute either actual communication or spying (Figure 10.1). Because the odorant produced by juvenile fish could contribute to part of the overall odors of the natal stream, this hypothesis is not mutually exclusive with the imprinting hypothesis. However, it does require that juvenile fish be in the stream system when the mature adults are migrating—something that is not true for some species of salmonids, such as Chum Salmon or Pink Salmon (O. gorbuscha) (Yamamoto and Ueda 2009).
Although Pacific and Atlantic salmon species have the ability to recognize kin and conspecifics (see preceding paragraphs), support for the pheromone hypothesis is mixed. In experiments on adult Coho Salmon, fish selected water conditioned by juveniles of their own population when tested against city water, but they did not select water conditioned by juveniles of their own population over water conditioned by juvenile Coho Salmon from another population (Quinn et al. 1983). A follow-up study did show that Coho Salmon can distinguish between populations based on chemosensory cues, so that pheromones could be involved, but not required, for successful homing to natal streams (Quinn and Tolson 1986).
In contrast, there is evidence that Norwegian Arctic Char seem to use pheromones produced by juveniles of their own population to locate their natal stream. Parental fish were removed from the Salangen River and transported to a hatchery in southern Norway with a different water source where eggs were fertilized and the young fish were raised. When fish reached the smolt stage, 96 were released in the marine environment. Subsequently over 1–3 years, 27 fish were recaptured with 63% of the recaptures in the upper Salangen River, including the natal area. The putative olfactory cues were pheromones produced by conspecifics in the natal area and by the natural downstream migration of smolts that coincided with the upstream movement of adults (Nordeng 2009).
Counter to most of the studies on salmonids, the pheromone hypothesis is strongly supported by work on lampreys (Petromyzontidae). Lamprey genera typically include both parasitic and nonparasitic species, with parasitic species generally showing longer migrations than nonparasitic taxa (Hardisty and Potter 1971). In addition, migratory lampreys do not necessarily return to their natal stream. Based initially on studies of Sea Lampreys, returning migrants select spawning rivers and locate spawning sites based primarily on their innate recognition of bile acid–based pheromones released by stream-resident larvae (Bjerselius et al. 2000; Vrieze and Sorensen 2001; Sorensen and Vrieze 2003). In addition, pheromones can act synergistically with natural stream odors. Adults are able to discern the odors at picomolar concentrations representing realistic concentrations in natural stream water and respond by swimming against current flow (rheotaxis). Once Sea Lampreys change from the migratory stage to the mature stage, they no longer respond to the larval pheromone, but do respond to odors released by mature members of the opposite sex.
The pheromone system in lampreys seems to be evolutionarily conserved, with high similarities among tested species in bile acid production and in detection responses by adults. The absence of species-specific differences in the production of bile acid pheromones is not surprising given the similar spawning and nesting requirements among lamprey species (Fine et al. 2004). The lamprey pheromone system has conservation and management applications. In areas such as the Great Lakes where Sea Lampreys are not native, the pheromone could be employed to attract and remove returning adult fish. In conservation efforts, the generalized lamprey pheromones could be used to attract rare native species of lampreys to appropriate spawning sites (Sorensen and Vrieze 2003; Fine et al. 2004).
VISUAL COMMUNICATION
Communication by visual means involves both the evolution of the receptors and the signals. It can include responses to color and color patterns, body shape, and body and fin movements. Given the wide range of colors exhibited by fishes, it is no surprise that fishes are able to see colors. In addition, many fishes are responsive to wavelengths in the ultraviolet (UV) range. Although most of the research on UV reception has involved tropical marine or freshwater fishes, UV reception occurs in juvenile salmonids (with the sensitivity of receptors for UV lost or reduced in adult fishes), in some minnows, and in cyprinodontids and goodeids (Siebeck et al. 2006).
Both scotopic (rod based, dim light) and photopic (cone based, bright light) vision arose early in vertebrate evolution, perhaps more than 540 mya (Collin and Trezise 2006). Fishes differ in their visual pigment systems, ranging from benthic fishes, such as ictalurids, that essentially lack sensitivity to low and midrange wave lengths, and which have high rod retinas that are sensitive in low light conditions (i.e., primarily scotopic vision), to fishes with 4–5 different visual pigments in the cones, resulting in well-developed color sensitivity (photopic vision). Examples of the latter include surface-inhabiting genera such as Cyprinodon, Fundulus, Gambusia, Poecilia, and Xiphophorus (Levine et al. 1980).
Colors in fishes can be caused by specialized pigments that are generally contained in cells, called chromatophores, but occasionally by free pigments in the body tissues. Common pigments are carotenoids (producing yellows, oranges, and reds) and melanins (producing browns, grays, and blacks). However, because fishes cannot synthesize the pigments or their precursors, pigment-based colors are diet dependent (Price et al. 2008). Structural colors, schematochromes, are colors produced by reflective structures such as layers of guanine, and cells containing such structures are iridophores (Bond 1996). Schematochromes are responsible for short-wavelength colors (blues and violets) and silvery colors (Price et al. 2008). In contrast to other vertebrates, pigment cells of bony fishes are under neuroendocrine control, so that the color signals can be changed rapidly (Moyle and Cech 2004).
The use of visual communication is lessened in visually complex environments, or in deep or turbid water. However, with the exception of Cavefishes (Amblyopsidae), lamprey ammocoetes (which burrow into the substratum), and blind catfishes, all North American fishes have well-developed eyes. Both larval and adult lampreys have lateral photoreceptors on the body that are responsive to light levels but are not likely involved in communication (Deliagina et al. 1995).
As a light beam passes through water, it is altered by scattering and absorption caused by water molecules and by dissolved and suspended matter (Loew and Zhang 2006). The quality of downwelling light in pure water or fresh water without significant amounts of dissolved organic compounds or particulate matter has a transmission maximum centered in the blue range (wavelengths of 450–490 nm). Water with small amounts of chlorophyll would appear green; whereas water with large amounts of tannins, chlorophylls, and lignins, as would be typical of marshes, swamps, and lowland rivers, has transmission maxima in the brownish-yellow, orange to red range. Compared to most marine habitats and clear, tropical lakes, many freshwater habitats are so strongly colored that spectral quality of light can change in only a few meters, resulting in microhabitat differences in color reception and the use of visual signals. Depending on the viewing direction, and thus the path distance of light through water, the quality of light can vary dramatically (Levine et al. 1980; Loew and Zhang 2006; McLennan 2007).
Defense of Territory
Visual signals, including frontal and lateral displays that make an individual look larger than it really is (i.e., inflationary displays), are part of agonistic behavior in many species. Although details of inflationary displays vary within and among taxa, there are some general characteristics, as shown by examples from salmonids, cyprinids, and elassomids.
Juvenile Atlantic Salmon actively defend feeding territories in streams during the day but settle to the bottom at night. Visual aggressive displays include frontal and lateral displays, both of which are accompanied by elevated fins (Keenleyside and Yamamoto 1962). During frontal displays, the more visual paired fins are all fully open, although the less visible dorsal fin is only partially raised. In lateral displays, all fins are fully extended. In addition to frontal and lateral displays, young Atlantic Salmon also charge or flee, nip, and chase. Besides the fin and body posturing, complex color changes occur during aggressive encounters among juvenile Atlantic Salmon. Active, aggressive fish tend to be pale; whereas a nonaggressive, fleeing fish turns pale yellow above the lateral line and gray below, and the parr marks are blurred and there are dark bands on the back. Aggressive fish have a pronounced vertical band through the eye in contrast to the overall dark eyes of submissive fish. Similar visual displays occur in other salmonid genera such as Salvelinus and Oncorhynchus (Puckett and Dill 1985; Grant et al. 1989).
Among cavity-nesting minnows, such as Fathead Minnow, a nesting male will confront an intruding male by performing a lateral display in which the fins are all elevated, similar to that described for salmonids. Rival males tend not to try to usurp the nest of obviously robust, dark-colored males with strong lateral banding, but show a greater propensity to attack males that appear weakened. As with salmonids, if the display fails to dissuade the intruder, the defending fish rams or nips it (Unger 1983). Surprisingly, male Fathead Minnows that have nests in close proximity to other males do not show a loss of body weight or apparent robustness over the breeding season in contrast to males that have solitary nests—even though solitary and group males do not leave the nest to feed. Successful male Fathead Minnows that are in close association with other males use a form of deceit in that they maintain a stable body weight and apparent bulk throughout the nesting period by using water to replace weight lost by depletion of energy reserves and muscle mass. Males nesting in isolation do not do this and thus, without the sham bulking with water, look much less robust, even though both group- and solitary-nesting males have equivalent dry weights and lose dry weight at similar rates. Group nesting males also maintain more intense color patterns. Even deceit has its limits, however, and solitary males generally have longer average nesting periods (19–20 days) than group-nesting males (11 days) (Unger 1983).
Among Everglades Pygmy Sunfish (Elassoma evergladei), dominant males sometimes defend feeding territories when prey are economically defendable (Rubenstein 1981). In addition to actual nipping, males often use lateral displays that are similar to those described previously. As two males approach each other, the caudal fins are spread, the dorsal fins are erect, pelvic fins are fully spread and held perpendicular to the long axis of the body, and the pectoral fins are used to position the fish by actively vibrating. In addition, the dominant fish may raise and lower the dorsal fin while at the same time alternatively moving the pelvic fins up and down. The lateral displays help to communicate the individual’s fighting ability in the absence of actual physical encounters.
Schooling and Aggregation
Visual signals often play an important role in the formation and maintenance of fish schools and aggregations. Fish associations can be formed on the basis of many factors, including familiarity, kinship, species, body size, parasite load, habitat, and diet features, and the choice may be based on chemosensory as well as visual cues (Hoar et al. 2000; Frommen and Bakker 2004; Ward et al. 2007).
Among centrarchids, laboratory experiments show that juvenile Rock Bass (Ambloplites rupestris) apparently use vision to recognize conspecifics (Brown and Colgan 1986). As a consequence, vision is likely important in the formation and maintenance of conspecific associations. Vision is also important in shoaling decisions in Threespine Sticklebacks, in addition to chemosensory cues. Based on European studies, nonreproductive Threespine Sticklebacks use their ability to see in the UV range to choose shoals. Threespine Sticklebacks and European Minnows (Phoxinus phoxinus) also choose shoals with body sizes most similar to themselves (Griffiths 2003; Modarressie et al. 2006).
Courtship and Reproduction
Where conditions permit, visual communication often has important roles in reproduction. Sexual status and quality of mates can be conveyed by colors or body shapes, and the types of signals include movements involved with initiation of courtship and recognition of the partner’s social status (Guthrie and Munz 1993). Visual communication using sexually dimorphic color patterns has been studied intensively in African cichlids and species of Poecilia, especially Guppies in the New World tropics (Endler 1992; Dalton et al. 2010). Among species occurring in North American freshwater, studies of visual communication have emphasized Sticklebacks; Bluefin Killifish (Lucania goodei); Pupfishes (Cyprinodon); and various minnow, sunfish, and darter species (Guthrie and Munz 1993; Foster 1995; Kodric-Brown and Strecker 2001; Fuller and Noa 2010).
The behavior and coloration of Threespine Sticklebacks have been intensively studied since the pioneering work of the German ethologist Niko Tinbergen (1952a, b). Male Threespine Sticklebacks defend a breeding territory, build a tubular nest, and use a combination of coloration and behavioral displays to entice females into the nest for spawning, followed by male parental care of the eggs and yolk-sac larvae and defense of the nest (Tinbergen 1952b; Foster 1994). What has only been understood more recently is the tremendous amount of adaptive variation in the components of the Stickleback courtship display system, as well as other aspects of their biology, in sharp contrast to the earlier view that behavioral components were basically invariant across populations of the same species (Foster 1995). Such variation can occur across small spatial scales, likely in response to changing selective pressures, and is an important reminder of the need to be cautious in making broad generalizations of sensory communication among populations of the same species, and among species within genera and families (Fitzgerald 1993).
Coloration in male anadromous Threespine Sticklebacks typically progresses through four distinct stages during the interval from nest construction, to courting, to spawning, to care of young. During nest construction the male is dull colored, with pale blue eyes, dull red throat, and a medium-gray body, and is similar in appearance to a nonterritorial male (McLennan and McPhail 1989; McLennan 2007). A courting male is bright overall; develops an intense red color across the ventral and lateral surfaces of the head and body; and has bright, cerulean blue eyes. In the latter stages of courtship the male develops a white flush over the body, perhaps as a signal of spawning readiness. Spawning males show a snowy white flush over the whole body so that the red colors are masked. Finally, the nest-tending and nest-guarding male has an increase in melanism, which masks the bright colors and gives the body an overwash of medium to dark gray, except for the blue eyes and the intense red on the throat. Behavioral displays include the zigzag dance, in which the male makes exaggerated movements from side to side during his approach to a female, and dorsal pricking, in which the male presses his dorsal spines into a female’s abdomen during backward-swimming thrusts or a female is positioned with only her snout above the male’s dorsum so that his spines do not actually prick her (Tinbergen 1952a, b; Foster 1995). Dorsal pricking can be initiated by the male or female.
The intensity of the red throat coloration is, at least in some instances, a visual signal communicating male quality to a female. For example, in laboratory experiments of European Threespine Sticklebacks, parasitized males have reduced color intensity and are not selected by females when given a choice between a healthy male and a parasitized male (Milinski and Bakker 1990). In addition, female preference for a male changes when the formerly healthy male is experimentally infected with parasites. The basis of the female’s choice is perhaps based on an indication of a male’s ability to care for young in his nest but could also indicate selection for genes carrying parasite resistance. In contrast, an anadromous population of Threespine Sticklebacks from the St. Lawrence Estuary, Quebec, did not show any relationship between male color intensity and the level of parasite infestation, nor between color intensity and aggressive dominance over other males. Females did prefer to mate with the most brightly colored males, but parasites have apparently not played a major role in the evolution of male nuptial coloration in this population (Fitzgerald et al. 1994).
The nature of the visual courtship signals in Threespine Sticklebacks is also influenced by local predation pressure. Recall that some populations of Threespine Sticklebacks include benthic and limnetic forms that differ in body shapes and foraging modes (Chapter 7). Benthic forms often are cannibalistic, forming foraging groups of several or more individuals (Foster 1994). In these populations, male courtship is less conspicuous, the zig-zag dance is absent or minor, and dorsal pricking is more common (Foster 1995). In addition, dorsal pricking is initiated more often by the female in cannibalistic populations, compared to noncannibalistic populations. Thus differences in foraging mode and cannibalistic tendencies affect the nature of visual signals used in courtship behavior.
Water characteristics have a strong effect on the transmission quality of visual signals. Because high levels of tannins filter out short-wavelength light (400 nm; blues and violets) more strongly than long-wavelength light (reds), the outcome is red-shifted horizontal light in tannin-stained habitats. In such an environment, the red nuptial coloration of male Threespine Sticklebacks would be rendered inconspicuous as seen against a reddish background. As predicted by the sensory drive hypothesis, Threespine Stickleback populations in high-tannin environments generally show an intense black nuptial coloration instead of the normal red color, and the color differences are heritable (Reimchen 1989; Scott 2001). The loss of red nuptial coloration and the development of melanistic males in the Chehalis River, Washington, was first attributed to convergence in threat displays between Threespine Sticklebacks and the Olympic Mudminnow (Novumbra hubbsi), endemic to the Chehalis River, that were thought to be competing for limited space (Scott and Foster 2000). However, studies in both the Chehalis River drainage, and in numerous lakes on the Queen Charlotte Islands, British Columbia, now show that the probability of populations with red nuptial coloration decreases as the level of tannins increases (lower transmission of blue light), while the probability of melanistic populations increases (Reimchen 1989; Scott 2001). Although the results seem to support the sensory drive hypothesis, the pattern could also reflect variation in dietary carotenoid pigments, which are often low in tanninstained water, given that fishes cannot directly synthesize carotenoid pigments but are dependent on their dietary source. If carotenoid pigments are limiting, this could favor the loss of red nuptial coloration (Scott and Foster 2000). The occurrence of melanistic males in water with high transmittance at 400 nm might be due to recent habitat changes, such as clearcutting resulting in the loss of tannin input into the stream (Scott 2001).
Bluefin Killifish, found in both clear springs and tannin-stained swamps in the southeastern United States, show a similar pattern to Threespine Sticklebacks of how sensory drive can impact visual communication. Males have distinctive red, yellow, blue, red-blue, or yellow-blue anal fins that are part of a visual courtship signal to females. Males with blue, or predominantly blue, anal fins are more abundant in tannin-stained swamps where the transmission of UV-blue light is low, thus causing the horizontal light quality to be red shifted. Under these conditions, red or yellow signals seen against a reddish horizontal background would be less visible. Males with predominantly red or yellow anal fins are more abundant in clear springs, where UV-blue penetration is high. Under these conditions, blue visual signals would be less distinct when seen against a bluish background in contrast to red or yellow signals (Fuller 2001, 2002). Female mating preferences also differ between clear and tannin-stained habitats. Females in clear springs show a general preference for males with red anal fins in contrast to females in tannin-stained swamps, which show a preference for males with blue anal fins (Fuller and Noa 2010). Female preference for males with red or yellow anal fins was much more subtle, although in female choice experiments, red males showed a slightly higher spawning success than yellow males, perhaps indicating an innate preference of females for red color. However, there was no effect of rarity on a female’s choice of red or yellow male color morphs (Fuller and Johnson 2009).

FIGURE 10.3. Male nuptial coloration in the Redspot Darter (Etheostoma artesiae).
The two examples of sensory drive emphasize how visual communication systems, in these cases courtship displays, can vary over space and time. For instance, in habitats occupied by Bluefin Killifish, wet years can increase the influx of tannins into streams, resulting in poorer communication of red visual signals and selecting for males with blue anal fins. In dry years, water would be clearer because of a decrease in incoming organic material, resulting in poorer communication of blue signals and selecting for red or yellow anal fins (Fuller and Noa 2010).
Darter species, especially within the large genera Etheostoma and Nothonotus, have an amazing array of color patterns—patterns that typically are shown by males and displayed during the breeding season (Figure 10.3) (Page 1983). This strongly suggests that visual signals based on male color patterns are important in reproduction and are a consequence of strong sexual selection. Somewhat surprising given the large number of darter species, there are relatively few studies that have investigated the role of male color patterns in darters and the importance of sexual selection, and even fewer that have tested the hypothesis that male breeding colors are part of behavioral isolating mechanisms in the evolution of darter taxa (Williams and Mendelson 2010).
Female Rainbow Darters (Etheostoma caeruleum) use visual cues to select males, with the most likely cue being male coloration (Fuller 2003). Sexual selection is also suggested in Splendid Darters (E. barrenense) and Banded Darters (E. zonale), where females use visual cues to select conspecific males when given a choice between conspecific and heterospecific males. Female choice based on male colors also indicates that visual signals are an important aspect of behavioral isolation in these two darter species and perhaps have been a major contributor to the extensive radiation of darters overall (Williams and Mendelson 2010).
Even though sexual selection seems to be responsible for male breeding colors in some darter species, this is not always the case. During the breeding season, male Orange throat Darters (E. spectabile) have orange throats and develop blue and red stripes on body and dorsal fins. However, females do not show any preference for brightly colored males over dull males, nor do they select larger over smaller males. Instead, the male breeding coloration is perhaps related to male-male interactions (Pyron 1995).
The role of visual signals in reproductive behavior is, of course, not limited to color patterns or body size. Females of various species of fishes choose to spawn in nests that already contain eggs, with freshwater examples including darters, minnows, sculpins, sticklebacks, and sunfish (Porter et al. 2002). One way to suggest the presence of eggs is to have egg-mimicking structures or pigments (Knapp and Sargent 1989). Among darters, structures mimicking eggs develop during the breeding season on the first or second dorsal fins of males in most species within the subgenus Catonotus, and on pelvic and pectoral fins in the subgenus Boleosoma. Egg-mimicking pigments, but not structures, develop during the spawning season on the pectoral fins of the Striped Darter (E. virgatum) (and in the subgenus Catonotus) (Page and Bart 1989; Page and Knouft 2000; Porter et al. 2002). Although putative egg-mimicking structures can be present in both sexes, they are most developed in males (Knapp and Sargent 1989). Female Fantail Darters (E. flabellare) prefer to spawn with males that have egg mimics (Figure 10.4) over males that had their egg mimics experimentally removed, and female preferences for males with egg mimics have been shown in other species as well (Knapp and Sargent 1989; Porter et al. 2002). Although knobs on fins have been suggested to have other roles, current evidence indicates that the egg mimics are primarily a visual signal by the male suggesting to the female that he has successfully mated and has eggs in his nest (Bart and Page 1991).

FIGURE 10.4. Egg mimics on the dorsal fin (arrow) of the Fantail Darter (Etheostoma flabellare).
ACOUSTIC COMMUNICATION
Fishes produce sounds to locate and choose mates (potentially important in reproductive isolation), and to show submission or aggressive responses and willingness to fight to competitors for territory, mates, or food (Ladich 1997; Amorim 2006). Although the mechanism of purposeful sound production is different in fishes compared to air-breathing vertebrates, both are similar in that all produce various unintentional sounds as part of their daily lives. For fishes, these include hydrodynamic sounds caused by swimming, pneumatic sounds caused by movement of air along the pneumatic duct in physostomous fishes, stridulatory sounds created by friction of mobile bony elements including jaw and pharyngeal teeth, cavitational sounds produced by the negative pressure gradient inside the mouth during suction feeding, stringed sounds caused by vibrations of stretched tendons in the fins, respiratory sounds caused by regular movements of the opercular bones during breathing, and percussive sounds as a consequence of striking the substratum with the body or fins. Although a basic part of daily life, unintentional sounds can also be preempted for use in signaling and communication (Fine et al. 1977; Kasumyan 2008). Fishes can perceive underwater sounds through the lateral line and through vibrations picked up by the air bladder, in those fishes having one. In addition, fishes can receive vibrations through the substratum (Fine et al. 1977; Whang and Janssen 1994).
Worldwide, specialized sound production is known in some 800 species of marine and freshwater fishes from 109 families (Kasumyan 2008). In addition to the possible use of normally unintentional sounds, purposeful sounds are produced by the contraction of specialized sonic muscles that are attached to the wall of the swimbladder in some species and by specialized bony elements used in stridulatory sounds. Fish sounds typically consist of low-frequency pulses that can vary in duration, number, and repetition rate, and higher states of arousal sometimes are accompanied by more rapid pulse rates. High-frequency sounds, more common in courtship than aggression, are typical of otophysans (North American orders: Cypriniformes, Siluriformes, and Characiformes) because of their specialized hearing mechanism, the Weberian apparatus. Sound repertoires are usually limited to one or two distinct sound types, but there are a number of examples of more complex sounds as well (Ladich 1997; Amorim 2006; Phillips and Johnston 2008a).
Although low-frequency sounds can propagate long distances in deep water, such as the open ocean, low-frequency communication is greatly constrained in shallow water. In addition, shallow water often has a high background noise caused by wind and wave action. The highest background noise in streams is where the water’s surface is broken; smooth runs, even when swift, are likely quieter than riffles. As a consequence, sensory drive is as important in acoustic signals as in the visual signals described previously. Water depth and ambient noise level are strong selective forces on the evolution of acoustic signaling and reception in freshwater fishes (Lugli and Fine 2003). For example, two freshwater goby species living in swift, rocky Italian streams show their maximum hearing sensitivity in a narrow band around 100 Hz and produce low-frequency sounds with most energy from 70 to 150 Hz, in the range of their maximum hearing sensitivity. A window in the ambient noise spectrum of the streams occupied by the gobies occurs at around 100 Hz at most noisy locations. This “quiet window” in the sound frequency spectrum falls between low-frequency turbulent noise and higher-frequency noise from bubbles associated with breaking water. Consequently, there is a match between the quiet window in ambient noise, the frequencies used in sound production, and the greatest hearing sensitivity (Lugli et al. 2003). A similar match between the frequency of the signal and the optimal hearing frequency occurs in Coosa Bass (Micropterus coosae) (Holt and Johnston 2011). Because of the generally high ambient noise and the shallow depth, fish sounds in streams or in the littoral zone of lakes are primarily limited to short-distance transmission on the order of 40–50 cm or less (Lugli and Fine 2003; Phillips and Johnston 2008b).
By far, the most information on sound production and audio communication is for marine fishes. Well-studied freshwater examples include South American and African cichlids, Asian gouramis, and several families of South American catfishes (Fine et al. 1977; Amorim 2006). In North American fresh waters, sound production and sonic communication have been studied in at least 32 species and eight families, including sturgeons, minnows, catfishes, cottids, pupfishes, sunfishes, darters, and drum (Table 10.1). However, this number likely greatly underestimates the actual number of fishes using acoustic communication. For instance, although nine species within the minnow genus Cyprinella are shown in Table 10.1—and a total of 19 examined so far, including unpublished data, have been shown to produce purposeful sounds—it is thought that all the species of Cyprinella (27–30 species) are sound producers (Phillips and Johnston 2009).
With few exceptions, sounds produced by North American freshwater fishes are made in the context of male-male aggression associated with reproduction—especially during male-male encounters over territory and during courtship and spawning (Table 10.1). Sunfishes (genus Lepomis) use sound as part of complex courting and spawning displays. Bluegill (L. macrochirus) and Pumpkinseed (L. gibbosus) both use a grunting or rasping noise, produced by the grinding of pharyngeal teeth, to mark transitions between aggressive and nonaggressive behaviors. The sounds apparently convey to the female the male’s high level of aggression and sexual arousal (Gerald 1971; Ballantyne and Colgan 1978). Calls of Lepomis species vary in duration, pulse rate, duration of sound, and number of sounds produced per unit time (Gerald 1971).
TABLE 10.1Examples of Acoustic Communication in North American Freshwater Fishes
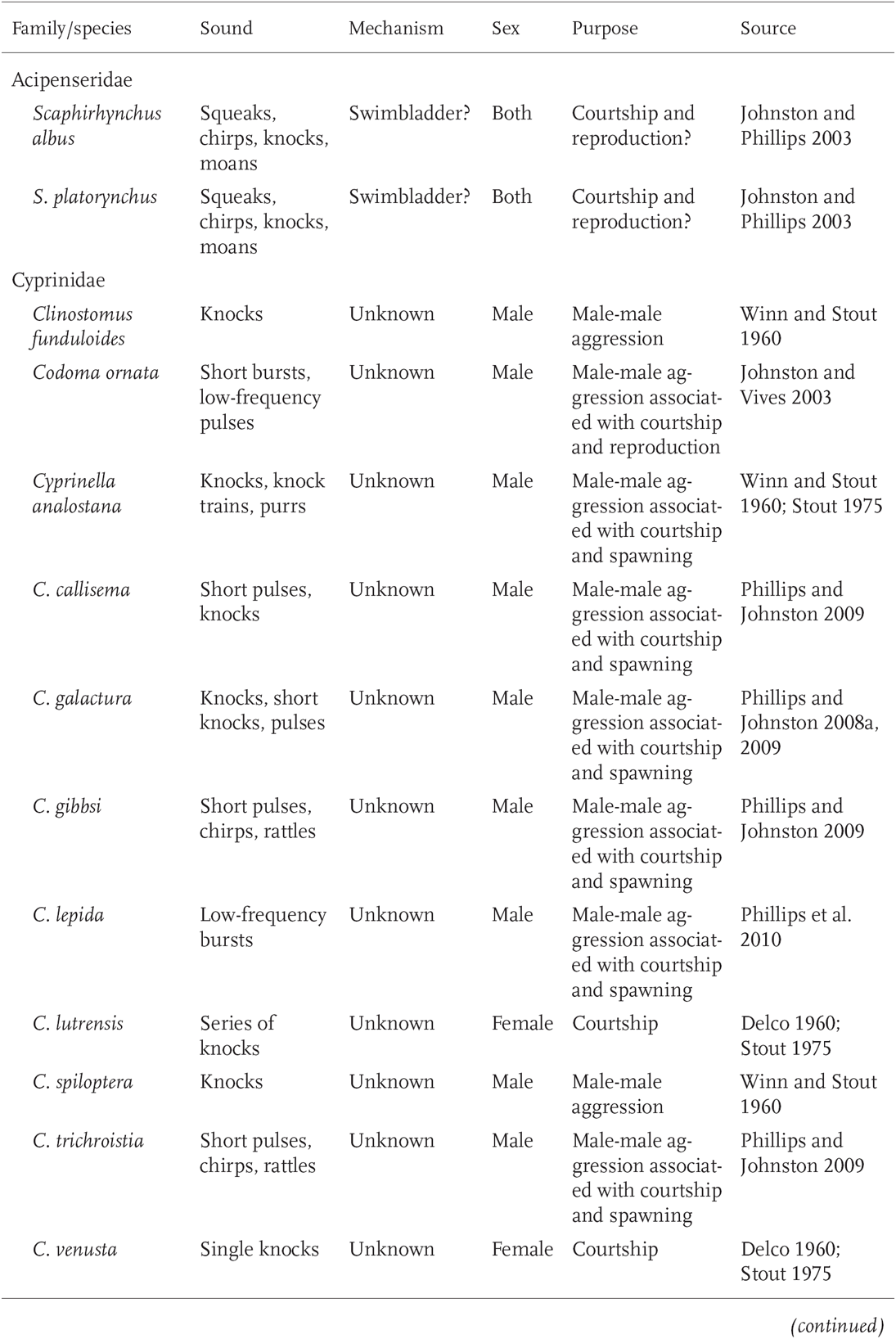
TABLE 10.1 (continued)
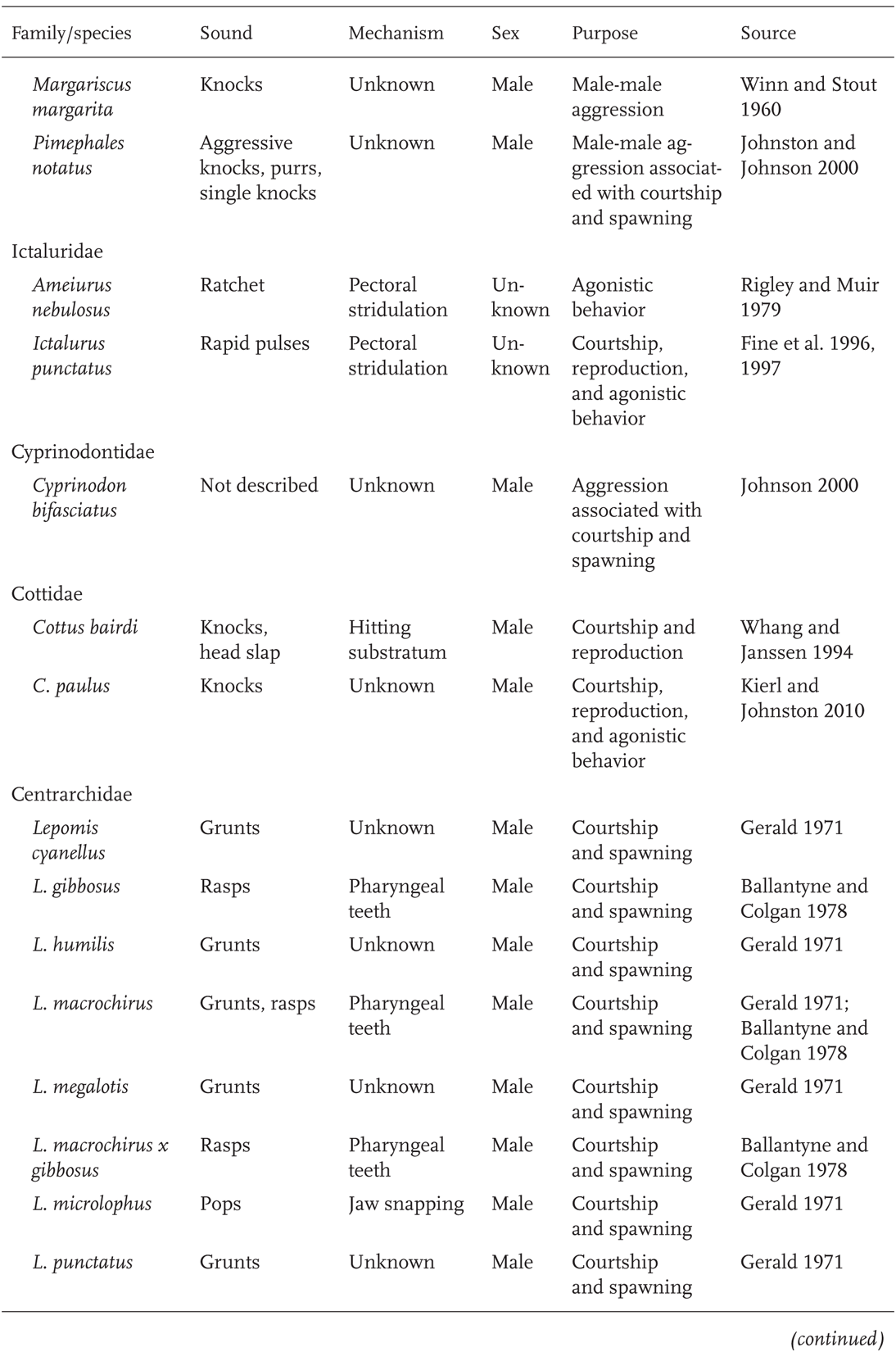
TABLE 10.1 (continued)

Sound production associated with reproduction, including male-male agonistic displays, is common in the shiner genus Cyprinella, as well as related taxa. Male Whitetail Shiner (C. galactura) make complex sounds that are associated with aspects of reproduction, including male-male or male-female aggression and male-female courting. Males vocalize during agonistic behaviors, such as dominance establishment, territory defense, and male-male assessment, but generally do not make sounds during highly aggressive actions such as lip locking and circle swims, perhaps because of competing energy demands. Sound production is also important in recruitment of females to a nesting site (Cyprinella are crevice spawners) and mate attraction displays, and to a lesser extent prior to and during spawning. Sounds made during courtship had higher dominant frequencies than those made during aggressive behaviors. Because higher-frequency sounds should attenuate more rapidly, calls used in courting might be more short range than aggressive calls (Phillips and Johnston 2008a).
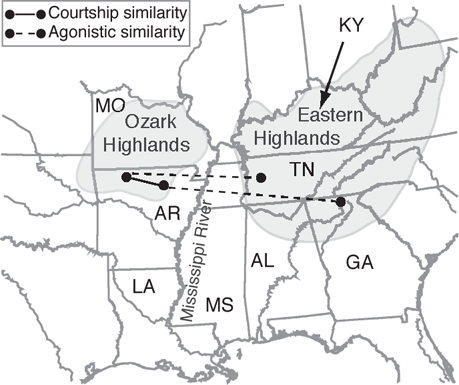
FIGURE 10.5. Geographic variation in courtship and agonistic calls in the Whitetail Shiner (Cyprinella galactura). Circles indicate approximate locations of the four populations. The Ozark Highland populations were more similar in courtship signals compared to those in the Eastern Highlands; adjacent populations grouped with more distant populations in agonistic signals Based on Phillips and Johnston (2008b).
Whitetail Shiners have a disjunct distribution occurring west of the Mississippi River in streams of the Ozark Plateau and Ouachita Mountains of Arkansas and Missouri, and in streams east of the Mississippi River including the Cumberland and Tennessee river drainages (Figure 10.5) (Gilbert and Burgess 1980; Mayden 1989). Although all populations share the same acoustic repertoire, calls varied among populations, primarily in pulse rate, pulse duration, and pulse interval. Courtship signals were more stereotyped than agonistic signals, and the Ozark populations showed greater call similarity compared to the more distant Eastern Highlands populations in the Tennessee drainages. Because they are used in mate recognition, courtship signals are more likely under stabilizing selection compared to agonistic signals, which may be more affected by genetic drift (Phillips and Johnston 2008b).
Microhabitats occupied by stream-dwelling darters can vary greatly in the level of background noise. Fringe Darters (Etheostoma crossopterum) occupy quiet pools with little or no flow, and the males make drumming and knocking sounds associated with male-male aggressive encounters during the reproductive season. The dominant frequencies of aggressive sounds vary rather widely from 78 to 496 Hz (mean = 151). Because the ambient background noise shows a quiet area in the range of 90–390 Hz, Fringe Darters are reducing the signal-to-noise ratio of their calls by generally fitting them within the quiet area. Sounds produced by Fringe Darters also include higher-frequency harmonics. Fantail Darters occupy fast-moving riffles, among the noisiest of aquatic habitats, and, as with Fringe Darters, produce drums and knocks during male-male aggression. In the riffles, the quiet window of ambient noise ranges from 150 to 330 Hz and the dominant frequencies of the aggressive sounds range from 172 to 374 Hz, again closely matching the quiet window. However, in contrast to Fringe Darters, Fantail Darter sounds generally lack harmonics, the higher frequencies of which would be masked in their noisier environment. Consequently, differences in signal characteristics of the two darter species, both in the subgenus Catonotus, seem to be shaped by the ambient noise spectra of their environments (Speares et al. 2010).
SUMMARY
Communication is an integral part in the daily lives of North American freshwater fishes. Among other roles, communication is involved in establishment and maintenance of dominance relationships and territories, mate attraction and selection, spawning, maintenance of social groupings, migration, and avoidance of predation. As with other organisms, including humans, true communication requires both a signaler and a receiver. The evolution of communication systems is the outcome of competing demands of maximizing signal value versus excessive risk of predation to an individual or to its young. Sensory drive refers to all processes causing biases in the evolution of communication systems, such as the constraints on the evolution of signal production by the nature of the receptor system, the potential for “eavesdropping” on signals by predators or competitors, and the impact of the environment on transmission and signal quality. North American freshwater fishes use chemical, visual, and acoustic means of communicating, with each system having advantages and disadvantages. Chemical signals received via olfaction or taste are widely used, in part because water is an excellent solvent for chemicals associated with living organisms. However, the transmission and persistence of chemical signals are influenced by the amount and direction of water flow. A specific class of chemical signals, pheromones, are secreted to the environment by the signaler and received by a conspecific in which they elicit a specific adaptive response. Pheromone-signaling systems are particularly important in reproduction, including mate attraction, courtship, and spawning, in a variety of fish groups. Pheromones are also involved in communicating social status and individual identity, the latter being linked to the evolution of MHC genes. Chemical communication, or sometimes spying, is also important in migration.
Visual communication is also widely used in fishes in territory defense, aggressive displays, courtship, spawning, and maintenance of schools and aggregations, and is advantageous because of the quantity of information that can be conveyed. However, visual communication is also strongly influenced by environmental conditions. As light passes through water, it is affected by the distance it travels and by what is in the water. In the highly tannin-stained waters of marshes, swamps, and certain lowland rivers, the shorter wavelengths, including blues and violets, are rapidly attenuated in contrast to the longer wavelengths of browns, yellows, oranges, and reds so that horizontal light is strongly red-shifted. One effect of this is that yellows and reds that are used in visual signals in clear water become less visible in the reddish background of tannin-stained water. Because of the strong linkage of visual systems with water quality, the nature of signals and their efficacy can vary both temporally and spatially within species.
Acoustic communication is perhaps the least known communication modality of North American freshwater fishes. Recently, the importance of acoustic communication, especially in behaviors associated with reproduction, has been demonstrated in a diversity of groups but especially in minnows, sunfishes, and darters. Purposeful sounds can be produced by the scraping of bony elements against each other, called stridulatory sounds, or by sounds associated with the swimbladder, either by passage of air through the pneumatic duct or by muscles attached to the swimbladder. In the majority of species, the actual mechanisms of sound production remain unknown. As predicted by the sensory drive hypothesis, the frequency of acoustic signals can be matched to optimum receptor frequency and to quiet areas in the background sound spectrum of the environment.
SUPPLEMENTAL READING
Burnard, D., R. E. Gozlan, and S. W. Griffiths. 2008. The role of pheromones in freshwater fishes. Journal of Fish Biology 73:1–16. A current review of pheromone research.
Fine, M. L., H. E. Winn, B. L. Olla. 1977. Communication in fishes, 472–518. In How animals communicate. T. A. Sebeok (ed.). Indiana University Press, Bloomington. An excellent background paper for understanding fish communication.
Ladich, F., S. P. Collin, P. Moller, and B. G. Kapoor. 2006. Communication in fishes. Vol. 1 and 2. Science Publishers, Enfield, New Hampshire. A current review of fish communication.
Fuller, R. C., and L. A. Noa. 2010. Female mating preferences, lighting environment, and a test of the sensory bias hypothesis in the Bluefin Killifish. Animal Behaviour 80:23–35. A fascinating study of the interrelationship of water quality, nuptial coloration, and the role of genetics and phenotypic plasticity in female mate choice.
Speares, P., D. Holt, and C. Johnston. 2010. The relationship between ambient noise and dominant frequency of vocalizations in two species of darters (Percidae: Etheostoma). Environmental Biology of Fishes 90:103–10. An intriguing study of how quiet windows in ambient sound levels shape the frequencies of fish vocalizations.
WEB SOURCES
Fish sounds, Dr. Carol Johnston, Auburn University. http://www.ag.auburn.edu/fish/about/facilities/ichthyology/sound-production.