ELEVEN
Interactions in Resource Acquisition I
NICHES, COMPETITION, AND TROPHIC POSITION
CONTENTS
Resource Limitation and Competition
Resource Partitioning and Other Observational Studies
Opportunistic Distributions and “Natural Experiments”
Field and Mesocosm Experiments
Food Webs, Trophic Position, and Competitive Exclusion
THE OCCURRENCE AND PERSISTENCE of individuals and populations depend on acquiring the spatial or trophic resources, all related to energy acquisition, required by each life-history stage. Potential impacts of changes in resource availability were covered in Part 2, and the functional morphological adaptations involved in obtaining food or using particular habitats were covered in Part 3. This chapter focuses on possible interactions involved in obtaining trophic or spatial resources, resource linkages in fish assemblages (i.e., food chains and food webs), and trophic positions. The rate of evolution of morphological structures and behaviors associated with feeding is in part influenced by competitive interactions among species (Liow et al. 2011), hence the close association between material in this chapter with chapters in Part 3. For instance, among darters in the genus Percina, the rate of evolutionary change in morphological structures associated with feeding is correlated with the number of co-occurring congeners, albeit the evidence does not identify whether greater co-occurrence of Percina species is a cause or consequence of the greater rate of morphological change (Carlson et al. 2009).
CHEMICAL ECOLOGY
Historically, the principal means of understanding energy sources, food web relationships, and trophic positions of fishes has been through direct analyses of stomach or intestinal contents (i.e., gut contents), and through visual observations of feeding. Such analyses provide resolution of taxa contributing to the diet and, depending on the study, can be used to determine spatial and/or temporal variation in resource use (Hynes 1950; Windell 1971; Bowen 1996). Over the last several decades, and especially since 1995, a new approach, stable isotope analysis (SIA), has been added to studies of trophic position, linkages, and trophic resource use (Box 11.1) (Peterson and Fry 1987; Dalerum and Angerbjörn 2005; del Rio et al. 2009). SIA and direct approaches to diet, such as gut analysis or visual observations of feeding, are best considered as complementary tools for understanding food habits, trophic position, and food webs. Direct approaches provide information on recently consumed food items, and, because ingested prey are generally killed whether they are eaten and assimilated or not, these direct approaches are perhaps better at showing the impact of predation on ecosystems (Franssen and Gido 2006). In contrast, SIA potentially provides time-integrated dietary information on prey items that are assimilated, if the food is allocated to the tissue being analyzed, and does not require identification of gut contents (Peterson and Fry 1987; Perga and Gerdeaux 2005).
BOX 11.1 • Stable Isotope Analysis (SIA)
By the 1980s, stable isotope ratios, first employed by geochemists, were starting to be appreciated by ecologists as another important tool in understanding complex ecological processes. Stable isotope ratios can provide clues about sources and transformations (fractionation) of organic matter, which in turn leads to inferences about habitat use, nutrient sources, food webs, and trophic position (Fry and Sherr 1984; Peterson and Fry 1987). Most simply, isotopes are mass unit variations of elements, such as 13C and 12C, and stable isotopes are those that do not decay over time (Jardine et al. 2003). Ecological studies primarily use isotopes of carbon, nitrogen, and sulfur. The common isotope of carbon is 12C (98.89%) and the rarer isotope is 13C (1.11%). The most abundant nitrogen isotope is 14N (99.64%) and the rarer isotope is 15N (0.36%). Sulfur exists in four stable isotopic forms, the most common of which are 32S (95.02%) and 34S (4.21%). Isotope ratios (amounts of heavier/lighter isotopes) are determined from mass spectrometry and are reported relative to established standards in the general format of
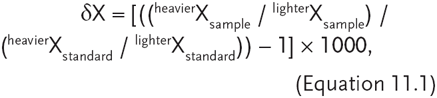
where δ refers to ± differences from known standards and X refers to a particular isotope (Cabana and Rasmussen 1996; Jardine et al. 2003).
Fractionation means a change in the ratio of heavy to light isotopes and usually arises when similar molecules of slightly different mass react at different rates. The resulting isotopic distributions reflect reaction conditions (process information) such as links in a food web. If fractionation is low or minimal, stable isotope distributions provide information on the origin of samples (source information), such as the origin of terrestrial or aquatic primary production that might be supporting a particular population. No change with increasing trophic level occurs with sulfur, so sulfur isotopes are good indicators of plant or bacterial sources of food and are used to track anthropogenic sulfur in sediments and food webs. Enrichment of the heavier carbon isotope (13C) in trophic level transfer, such as from primary producer to primary consumer tissues, is low (usually in range of one part per thousand [‰] and typically 0.2‰ for freshwater consumers). Consequently, δ13C also is used for source information (Peterson and Fry 1987; Jardine et al. 2003).
In contrast, the heavier nitrogen isotope (15N) is enriched in consumer tissues from 3 to 5‰ relative to the diet because the lighter isotope is preferentially excreted (Peterson and Fry 1987; del Rio et al. 2005). As a result of the fractionation difference between carbon and nitrogen isotopes, δ15N is generally used as a time-integrated measure of trophic level among populations of the same consumer species (i.e., process information). There is the potential for local variation in the 15N value, so that it is sometimes important to adjust for between habitat variation of primary producers or other organisms that are in low trophic levels. Overall, δ15N provides a measure of food-chain length related to bottom-up mass transfer and, in contrast to food chain lengths based on stomach content analyses, does not require detailed taxonomic information on all species present in the food web. In addition, some trophic links based on food habits can be of negligible importance in terms of mass transfer because, although the items are eaten, they may not be assimilated or may be only slightly assimilated (Peterson and Fry 1987; Cabana and Rasmussen 1996; Jardine et al. 2003).
Although direct approaches to dietary analysis show recently consumed items, and SIA provides a long-term view, there is much variability in how long a time period is actually integrated by SIA. The time required for isotopes in fish tissues to reflect changes in isotopes of food sources is related to rates of metabolic replacement and growth. Whereas new growth reflects the isotopic values of current food items, turnover in existing tissues through metabolic replacement can be much slower. The turnover of isotopes in fish tissues is related to the rate of protein turnover, which in turn is related to the specific metabolic rate of each tissue and allometrically related to the body size of the animal. In general, structural elements such as collagen, striated muscle, and red blood cells should have lower rates of isotopic incorporation compared to visceral organs, such as the liver, the digestive tract, and plasma proteins. Also, protein intake generally has a positive effect on protein synthesis and thus the isotopic incorporation rate. The degree that different tissues reflect dietary isotopic ratios is also variable and can be related to lipid content and amino acid composition. Consequently, each tissue potentially differs in the time lag before it reflects dietary isotope values, and each tissue can provide a different measure of dietary isotopes (Tieszen et al. 1983; Peterson and Fry 1987; Dalerum and Angerbjörn 2005; del Rio et al. 2009).
In general, changes in isotopic ratios of tissues require 3–12 months or longer before they reflect changes in diet, depending on species and the tissue being analyzed (Jardine et al. 2003). In Swedish lakes, Roach (Rutilus rutilus), Perch (Perca fluviatilis), and Bream (Abramis brama) all showed an approximate three-month lag before the δ15N value of consumer tissue was highly correlated with the diet as determined by gut analysis (Persson and Hansson 1999). A one-year feeding experiment using a Canadian population of Broad Whitefish (Coregonus nasus) determined how fast sulfur, carbon, and nitrogen isotope ratios responded to a change in isotopic ratios of the diet (Hesslein et al. 1993). Most of the change in SIA following the change in isotope ratios of the diet was due to growth rather than metabolic replacement, and, in contrast to the Swedish study, the half-life (the median residence time in the tissue) for metabolic replacement of sulfur, carbon, and nitrogen isotopes in muscle tissue was greater than one year.
The importance of growth periodicity on tissue changes in stable isotopes is emphasized by a study of European Whitefish (Coregonus lavaretus) in Lake Geneva (Perga and Gerdeaux 2005). Growth occurs from March to September and the study tested predictions that skeletal muscle isotopic composition would more closely reflect isotopic values of prey, primarily zooplankton and benthic chironomids, during the growth period, compared to the liver isotopic value, which would reflect current prey and thus show greater annual variability. The isotopic composition of prey varied continuously throughout the year and isotopic turnover in European Whitefish tissues, based on the δ15N value, occurred in liver tissue after one month, but took 4–5 months for muscle tissue. The results supported the prediction that the isotopic ratio of liver tissue would be more variable than for muscle tissue, with carbon and nitrogen isotopes in the liver having three times the variation of those in muscle tissue. Overall, the results suggested that the δ13C portion of the diet was not being incorporated into muscle tissue in autumn and winter. European Whitefish muscle δ15N varied during the winter, but some variation was likely caused by the fish catabolizing tissue proteins and not fully related to changes in the isotopic ratio of the prey. Consequently, dorsal muscle tissue provides a time-integrated image of the isotopic composition of food consumed during the period of active growth (March to September in Lake Geneva). Feeding does occur in the winter but is allocated to basal metabolism and reproduction, emphasizing how isotopic ratios of different consumer tissues are affected by how nutrients are routed in the body once assimilated. The take-home message from this study is that “isotope composition of muscle cannot provide reliable information about the food consumed during seasons when there is no somatic growth” (Perga and Gerdeaux 2005).
Stable isotope analysis can integrate a rather long (well more than one year) feeding period, especially in slow-growing fishes or populations, and the response time can also vary greatly among consumer tissues. It is also important to recognize that isotopic variation can be caused by various factors other than a change in the diet of the focal species. It can be due to a dietary change of the prey species, without any change in the diet of the focal species, or even by changes occurring several trophic levels removed from the focal species (Dalerum and Angerbjörn 2005).
Thus depending on the tissue analyzed, SIA integrates long-term assimilation of nutrients and does not necessarily reflect short-term feeding patterns. The latter would only occur if organisms tended to specialize on particular food types rather than feeding opportunistically. For instance, the general correspondence of SIA and gut content analysis in Northern Pike (Esox lucius) inhabiting various boreal lakes indicates that short-term feeding may not be simply opportunistic but that it reflects long-term individual differences so that some individuals are longterm invertebrate specialists whereas others are long-term fish specialists (Beaudoin et al. 1999).
RESOURCE LIMITATION AND COMPETITION
In many instances, access to resources, or their availability, can be strongly influenced by individuals of the same or different species. Competition occurs when the negative interactions depress individual fitness, population growth rates, or overall population sizes of the two groups (individuals, populations, and species). In freshwater fishes, this can occur primarily through the use, and consequent depletion, of shared resources, or by the aggressive responses of individuals that might keep others from having access to resources. In the first case, resources are limiting by their common use by the same (intraspecific) or different (interspecific) species, without active interactions between the individuals. This is referred to as exploitation competition, or sometimes as consumptive or passive competition, such as the common use of drifting insects by several species of fishes. In contrast, when resources are limited because of access to them, such as when the defense of a feeding or nesting territory by the territory holder keeps other individuals from using the resources, interference competition takes place; this is also known as active competition (Schoener 1974; Pianka 1988; Begon et al. 1996). Interference competition can be subdivided into territorial competition, in which an individual aggressively defends, or shows the potential to defend, some spatial resource against other individuals, and encounter competition, in which interactions among mobile individuals results in some type of harm, such as lost time or energy, theft of food, or injury or death (Schoener 1983). A third type, preemptive competition, is recognized as separate from exploitation and interference, although it shares elements of each.
In preemptive competition, some type of space, such as a nesting site or foraging site, is passively occupied by an individual so that other individuals do not occupy the space unless the occupant leaves. This is similar to exploitation because the shared use of a resource pool (such as all the potential nesting holes), limits overall resource availability, but unlike units of food, units of space can be reused. Because preemptive competition also includes avoidance of use, it fits the category of interference as well (Schoener 1983; Gotelli 2001).
Importantly, although in at least some cases the potential for competition increases with the overlap in resource use, high overlap by itself does not indicate that competition is occurring. The key aspect is whether the resources in question are in limited supply or the organisms show evidence of negative interactions, such as reduced growth, survival, or reproductive success (Wiens 1977; Schoener 1983; Begon et al. 1996). Competition is usually viewed as being symmetrical, with both sides affected negatively; however, as shown later in this chapter, the impact of competition, although still a negative response, is not necessarily equal on both sides; often one group of competitors is affected much more strongly than the other.
The presence and importance of competition in fish assemblages have been assessed through four principal means. Most of the early studies of resource use and the potential for competition occurred via observational field studies of resource partitioning (which cannot prove the existence of competitive interactions, but can show the potential for competition if large overlaps occur in resource use and if resources are limiting), and through so-called “natural experiments,” including character displacement, in which different combinations of species occur in relatively close proximity. Manipulative field studies and/or laboratory experiments have also been important in assessing the role of competition in fish assemblages. Although all approaches have their strong and weak points, perhaps the strongest approach is a combination of well-designed observational studies that suggest important hypotheses that can then be tested by properly designed field or laboratory experiments.
Ecological Niches
The niche concept is central to ecology, and niche terminology continues to pervade the ecological literature. For instance, one-third of the papers in the 2010 issues of Ecology, one of the leading ecological journals, mentioned the term niche. The principal issue with the niche “concept” is that there is not one concept—in fact many different niche concepts have been described, some appropriate to population studies and others to community-level studies, so that an indication of what concept is being applied is needed (Box 11.2) (Hurlbert 1981). In addition, attempts to synthesize niche concepts have tended to oversimplify usages, further muddling the understanding of niches (James et al. 1984). Finally, most niche concepts theoretically require determination of a species’ position (use or fitness) along a large number of resource axes, something that is logistically difficult or impossible (Godsoe 2010).
BOX 11.2 • Historical Development of Niche Concepts
Identifying the first appearance of a scientific term is often fraught with challenges and the ecological niche is no exception. The first use of niche in an ecological sense was in 1910 by an experimental geneticist, Roswell H. Johnson, writing about limits to the distribution of ladybugs (Gaffney 1975; Hutchinson 1978). However, Johnson did not use the term again and his first mention of niche apparently escaped notice by his contemporaries (Cox 1980). The strongest candidate for being the father of the ecological niche concept is Joseph Grinnell, a towering figure in vertebrate biology at the University of California in the late nineteenth and early twentieth centuries. In a paper on the distribution of a small bird, the Chestnut-backed Chickadee, Grinnell (1904) essentially described the role of niche overlap and resource competition in limiting species’ distributions, although he did not use the term niche. His first use of the term “ecological niche” occurred in a 1913 paper published with H. S. Swarth on distributions of birds and mammals at the limits of their ranges in the San Jacinto mountains of Southern California (Cox 1980). Grinnell further refined his use of ecological niche in two papers published in 1917, stating for instance, “It is of course, axiomatic that no two species regularly established in a single fauna have precisely the same niche relationships” (Grinnell 1917a). It is clear from this paper, and also from Grinnell (1917b), that he was not just equating niche with habitat or place as some recent authors have proposed, but that his view included food, climate, habitat, and so on. Thus, in these and later papers, he used niche to designate the place in an association of biotic and physical factors occupied by a single species. In essence, the Grinnellian niche comprises “the range of values of environmental factors that are necessary and sufficient to allow a species to carry out its life history” and determination of these values requires studies of variation in resource use among populations over the geographical range of the species (James et al. 1984).
Charles Elton, another leader in ecological thinking in the early and mid-twentieth century, first used the term ecological niche in a 1924 paper where he referred to lemmings as occupying the same ecological niche as mice and rabbits of lower latitudes (Cox 1980). Elton viewed the niche as an organism’s “place in the biotic environment, its relations to food and enemies” (Elton 1927). Consequently, his was more of a functional concept; in particular, he tended to emphasize the position of an organism in a food chain, and the niche was not necessarily concerned with competitive exclusion. Other contributors to the niche concept include the Russian ecologist Georgii Gause who formally developed the concept of the competitive exclusion principle, although naturalists, such as Grinnell and Darwin, had already expressed similar thoughts that two species with identical niches could not coexist (Kingsland 1985). Thus the niche became “a unit structure over which species fought for possession” (Gause, in Kingsland 1985). Gause’s work stimulated G. E. Hutchinson and later his student Robert MacArthur to look more closely at ecological relationships of organisms to see how resource separation might occur.
Hutchinson (1957b) proposed a formal definition of the niche as being an n-dimensional, geometric space or hypervolume. Within the hypervolume an individual or population could survive indefinitely. In spite of challenges offered by the Hutchinson’s hypervolume concept, it greatly stimulated a quantitative approach to studies of the niche (Hurlbert 1981). Hutchinson viewed the preinteractive, or fundamental, niche as the entire set of conditions under which a given organism can live and replace itself, and the interactive, or realized, niche as the actual set of conditions under which an organism exists, as influenced by interactions of other species. As envisioned by Hutchinson, the realized niche volume is always less than the fundamental niche. However, the concept of facilitation (see Chapter 13) alters this view because it shows that neighboring species do not necessarily have a negative impact. When neighboring species have a positive impact, this can lead to the paradox that realized niches are larger than fundamental niches (Bruno et al. 2003).
Actual measurement of niche volume is problematic. The Hutchinsonian niche is basically a synecological concept, determined among species within a single community or several communities, and reflecting the interactions of other species. As such, it would be a realized niche. The Grinnellian niche is an autecological concept, being determined among many different populations of a species with many different realized niche states; it comes closest to approximating the fundamental niche (James et al. 1984).
Recently, the multitude of niche concepts have been distilled down to three: the recess/role niche, the population-persistence niche, and the resource-utilization niche (Schoener 2009). The recess (i.e., cubbyhole)/role niche has its major focus on the environment and corresponds with Grinnell’s autecological use of the ecological niche in that a species has a set of behavioral, physiological, and morphological adaptations for exploiting particular food and habitat resources in a community. If such resources are not used, then the recess niche would be empty. This concept also leads to the consideration of ecological equivalents—different species in disparate localities that have the same or similar niche characteristics.
The population-persistence niche has its focus on species’ populations rather than the environment. It emphasizes interactions among and within species, especially competition, and corresponds closely with the multidimensional Hutchinsonian niche. With this concept, the niche is a property of the species so that there can be no empty niches. The synecological population-persistence niche offers a quantitative way to characterize niches, with a focus on macrohabitats, and lends itself well to multivariate statistical approaches (Green 1971; Hutchinson 1978).
The resource-utilization niche has its origin in an influential paper by Robert MacArthur and Richard Levins (1967). It is also quantitative and multidimensional, but rather than focusing on fitness per se, this concept emphasizes how organisms are actually using resources and the limiting similarity among niches to allow for coexistence of species. The relative use of a particular resource can be plotted along an axis—similar to the Hutchinsonian niche (Box 11.2), but with the response variable being relative use instead of fitness (the latter being much more difficult to measure). This concept is also similar to the Grinnellian niche in terms of what is being measured; the difference is that Grinnell was focusing more on what the environment offered, rather than resources used by an organism (Schoener 2009). Recent approaches to niche modeling using GARP or other algorithms (see Chapter 4) are based primarily on Grinnellian and resource-utilization niche concepts because they quantitatively describe resources used by a species, allow for empty niches, and can be used to examine limiting similarity. They include aspects of the population-persistence niche in that both usually (but certainly not always) focus on the macrohabitat scale.
Compared to vertebrate organisms with extended parental care so that young are able to reach near-adult size before actively competing on their own, most fishes are actively foraging over several orders of magnitude in body size, with a concomitant wide range of food size and kind, and often progress through a range of habitats as they increase in size. Thus niche width, or the breadth of resources used by an organism throughout its life (the ontogenetic niche), would be much greater in fishes compared to most other vertebrates. This would seem to be especially true along the food-size axis, so that resource separation among species along trophic axes should be less common (Werner 1977; Werner and Gilliam 1984). However, the prediction of reduced importance of trophic separation in fishes is not well supported, perhaps because studies of resource partitioning have not addressed the full age/size range of species (Ross 1986).
Resource Partitioning and Other Observational Studies
Resource partitioning refers to how organisms within an ecological community differ in their use of resources and derives from the basic questions of how species coexist and how many species can occur together (Ross 1986; Schoener 1974, 1986). During their initial popularity, the basis for studies of resource partitioning was to determine the limiting bounds placed by interspecific competition on the number of stably coexisting species (Schoener 1974). More recently, the importance of competition as the force behind observed differences in resource use is not accepted a priori (Schoener 1986).
Observational studies of resource partitioning in North American freshwater fishes have been based about equally on habitat, food, and temporal (seasonal and diel) variables (Ross 1986). An early quantitative study of spatial resource separation examined habitat use by fishes greater than about 20 mm TL in Lawrence Lake and Three Lakes, two small Michigan lakes. The common fish species showed horizontal (distance from shore) differences in occurrence as well as vertical (water column position) differences. Green Sunfish (Lepomis cyanellus) occurred closest to shore in shallow water, followed by Longear Sunfish (L. megalotis) and Pumpkinseed (L. gibbosus) in slightly deeper water and normally within a half-meter or less from the bottom. Minnow species and Yellow Perch occurred more in the water column but still in water ≤ 1 m deep, and Largemouth Bass (Micropterus salmoides) and Bluegill (L. macrochirus) occurred farthest from shore in deeper, more open water (Werner et al. 1977). Based on other studies, these species also show separation by food kind and size. For instance, among the centrarchids, Bluegill feed on small invertebrates primarily using suction feeding, Largemouth Bass feed on larger prey using both ram and suction feeding, and Green Sunfish use prey intermediate in size between Largemouth Bass and Bluegill (see Chapter 8). Both Pumpkinseed and Longear Sunfish are benthic specialists, but only Pumpkinseed has enlarged molariform teeth allowing it to feed on heavybodied mollusks. There are also temporal differences in feeding, with Black Crappie (Pomoxis nigromaculatus) primarily being nocturnal predators on a broad range of prey occurring in the open water column (Werner et al. 1977; Ross 2001).
Observational data also show evidence of strong habitat separation among stream fishes. For instance, eight minnow species in a section of a southeastern, blackwater stream separate along a habitat dimension that primarily reflects their association with aquatic vegetation. Bluenose (Pteronotropis welaka) and Flagfin (P. signipinnis) shiners have greater association with submerged aquatic vegetation, and Cherryfin (Lythrurus roseipinnis) and Blacktail (Cyprinella venusta) shiners are less associated with vegetation. Species most similar in terms of association with vegetation show separation along the second resource axis of water column position (Figure 11.1). The only species pair that was essentially the same along the two resource dimensions differed in time of feeding activity, with the Longnose Shiner (Notropis longirostris) feeding during the day and the Longjaw Minnow (N. amplamala) feeding primarily at night (Baker and Ross 1981). Temporal niche separation could occur if resources, primarily aquatic insect drift in this case, were replenished between day and night, although whether the complementarity in foraging time is actually related to resource limitation is unknown (Ross 1986).
Vertical habitat segregation of fishes seems to be common and, as the previous studies have suggested, potentially could be a result of interactive segregation. However, such differences could also be the consequence of phylogenetic history or other noninteractive factors, such as individual-based optimal foraging. For instance, nine species (all in different genera) in a 37-m section of an Appalachian stream, Coweeta Creek, North Carolina, segregated into a water column guild (five species) and a benthic guild (four species) (Grossman and Freeman 1987). Guilds were stable seasonally and annually, and guild members apparently did not differ in habitat use. The differences in microhabitat use between guilds were attributed to common selective pressures or to phylogenetic constraints on the species and not to ongoing biotic interactions, with the possible exception of Warpaint Shiner (Luxilus coccogenis) and Rosyside Dace (Clinostomus funduloides). However, in a later paper, individual-based optimal foraging models better explained habitat differences between these two species than did interspecific interactions (Grossman et al. 2002).

FIGURE 11.1. An estimation of realized niche shapes for eight species of southeastern cyprinids using multiple discriminant analyses. Based on Baker and Ross (1981); used with permission from Ross and Matthews (in press).
Water column fishes in Coweeta Creek show high overlap in microhabitat use, suggesting two possibilities—resources might not be limiting or that species might be competing for food (Grossman and Freeman 1987). The importance of competition in structuring microhabitat use of fishes comprising the water column guild of Coweeta Creek were further studied by underwater observations, with the major focus on the introduced Rainbow Trout (Oncorhynchus mykiss) and the native Rosyside Dace, the most abundant water column species in the assemblage (Freeman and Grossman 1992). Rosyside Dace spent less time in feeding areas and had lower feeding rates when Rainbow Trout were present compared with when they were absent. The Rainbow Trout were too small to act as predators and, furthermore, rarely were overtly aggressive toward the dace. The presence of Rainbow Trout apparently lessened the value of a particular feeding site for Rosyside Dace, perhaps by depleting local food resources. In contrast, Rosyside Dace entered and left foraging sites independent of other cyprinids and aggression was rare (Freeman and Grossman 1992). The impact of Rosyside Dace on Rainbow Trout was analyzed using a laboratory stream system. In this study, Rosyside Dace did not impact habitat selection by Rainbow Trout (Grossman and Boulé 1991). A priori, the overall results suggest asymmetrical, exploitative competition of Rainbow Trout on Rosyside Dace; however, the presence of exploitative competition would depend on the availability and the foraging value of alternative sites—that is, are Rosyside Dace simply moving away from Rainbow Trout without suffering a loss in energy intake. The fact that both species seem to be foraging at water velocities close to their energetic optima supports this, although whether or not Rosyside Dace suffer a fitness penalty in the presence of Rainbow Trout (thus indicating competition) has not been tested (Freeman and Grossman 1992).
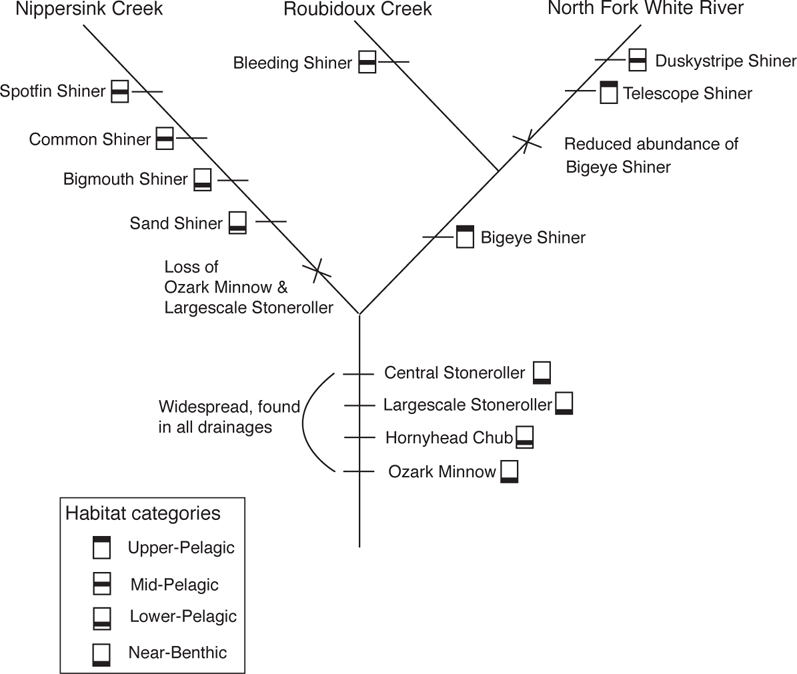
FIGURE 11.2. The importance of evolutionary history in the interpretation of ecological characters. Illustrated by an area cladogram of three streams and showing predominant minnow species overlain with their water column positions. Modified with permission from Gorman 1992; copyright Leland Stanford University.
Vertical habitat segregation is also apparent in a suite of minnow species in two streams of the Ozark Highlands, Roubidoux Creek and North Fork White River (see Chapter 3; Figure 3.5) and one in northern Illinois, Nippersink Creek (Gorman 1992). However, in addition to field observations, Gorman also included laboratory studies of habitat preferences and interactive habitat, and examined historical influences on ecological patterns using phylogenetic relationships of the minnows and an area cladogram showing relationships of the streams the minnows inhabited (Figure 11.2). Four species (Central Stoneroller, Campostoma anomalum; Largescale Stoneroller, C. oligolepis; Hornyhead Chub, Nocomis biguttatus; and Ozark Minnow, Notropis nubilus) occur in all three streams and, based on the area cladogram, composed part of the ancestral fauna. All of these species are bottom or near-bottom inhabitants that shift little in vertical water column position in the presence of other species. In contrast, species occupying the middle and upper regions of the water column are more recent additions to the fauna and show greater evidence of interspecific interactions. This suggested to Gorman (1992) that “niche partitioning involving mid-pelagic species has not yet reached an evolutionary equilibrium.” The near absence of Bigeye Shiner (Notropis boops) in the North Fork is perhaps due to its replacement by the Telescope Shiner (N. telescopus), another large-eyed pelagic minnow. However, resolution of this question would require knowing if the Bigeye Shiner occurred in the drainage before the arrival of the Telescope Shiner. In contrast to purely observational studies, studies such as Gorman (1992) allow a fuller understanding of the differences in resource use among co-existing species, in this case showing that ecological differences among the benthic guild are not due to contemporary interactions, but that ecological differences among the more recent water column guild could be due to interactive segregation.
Most studies of resource overlap among freshwater fishes have focused on juvenile and adult life-history stages, yet intra- and interspecific interactions among early life-history stages (larvae and postlarvae) are potentially critical (Ross 1986). In arid, western rivers, such as the Rio Grande, habitats for larval fishes can be ephemeral, and reproduction is often tied closely to the hydrograph. Larval and juvenile stages of six fish species in a section of the Rio Grande in New Mexico showed high habitat overlap, occurring primarily in slow or no velocity habitats (Pease et al. 2006). These habitats occurred during high discharge, when backwater areas were inundated, or during low discharge, when flow was greatly reduced in the main channel. Even though they used similar, low-velocity habitats, species partitioned habitats temporally based primarily on differences in peak spawning periods related to discharge patterns. For instance, White Sucker (Catostomus commersonii) initiated spawning in early April at generally higher river discharge compared to Red Shiner (Cyprinella lutrensis), which began spawning near mid-June at low river discharge. Temporal separation of larvae of native and nonindigenous fishes is also common in other southwestern streams (Gido and Propst 1999). In terms of trophic separation of fishes in the Rio Grande, there were no obvious differences in carbon and nitrogen isotope ratios among larval and juvenile stages of the six studied species (four cyprinids and two catostomids), and carbon isotopes for larvae were nearly identical to those obtained from adult fishes. Carbon isotope ratios indicated that 80% of dietary carbon for larval and juvenile fishes was from benthic algae and 20% from emergent macrophytes. The six species also overlapped in nitrogen ratios, indicating that they were all feeding at approximately the same trophic level and thus supporting the idea of general overlap in trophic position. Larvae and early juveniles of all six species feed on microinvertebrates, including species of rotifers, copepods, and cladocerans; however, without supporting studies of actual food items, overlap in trophic position and in carbon sources does not necessarily imply overlap in how the carbon is packaged (i.e., the actual food taxa) (Pease et al. 2006).
Observational field studies addressing interactive segregation among fishes continued to be prevalent in the ecological literature through the 1980s and mid-1990s. Of 37 papers reviewed by Matthews (1998) that dealt with habitat and/or food segregation of North American freshwater fishes, 32 (86%) found evidence of segregation and 14 (44%) of these studies inferred that competition was involved. However, there has been a trend to support field studies with historical information and/or manipulative field or laboratory studies, as some of the examples in this section have already shown.
A problem with observational studies, relative to the question of competition, is their usual lack of a clear null hypothesis from which to judge observed differences among cohabiting taxa in such things as resource use. In nonexperimental research, data are collected and evaluated for their consistency with specific hypotheses or with the operation of a specific causal process, but without the knowledge of what values might be attributed to similar data in the absence of these causal processes. This often results in a nonnull hypothesis being the tested hypothesis. Preferably, null models (models generating the distribution of values for the variable of interest in the absence of a putative causal process) should be used as a first step in evaluating nonexperimental evidence (Connor and Simberloff 1986). However, null models are not without their own problems, especially because the way a null model is constructed can control the outcome, and a profuse literature has formed around this issue (see review by Gotelli and Graves 1996).
Opportunistic Distributions and “Natural Experiments”
“Natural experiments”—that is, the comparison of nonmanipulated areas—are offered in situations where a species occurs in different combinations with other species or resources. Although such opportunistic, observational studies usually lack suitable controls, as well as having other problems with experimental design (Hairston 1989; Underwood 1998), they often can suggest the presence of competition and lead to more tightly focused, experimental tests. Interpretations of such opportunistic distributions are strengthened if multiple sympatric and allopatric sites are available, a situation that is typically rare.
In a broad sense, “natural experiments” include niche shifts and character displacement, where niche shifts allow organisms to rapidly alter their trophic resource use or reproductive behavior in response to the presence or absence of heterospecifics, and character displacement refers to the reduction of overlap in resource use or in reproductive phenotypes between species through the process of natural selection (Brown and Wilson 1956; Werner and Hall 1976; Pfennig and Pfennig 2009). Phenotypically variable traits that remain under persistent, strong selection can lose their variability and become genetically fixed, so that phenotypic shifts can lead to evolutionary divergence resulting in character displacement (Pfennig and Pfennig 2009).
Niche Shifts
The spread of Red Shiner into watersheds in Arizona and New Mexico where it is not native has coincided with a dramatic decline of Spikedace (Meda fulgida), a small minnow endemic to the Gila River basin of Arizona and New Mexico (Colorado River drainage). Two hypotheses have been proposed for the rapid decline of Spikedace—displacement by competition with Red Shiner or replacement of Spikedace by Red Shiner following major environmental alteration (Douglas et al. 1994). By studying habitat use in allopatric and sympatric populations, Douglas et al. demonstrated that Spikedace shifts its habitat use where it co-occurs with Red Shiner. Importantly, the initial part of the study was a rigorous comparison of habitat availability in the sympatric and allopatric sites, thus reducing the chance that any observed differences in habitat use were simply caused by differences in habitat availability. In allopatry, both species seem to be selecting less common but similar habitats that are significantly shallower and have slower current flow and finer substrata than the average habitats available. When the populations of the two species are in sympatry, Red Shiners do not change their microhabitat but Spikedace are displaced, apparently through interference competition, into deeper microhabitats with swifter currents and larger particle sizes.
The hypothesis of interference competition is also supported by interactions of the Tessellated Darter (Etheostoma olmstedi), native to Atlantic drainages, including the Susquehanna River of Pennsylvania, and the Banded Darter (E. zonale), which has been introduced from the Lake Michigan and upper Mississippi watersheds. The Banded Darter is more of a habitat generalist compared to the Tessellated Darter, and excludes the Tessellated Darter from riffle and run habitats, restricting it to shallow pools and stream margins (Van Snik Gray et al. 2005). By comparing three sites where the Tessellated Darter did not co-occur with the Banded Darter, and one site where both occurred, and after controlling for habitat availability, Van Snik Gray et al. showed that in allopatry the Tessellated Darter occurred in significantly deeper microhabitats, with higher current speeds and larger particle sizes than when in sympatry. Tessellated Darters also showed niche compression in sympatry with the Banded Darter. Results of the field study corroborated an earlier laboratory study of habitat selection. In addition, the laboratory study supported the hypothesis of interference competition, showing that Banded Darters performed agonistic behaviors to Tessellated Darters but that the latter did not show aggressive responses toward Banded Darters (Van Snik Gray and Stauffer 2001).
Character Displacement
Studies of character displacement and release take a similar approach to natural experiments but with an evolutionary rather than ecological time scale. Ecological character displacement occurs in sympatric species through genetically controlled, phenotypic divergence (Schluter and McPhail 1992; Schluter 2000; Pfennig and Pfennig 2009). Ecological character release is a special case of character displacement involving a shift in characters in allopatric compared with sympatric populations caused by the absence of other restricting species (Robinson and Wilson 1994; Robinson et al. 2000). Both displacement and release generally invoke competition as the driving force so that the distinction between the two is simply the historical pattern of events. Although it is also true that most studies of character displacement and release are unable to provide independent evidence that competition is responsible for the shifts in phenotype, the current consensus is that competition is most often the driving factor (Robinson and Wilson 1994; Schluter 2000; Pfennig and Pfennig 2009). In addition, character release and the associated trophic polymorphism are overwhelmingly more common in species-poor, relatively recent, high-diversity (i.e., large, deep lakes) environments, with 94% of the examples found above 39ˆ N latitude, not coincidently the maximum southern extent of the Wisconsinan ice sheet (Griffiths 1994; Robinson and Schluter 2000).
Examples of ecological character displacement or release involving benthic and limnetic forms are particularly well demonstrated among populations of Threespine Sticklebacks (Gasterosteus aculeatus complex, Gasterosteidae) and Sunfishes (Lepomis spp., Centrarchidae) (Schluter and McPhail 1992; Robinson et al. 1993). Apparent character release occurs in Arctic Char (Salvelinus alpinus), with some morphotypes differing on the basis of body depth and size (Griffiths 1994).
At least six lakes in southwestern British Columbia, along the Strait of Georgia, have species pairs of the Threespine Stickleback complex, with one member of a pair primarily benthic and the other limnetic (see also Chapter 7) (Schluter and McPhail 1992; Rundle et al. 2000; Baker et al. 2005). In these, as in other Stickleback populations in North America, colonization of freshwater habitats was by the marine anadromous form over the last 12,000–20,000 years as the Cordilleran ice sheet began to retreat from northwestern North America (Bell and Foster 1994; Clague and James 2002; Bell et al. 2004). Mitochondrial DNA analysis indicates that neither limnetic nor benthic types form a single lineage among lakes. Instead, relationships of species pairs are closer within lakes, indicating that species pairs apparently evolved independently on a lake-by-lake basis from the ancestral anadromous stock (Taylor and McPhail 1999; Wund et al. 2008). Limnetic sticklebacks obtain most of their prey from the plankton, except during the breeding season when males move into the littoral zone of lakes to establish nests on the bottom and attract females. The larger and deeper-bodied benthic forms, which also have fewer and shorter gill rakers, occur in structurally complex habitats and obtain most of their food from benthic invertebrates (Bentzen and McPhail 1984; Wootton 1994). In lakes with only a single species or ecotype, resource use tends to be more generalized over benthic and planktonic habitats. Overall, the results strongly suggest that the divergence of benthic and limnetic forms of Sticklebacks occurred via resource competition, a finding supported experimentally by Schluter (1994) and Bolnick (2004) (see the section that follows).
In the Adirondack region of the northeastern United States, numerous lakes were formed as Pleistocene glaciers withdrew some 17,000 years ago (Dyke et al. 2002). Some lakes were colonized by both Bluegill and Pumpkinseed, but others were colonized only by Pumpkinseed. Where they co-occur, Bluegill occupy the water column and feed primarily on planktonic prey, and Pumpkinseed occupy the benthic littoral zone and feed mostly on benthic invertebrates (see also Chapter 7) (Robinson et al. 1993, 2000). In lakes where Bluegill are absent, Pumpkinseed show character release, retaining the benthic form but also having a limnetic form that acts like a Bluegill—occurring in the water column and feeding primarily on plankton. The role of competition-driven, disruptive selection is suggested by better condition and faster growth rates of the more extreme limnetic forms of Pumpkinseed compared to fish with intermediate body forms (Robinson et al. 1996).
Arctic Char, found at high latitudes in northern Europe and in northern North America, show various polymorphisms in body shape and size, growth rate, age and size at sexual maturity, body coloration, and food and habitat use. At least some of these polymorphisms are thought to be caused by reduced interspecific competition (i.e., character release) in concert with intense intraspecific competition (Griffiths 1994; Power et al. 2009). Different phenotypes generally are found in relatively young systems that offer discontinuous foraging niches and low potential for interspecific competition. Widely documented in Europe, only five instances of sympatric morphs of Arctic Char have been studied in North America, although there are likely numerous systems where polymorphisms exist. Lake Aigneau, a moderately deep lake in northern Quebec, supports two phenotypes of Arctic Char, but is somewhat unusual for lakes with multiple Arctic Char phenotypes in having a relatively diverse fish fauna (10 species, including three salmonids) (Power et al. 2009). There are two size groups of Arctic Char: a small size group with a modal fork length of 210–220 mm having terete bodies and residual parr marks, and a large size group with a modal fork length of 600–640 mm, having deeper, more robust bodies; large heads; and dorsal humps (Figure 11.3A). Fish in the two size groups show almost no overlap in occupied depths during the summer, with fish in the smaller size group at depths ≤ 5m in the littoral zone of Lake Aigneau, and those in the larger size group typically at depths > 15 m in the profundal zone. The two groups also differ in age at maturity, with 50% maturity occurring by age 4 in the littoral zone fish and age 11 in the profundal fish, and in longevity, with littoral zone fish reaching six years and profundal fish to 21 years. Minimal interbreeding and genetic divergence, as determined by mtDNA haplotypes and microsatellites, characterize the two size groups. Not surprisingly given the different habitats, trophic niches also differ. Littoral-zone fish consumed a summer diet dominated by chironomid larvae and pupae whereas profundal fish had empty, shrunken stomachs; no evidence of recent feeding activity; and depleted tissue-nitrogen levels—all of which suggest summer fasting. Stable isotope analysis shows different isotopic ratios for the two groups, with profundal fish averaging one trophic level higher than littoral fish (Figure 11.3B). Further, the isotope ratios indicate heavy reliance by the profundal Arctic Char on the littoral Arctic Char. Overall, the large, profundal fish seem to be fasting during the summer and then feeding on the small morph of Arctic Char in the winter when ice drives the small fish into winter refugia in the limited area of deep water, providing the large fish with an abundant food supply. The pattern is likely driven by intense exploitative competition for available summer resources, favoring the adoption of winter feeding by the large fish. The genetic differences support the possibility of different allopatric origins for littoral and profundal fish, with more recent colonization by the latter, but the evidence is not complete enough to eliminate the competing hypothesis of ecologically driven sympatric divergence. The Lake Aigneau Arctic Char are distinct from most polymorphic forms of Arctic Char—normally the small form occurs in the energetically limited habitat of deep water and the larger form occurs in littoral or pelagic habitats (Power et al. 2009).

FIGURE 11.3. Character release and intraspecific competition.
A. Length frequency distributions (fork length) of littoral (light gray) and profundal (black) Arctic Char (Salvelinus alpinus). Littoral fish are grouped in 30 mm size intervals; profundal fish in 50 mm size intervals.
B. Trophic separation based on stable isotopes of carbon and nitrogen of littoral (light gray) and profundal (black) Arctic Char. Error bars are ± 2 SE. Based on Power et al. (2009).
Field and Mesocosm Experiments
Well-designed field or mesocosm experiments are not without their own problems, but they generally are considered to provide the best evidence for competition (Schoener 1983; Connell 1983; Hurlbert 1984; Eberhardt and Thomas 1991). In addition to the need for experimental arenas to closely match major attributes of natural habitats, other key points for experimental studies of competition include the need for proper controls so that only the effects of an added competitor are being measured and not potential impacts from capture and handling. Experiments also need to have proper and sufficient replication so that any effects from a competitor are not masked or attributed to differences among the sites used for controls and treatments—in fact, “the need for replication in any field experiment is paramount” (Underwood 1986). The same need for replication applies to mesocosm experiments, laboratory streams, and so on. A still too common problem of ecological experiments is pseudoreplication—the result of confusing experimental units and evaluation units, or the case of not replicating treatments (the experimental units), although samples (evaluation units) might be replicated, and then testing for experimental effects using inferential statistics. Pseudoreplication is a problem of the experimental design in combination with using an inappropriate statistical analysis for testing the hypothesis of interest. Finally, the assignment of replicates to treatments and controls must be randomized so that potential biases are avoided (Hurlbert 1984, 2009; Fausch 1998). Basically, experimental designs used in competition experiments fall into two groups, additive and substitutive (Box 11.3).
BOX 11.3 • Design of Competition Experiments
Competition experiments, as with all experiments in ecology, are only as meaningful as their design, analysis, and interpretation, which are, of course, based on the hypothesis to be tested. In addition, successful field, mesocosm, or laboratory experiments require a strong measure of good judgment, sound biological insight of the subject organisms and their habitats, and a solid dose of artistry as well (Hurlbert 1984, 2009; Underwood 1997). Other useful references on ecological experiments include Hairston (1989), Underwood (1990), and Fausch (1998).
The two basic questions concerning interspecific competition—does competition occur when two species are placed together, and is the level of this competition greater than just adding the same number of a single species—are most simply tested by two different types of experiments (see part A of the following figure). Additive designs are useful in testing for the presence of competition, especially in the situation of competition between native and nonnative species, or between potential competitors that differ greatly in size. In this design, the only change is the addition of equal numbers of the two species to the test habitat (stream, pond, enclosure, etc.); intraspecific competition is held constant. However, because the number of individuals is greater in the treatment than in the control groups, this design cannot differentiate between intra- and interspecific competition. Substitutive designs (B) maintain the same number of individuals across the treatment and control groups, thus allowing the level of interspecific competition to be adjusted relative to the level of intraspecific competition. For instance, this design would be used where competition is known to occur between native species having similar sizes, habitats, and food use. Ideally, the two designs would be combined so that the experiment would test both the existence and the relative strength of interspecific competition as well as intraspecific competition (see part C below)(Underwood 1978; Fausch 1998).

Experimental designs in the study of competition showing the proportions of individuals of each of two species that might be used in additive, substitutive, and combined designs. The number of individuals are, of course, chosen by the experimenter. Based on Underwood (1978, 1986), Connell (1983), and Fausch (1998).
Interspecific Competition
An early additive experiment testing the occurrence of competition used juveniles of three sunfish species (Lepomis) to test for niche shifts in the presence and absence of interspecific competition, while keeping intraspecific competition relatively constant (Werner and Hall 1976). The experiment was conducted in four small ponds that were as identical as possible. One pond received 900 individuals each of Bluegill, Green Sunfish, and Pumpkinseed for a total of 2700 fishes. In the three other ponds, 900 fish of each species were stocked alone. The principal response variables were growth (changes in body weight over the experiment), prey size, and habitat use. An increase in growth rate and/or breadth of habitat use in allopatry versus syntopy would indicate a release from interspecific competition. All species showed increased weight gain and use of larger prey in allopatry (measures that relate to overall fitness), but the level of competitive release was greatest for Bluegill, which showed more than a 200% increase in the size of prey it consumed (Figure 11.4A). In allopatry, the three species used similar foraging habitats, as determined from the nature of prey in stomach content samples, with all three species getting ≥ 40% (dry weight) of their diets from vegetated areas. However, Bluegill and Pumpkinseed shifted their habitat use when all three species were together (Figure 11.4B). When together, Green Sunfish continued to feed on invertebrates characteristic of vegetation, but Bluegill shifted primarily to open water prey, and Pumpkinseed shifted to benthic prey. The principal conclusions were that sunfishes show considerable niche flexibility, that such flexibility would be adaptive in allowing fish to respond to seasonal changes in resource availability, and that such niche shifts suggest the importance of competition in structuring natural assemblages that include these species. Although aspects of the experimental design were criticized, but surprisingly not for the total absence of replication, the overall conclusions have been supported by subsequent studies (Maiorana 1977; Werner and Hall 1977a).
The apparent asymmetric competition between Bluegill and Green Sunfish was further examined with a substitutive, but still unreplicated, design by establishing three approximately 50 m3 enclosures in the vegetated zone of a small pond. Overall densities were kept the same, with one enclosure receiving 250 individuals of each species, one receiving 500 Bluegill, and one receiving 500 Green Sunfish (Werner and Hall 1977b). Although both Green Sunfish and Bluegill favored vegetation-dwelling prey, the more territorial and aggressive Green Sunfish was able to force Bluegill to shift to alternative habitats offering different prey spectra. There was little effect of Bluegill on the growth in length of Green Sunfish, but Green Sunfish had a strong impact on growth of Bluegill, thus confirming the strong asymmetry in competition (most likely interference competition) favoring Green Sunfish in vegetation. Bluegill showed greater niche flexibility and were able to forage efficiently on smaller prey typical of the open water column.
A later study tested the hypothesis that movement of juvenile Bluegill and Pumpkinseed from open water to vegetation, which occurs as a consequence of predator avoidance, resulted in competition among small fishes in the vegetation (Mittelbach 1988). Twelve cages arrayed along the vegetated littoral zone of a lake were stocked with two juvenile Pumpkinseed and varying numbers of Bluegill, allowing for two replicates. Growth of both species declined linearly in response to increased fish density in the cages, indicating that the concentration of small fishes in the vegetated littoral zone can lead to competition for food. In addition, increases in fish density resulted in decreases in invertebrate body length and decreases in the density of large invertebrate prey in the cages. Further, the density of large invertebrates was related positively to growth rates of both sunfish species. The mode of competition among these small sunfish was most likely exploitative (passive) (Mittelbach 1988).
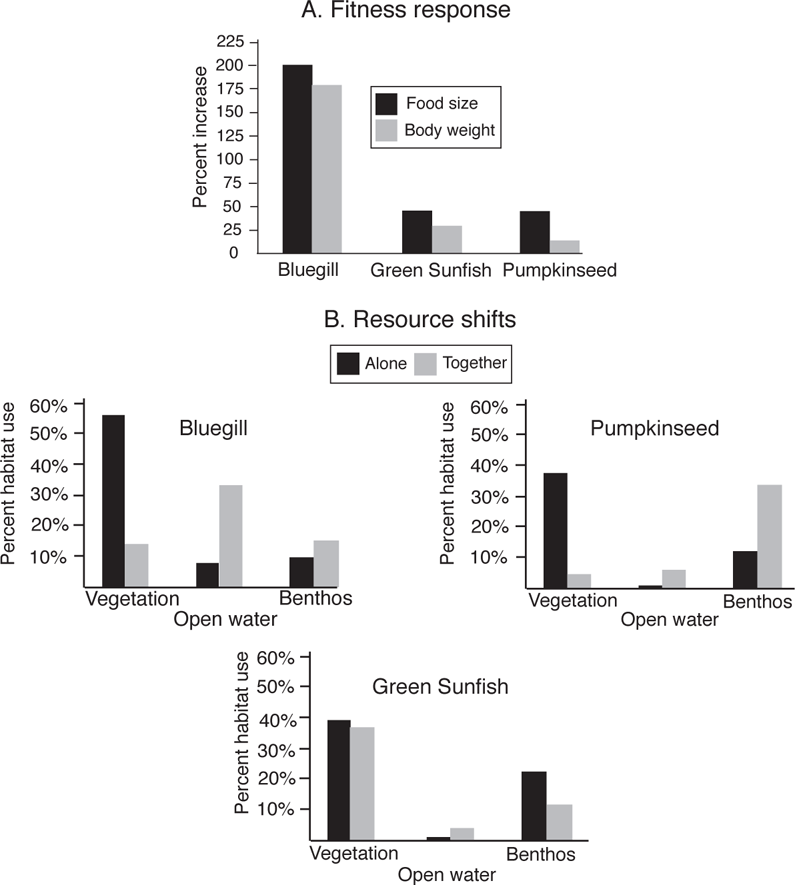
FIGURE 11.4. Experimental tests of competition in juvenile sunfish species.
A. Measures of competitive release, shown by percent weight change and percent change in prey size, of Bluegill (Lepomis macrochirus), Green Sunfish (L. cyanellus), and Pumpkinseed (L. gibbosus) when raised together or in isolation.
B. Directions of habitat shifts of the three species when grown together or separately. Habitat use was determined from the nature of prey consumed; percentages for each species do not sum to 100% because prey not typical of one of the three foraging habitats are not included. Based on Werner and Hall (1976).
Even though competition is considered a negatively symmetrical response, the impact can vary greatly between pairs of competing species, as shown by the interaction between Green Sunfish and Bluegill. The strength of competition can also be unequal between size groups or individuals of the same or different species, and the direction of the impact can change with life-history stage. Asymmetry in both inter- and intraspecific competition has been demonstrated in studies of adult-sized Mottled Sculpin (Cottus bairdi), Kanawha Sculpin (C. kanawhae), and Fantail Darter (Etheostoma flabellare), species that are common riffle inhabitants of many southeastern streams and co-occur in some. Using 16 replicated, outdoor experimental streams, Mottled Sculpin showed the effects of intraspecific competition with decreases in growth, condition, and survivorship when their density was increased from 5 to 10 fish per stream. Interspecific competition showed the same negative outcomes when five Mottled Sculpin were placed together with four Kanawha Sculpin, a somewhat larger species. Surprisingly, when five Mottled Sculpin were placed with five Fantail Darter, the Mottled Sculpin showed negative responses in survival, body mass, and total length that were stronger than their response to Kanawha Sculpin, although the actual mechanism of competition (active versus passive) is not known. This finding is counter to the generally held view that competition is strongest between closely related species and the conclusions of the study are particularly strong given the robust experimental design (Resetarits 1995).
The level of competition and even the type of interaction between Mottled Sculpin and Fantail Darter change with life-history stage. Based on findings from 20 replicated, outdoor experimental streams, juvenile Fantail Darter and Mottled Sculpin compete at densities that approximate natural conditions, although the nature of the response differs between the species. Juvenile Fantail Darter had a negative effect on growth of juvenile Mottled Sculpin, but did not affect sculpin survival or relative condition (determined by the final individual body mass compared to predicted body mass of a wild fish of the same length). In contrast, juvenile and adult Mottled Sculpin did not affect survival or growth of juvenile Fantail Darter, and juvenile sculpin only had a slight negative effect on the relative condition of darters. Adult Fantail Darter facilitate survival of juvenile Mottled Sculpin, although they had a negative effect on final total length and body mass. The reason for the increase in survival is not known. These results emphasize the natural complexity of species interactions. Overall, the impact of competition between Fantail Darter and Mottled Sculpin is asymmetrical—Fantail Darter impact Mottled Sculpin much more than the reverse, even though the darters are smaller than the sculpin. In addition, the intensity of competition and even the nature of the response (i.e., competition versus facilitation) change depending on the response variables (Resetarits 1997).
Competitive interactions within an assemblage composed of native and nonindigenous salmonids of Brook Trout (Salvelinus fontinalis), Brown Trout (Salmo trutta), and Coho Salmon (Oncorhynchus kisutch) were studied in natural streams flowing into Lake Michigan, and in a laboratory stream (Fausch and White 1986). In syntopy, Coho Salmon (introduced from western North America) could defend profitable foraging sites against Brook Trout (native to Michigan streams) and Brown Trout (introduced from Europe). Coho Salmon emerged earlier than Brown Trout and were 7–28% larger than Brown Trout throughout the first growing season. Coho Salmon also emerged earlier than Brook Trout and again were larger, although sympatric populations of Coho Salmon and Brook Trout were uncommon in the tributaries, likely because Brook Trout were being displaced by Brown Trout (Fausch and White 1981, 1986). In the experimental stream, Brook Trout, in contrast to observations in natural streams, could displace Brown Trout from the most profitable foraging sites. Under allotopic conditions, both trout species increased their breadth of foraging sites, including those sites with the highest profitability, but Coho Salmon, the dominant competitor, showed little change in position. Shifts in habitat use under syntopy were due to aggressive behavior of the dominant fish, thus making this an example of asymmetrical interference competition (Fausch and White 1986). In fact, asymmetry is the general rule among competitive interactions (Schoener 1983).
The impact of introduced species of Pacific salmon, and especially Coho Salmon, as well as native fishes on native populations of Atlantic Salmon (Salmo salar) along the Atlantic Coast have been addressed experimentally in a number of studies. However, many studies have lacked sufficient replication, making the results difficult to evaluate. Of studies suitably replicated, there seems to be sparse evidence of competitive effects at any spatial or temporal scale. The most likely competitor of juvenile Atlantic Salmon would be juvenile Coho Salmon, given their size advantage (earlier emergence and greater size at hatching) and their innate aggressiveness (Fausch 1998).
The role of interspecific competition in driving vertical habitat use of Cutthroat Trout (Oncorhynchus clarkii) and Dolly Varden (Salvelinus malma) has been assessed over a 28-year period from 1974 to 2001 using whole-lake transplants (Figure 11.5). From 1974 to 1976, sympatric populations of the two species in Loon Lake, British Columbia, were used to create two allopatric populations in two similarly sized nearby Lakes—Eunice Lake received Cutthroat Trout and Katherine Lake received Dolly Varden (Andrew et al. 1992; Northcote 1995). In sympatry, Cutthroat Trout foraged in the shallow littoral and epipelagic (< 5 m) zones whereas Dolly Varden foraged in the deeper pelagic and epibenthic zones. In laboratory studies, Cutthroat Trout are more effective at capturing surface prey and littoral benthos compared to Dolly Varden, but the latter have better visual acuity under low light conditions and thus are more effective at finding prey in deep water (Northcote 1995). After eight years, the experimental allopatric population of Dolly Varden had expanded their habitat use in Katherine Lake to include shallow littoral habitats. In contrast, the vertical distribution of Cutthroat Trout in the allopatric population remained essentially the same as that of the naturally sympatric population. The results suggested that the change in habitat use of Dolly Varden could be the result of release from interspecific competition, but the observed difference in habitat could also be due to differences between the three lakes. In addition, the evolved differences in foraging and visual acuity suggest why Dolly Varden were using pelagic and epibenthic habitats, but not why they were apparently restricted in resource use by Cutthroat Trout (Andrew et al. 1992; Northcote 1995; Jonsson et al. 2008). To control for lake effects, in 1994, reciprocal transplants were done between trout and char in the two allopatric populations and the habitat use of the trout and char was assessed in 2001. The results showed that Dolly Varden were displaced from the littoral and epipelagic zones in the presence of Cutthroat Trout but that the vertical distribution of Cutthroat Trout remained essentially the same between the two experimental sympatric populations. The asymmetric competition presumably results from reduced foraging success in shallow water caused by interference from Cutthroat Trout so that deeper foraging habitats become relatively more profitable to Dolly Varden (Jonsson et al. 2008).
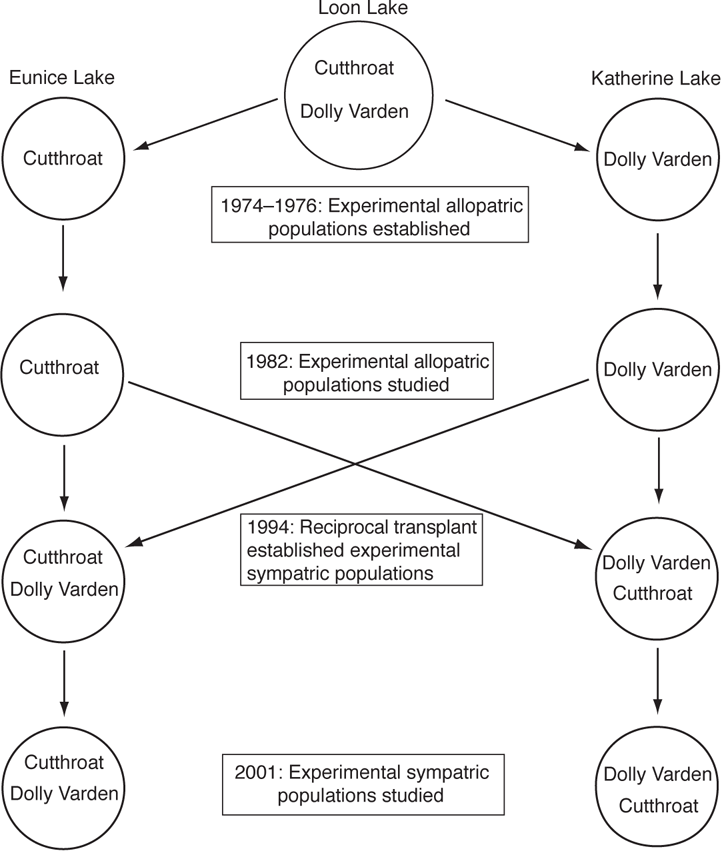
FIGURE 11.5. Design of a 28-year reciprocal transplant experiment to study interactive segregation in Cutthroat Trout (Oncorhynchus clarkii) and Dolly Varden (Salvelinus malma). The study involved Loon Lake, where the two species are naturally sympatric and sequential experimental allopatric, and then sympatric populations in two formerly fishless lakes, Eunice Lake and Katherine Lake. Based on Andrew et al. (1992), Northcote (1995), and Jonsson et al. (2008).
Although effects of interspecific competition on fishes most often focus on interactions with other fish species, interspecific competition between fishes and organisms in other taxa can also be important. Crayfish of various species have been introduced into the Colorado River basin and may feed at the same trophic level as fishes. The Virile Crayfish (Orconectes virilis), an opportunistic omnivore that has one of the broadest natural ranges of North American crayfish east of the continental divide, has been introduced into western drainages, including the Colorado River basin (Taylor et al. 1996; Lodge et al. 2000; Larson and Olden 2011). Competitive interactions among the Virile Crayfish, Gila Chub (Gila intermedia), and Flannelmouth Sucker (Catostomus latipinnis) were studied in 200 aquaculture tanks using a replicated, combined substitutive and additive design (Box 11.3) (Carpenter 2005). All tanks received the same daily food ration of chironomid larvae, and the response variable was the change in body mass of the three species over the course of the experiment. Although Virile Crayfish reduced the growth of both fish species by competition for food, the degree of competition varied between the two species. Gila Chub were more affected by intraspecific competition than interspecific competition, whereas Flannelmouth Sucker were more affected by interspecific competition with Virile Crayfish than by intraspecific competition. Virile Crayfish growth was not altered by the presence of either fish species. The increased level of interspecific competition on Flannelmouth Sucker compared to Gila Chub likely is a consequence of the former species being more benthic. Because of the inherent aggressiveness of Virile Crayfish, competition most likely occurs via interference (Carpenter 2005).
Intraspecific Competition
Intraspecific interactions also can impact the structure of fish assemblages (Fausch and White 1986; Freeman and Stouder 1989; Matthews 1998). The effect of intraspecific competition was tested using Red Shiners, an extremely widespread minnow in the midwestern United States extending into northern Mexico (Matthews 1985b). Red Shiners were stocked at densities from about 3 to 30 individuals/m2 in large experimental streams (with two replicates) (Matthews et al. 2001). Fish showed moderately reduced overwinter survival that was negatively related to initial stocking density (Figure 11.6). Although fish showed little growth between initial fall stocking through the end of the year at any density level, fish density did have a strong, negative linear effect on survival and spring growth (Figure 11.6). Also, at high population densities, growth was skewed with fewer individuals growing large enough to reach sexual maturity. In a subsequent experiment (W. J. Matthews, unpublished data), Red Shiners with free access to a series of pools and riffles in predator-free experimental streams typically formed groups with densities lower than those that had resulted in low modal growth in the groups in the previous experiment (Matthews et al. 2001). This finding suggests that fish may avoid excessively dense aggregations (unless factors like predators or differences in habitat quality provide stronger stimuli). The actual mechanism or mechanisms responsible for the growth depression relative to fish density is unclear. Fish density did not have any effect on algal standing crop or density of benthic invertebrates and, in fact, had a positive effect on benthic primary productivity. The latter effect disappeared after 5–7 months and its occurrence was likely caused by the transfer of nutrients from surface prey (Red Shiners feed on surface and water-column prey) to the benthic compartment, with the disappearance of the effect occurring after other nutrient sources became more available (Gido and Matthews 2001). The actual mechanisms for growth suppression could have been due to interference or exploitation competition for surface prey (which were not measured), or to interference competition for benthic prey, but the suppression was not due to exploitation competition for benthic prey (Matthews et al. 2001).

FIGURE 11.6. Effects of intraspecific competition in Red Shiner (Cyprinella lutrensis) on growth and survival of fish in a replicated experimental stream. Based on Matthews et al. (2001).
Morphological and behavioral divergence of Threespine Sticklebacks in northwestern lakes also provide opportunities to assess the presence of competition and its role in promoting such divergences. In various lakes, Threespine Sticklebacks have diverged into benthic and limnetic morphologies, whereas in other lakes, populations are polymorphic with intermediate as well as extreme morphologies (See Chapter 7). The role of competition as a driver in morphological divergence was first shown experimentally by a pond experiment using fish from a polymorphic (both benthic and limnetic forms) population of Threespine Sticklebacks in Cranby Lake, British Columbia, using a basic additive design (Schluter 1994). The study tested the prediction (based on a competition hypothesis) that individuals at the limnetic extreme would suffer disproportionately when limnetic fish from Paxton Lake (having distinct benthic and limnetic forms) were added. Because fish at the two extremes are uncommon in Cranby Lake, additional fish at the limnetic and benthic extremes were produced by hybridizing Cranby Lake fish with benthic and limnetic fish from Paxton Lake. The study used two ponds, each divided into equal treatment and control sections (Figure 11.7A). In both of the ponds, the added numbers of the limnetic form resulted in lowered survival and decreased growth in the more extreme limnetic fish but the treatment effect diminished gradually in fish that were morphologically more distant from the limnetic body form (Figure 11.7B). Although the study only had two replications, the results are strong support that morphological diversification in Threespine Sticklebacks could be driven by resource competition (Schluter 1994, 2010).
Another pair of studies with Threespine Sticklebacks further tested the role that competition might play in disruptive selection, testing the prediction that populations with elevated intraspecific competition should show faster niche expansion or stronger diversifying selection. In the first study, pairs of large enclosures were established in one lake in 2001 and in five different lakes in 2002—all on Vancouver Island, British Columbia (Bolnick 2004). The enclosures went from shore to open water and thus included a range of habitats. Some fish were removed from one randomly chosen enclosure out of a pair of enclosures to create a low-density treatment; fish were added to the other enclosure of the pair to form a high-density treatment. Fish in both enclosures of a pair had natural phenotypic distributions. At the end of the 2001 experiment, fish in the high-density experiments showed evidence of disruptive selection in gill-raker length (limnetic forms have longer gill rakers than benthic forms), were smaller, and had lower relative gonad mass than those from the low-experiments. The high-density enclosure in 2001 also had lower zooplankton density than the lowdensity enclosure. Results were more variable in 2002, but generally provided support for the hypothesis of competition-driven disruptive selection (Bolnick 2004).
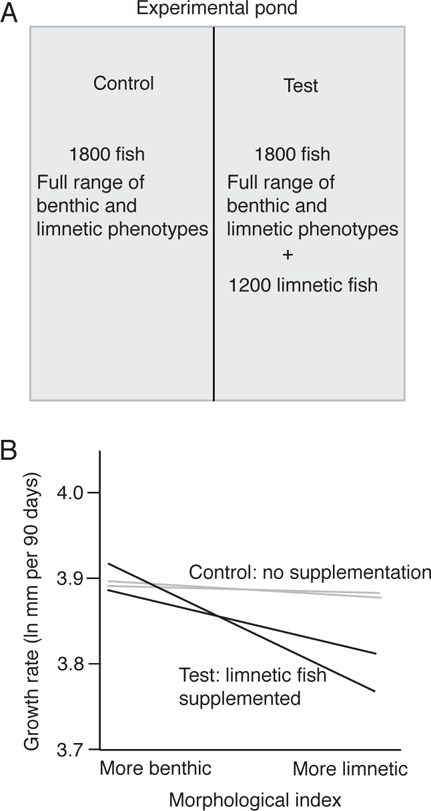
FIGURE 11.7. Competition within limnetic morphologies of Threespine Stickleback (Gasterosteus aculeatus).
A. Control and treatment in the two halves of an experimental pond (two ponds were used in the experiment).
B. Growth responses of different morphologies of fish in response to supplementing the numbers of limnetic fish in two different ponds. Based on Schluter (1994, 2010).
The second study used five pairs of enclosures placed in the shallow water of a single lake on Vancouver Island; one enclosure of a pair received 30 fish (low density) and the other 90 (high density) (Svanbäck and Bolnick 2007). After two weeks, the high-density treatments had lower densities of benthic invertebrates and zooplankton and fish in this treatment had reduced gut contents and lower growth rates. The fish in the low-density treatments did not differ in growth rate or gut content from fish taken outside of the enclosures; the only artifact of caging was a lower zooplankton density than outside the low-density enclosures. Importantly, fish in the high-density treatment had greater variation in diet due to increased individual specialization leading to an overall increase in population niche breadth. In contrast, the diet variation and niche breadth in wild-caught fish were similar to fish in the low-density enclosures. Fish survival did not differ among the treatments so that differential survival was not the reason for changes in niche characteristics. The study confirms that competition for limited resources can lead to increased diet variation among members of a single population and that such changes can occur in a matter of weeks, essentially as soon as fish can detect a change in prey availability (Svanbäck and Bolnick 2007).
Intraspecific interactions between larval and adult fishes are also important, and can have both positive and negative effects, but are as yet poorly studied. Adult male Bluegill build circular nests in the littoral zone of lakes and ponds, or quiet areas of streams, and the male defends the developing embryos and early larvae against predators (Avila 1976). However, once the larvae leave the nest, their interactions with adult Bluegill can be positive or negative. Adults can have a positive impact on conspecific larvae by consuming predators of larvae, such as predatory insects, or by increasing the abundance of small zooplankton through removal of large zooplankton. Potential negative effects include competition for large zooplankton and cannibalism. In pond experiments conducted on adult and larval Bluegill (during the experiment larvae transitioned to postlarval YOY) at the Kellogg Biological Station, Michigan, the presence of adult Bluegill had a strong negative impact on the final mass of larvae through competition for large zooplankton prey, such as Chaoborus and Daphnia, which were the dominant larval prey in absence of adults (Rettig and Mittelbach 2002). As adult density increased, the prey biomass in stomachs of larval Bluegills decreased, resulting in a decline in average mass of larvae. However, larval survival was greatest at intermediate adult densities, with the low survival in the absence of adults being related to the high density of predatory insects. Because adult Bluegill consume the large-bodied, predatory insects, the density of predatory insects declined as adult density increased; however, because of the competitive interaction, the overall production of larvae peaked at intermediate adult densities (Rettig and Mittelbach 2002).
FOOD WEBS, TROPHIC POSITION, AND COMPETITIVE EXCLUSION
Food webs and food chains describe the complexity of trophic interactions occurring within a community and thus indicate how matter and energy move through an ecosystem (Krebs 1985; Post 2002). The understanding of these food webs has been greatly influenced by the pioneering works of Elton (1927), on trophic pyramids, and Lindeman (1942), on the dynamics of energy flow in communities (Cook 1977). Food webs are dynamic, changing spatially as well as temporally, and resulting in the formation and breakage of linkages among trophic groups. Temporal changes include seasonal and annual variation related to variation in the physical environment (temperature, water chemistry, precipitation amounts, runoff, water level, stream discharge, and floodplain access in rivers) or biotic environment (spawning and recruitment of young, migratory patterns, energy demands, and impacts of fisheries) (Power et al. 1995; Winemiller 1996). Also, the introduction of nonindigenous fishes and invertebrates can have widespread, and often unexpected, impacts on food webs, providing the potential for elimination of native species (Vander Zanden et al. 2003).
Webs and Chains
Food webs and food chains comprise trophic groups and the linkages between them. There are several types of food chains and food webs, based on what is emphasized in the data. A connectance web essentially shows all known linkages and trophic groups from primary production and detritus up to the top-level consumers (essentially who eats whom) and, because it is based on presence/absence data, is qualitative (Figure 11.8A). Two other webs are quantitative and can represent the size of the trophic groups and the degree of energy flow or interaction between them. An energy web emphasizes the amount and direction of energy flow between trophic groups, again starting from primary producers and detritus and moving upward to top-level consumers, essentially following the work of Lindeman (1942) (Figure 11.8B). Often, only surrogates of energy flow are available, such as the number, volume, or biomass of consumed organisms. The importance of a linkage or the size of a trophic group can change based on the currency used to evaluate them. For instance, trophic group size based on the number of individuals will be biased toward small organisms, whereas trophic group size based on biomass will be biased toward large organisms (Winemiller 1996; Post 2002). Finally, an interaction web (or functional food web) is based on the strength of the interaction between levels and is generally a top-down web that is determined by experimental manipulation. Interaction webs offer an estimate of the functional food-chain length as shown by the impact of a species or a group of species on some community attribute, such as the population size of herbivores (Paine 1980) (Figure 11.8C).

FIGURE 11.8. Hypothetical aquatic food webs.
A. Connectance web.
B. Energy web, with the amount of energy transfer between levels shown by the width of the arrows.
C. Interaction web, with the strength of the interaction between trophic levels shown by the width of the arrows. Based on Post (2002) and Woodward and Hildrew (2002).
A food chain refers to a single pathway showing the linkages and trophic groups between the bottom and top of a food web. As such, a single food web can contain multiple food chains. Both connectance and energy webs provide estimates of realized food-chain length, which is based on all the different trophic pathways that lead from the base of a food web to a consumer. For instance, the three possible realized food chains from Figure 11.8B are link-1 (benthic algae to herbivorous aquatic insects to predaceous aquatic insects to large fishes—four levels), link-2 (benthic algae to herbivorous aquatic insects to small fishes to large fishes—four levels), or link-3 (benthic algae to snails to large fishes—three levels). Stable isotope analysis provides an improved way to estimate energy webs and food-chain length by providing a summary of energy flow and integrating the outcome of complex interactions. A functional food chain is based on an interaction web (Post 2002). Because interaction webs and functional food chains emphasize the role that a predator has on community properties, they are treated in more detail in Chapter 12.
The control over the length of a food chain has been a topic of interest at least from the pioneering works of Elton and Lindeman. Factors thought to be important include the history of community organization (what organisms have had access to a community), the amount of available resources, habitat stability, and the size of the ecosystem (Post 2002; Power and Dietrich 2002). The effect of resource availability seems to only prevail at extremely low resource levels, such as might occur in deep caves, extremely high altitudes or latitudes, or extreme deserts, and is not likely a factor in most North American freshwater ecosystems. Because most aquatic food webs are size-structured (i.e., body size increases with trophic position), food chain length is influenced by the range in body size between organisms at the bottom and top of the food web. Although intuitively appealing, shorter food chains have not generally been linked with increasing levels of disturbance, although disturbance has been associated with the loss of top predators in small ecosystems (Post et al. 2000; Post 2002). Undoubtedly, part of the problem is in identifying what constitutes a disturbance (see also Chapter 6). For instance, in rivers of northern California, and likely in other Pacific coast streams, the lack of flooding, rather than flooding, is the disturbance. The lack of flooding shortens the dominant food chain to two levels—primary producers and a large, predator-resistant herbivorous caddisfly, in contrast to a 3–4 level food web that occurs during years with winter flooding, moving from primary producers, to small herbivorous aquatic insects, to larger aquatic insects, to juvenile salmonids (Power 1992a; Wootton et al. 1996; see Chapter 12). There is also an effect of ecosystem size on food-chain length, although where changes/additions occur is not well explained (Post et al. 2000; Post 2002).
In contrast to a clearer separation of trophic position just shown, a study comparing trophic positions of small-bodied fishes from four streams in Kansas, Oklahoma, and New Mexico showed much more variability and overlap. Trophic position was based on δ15N from muscle tissue, a synthesis of published studies on food habits of the species, and gut content analyses from a single collection of fishes from each stream. For purposes of comparison, data were analyzed using the broad trophic categories of algivore/detritivores, omnivores, and insectivores. All three approaches to determining trophic position generally agreed, but the relationship between the percentage of invertebrates consumed and δ15N was only weakly correlated (r = 0.42). Trophic separation was blurred, primarily because the small-bodied fishes in these four streams occupied only two trophic levels and all streams were dominated by omnivores. Omnivorous fishes can show high variability in feeding depending on time of year, flow patterns, and food availability. Because of the highly variable hydrology of the study streams, there has likely been strong selection for omnivory (Franssen and Gido 2006).
Changes in food webs as a consequence of nonindigenous fish and invertebrate introductions can be extensive. One of the betterstudied systems is that of Lake Tahoe, a large lake located in the Sierra Nevada Mountains on the California/Nevada border. The changes in the food web of Lake Tahoe over a 130-year period from the late 1800s and early 1900s to 1998–2000, have been studied using stable isotope analysis of muscle tissue from formalin-preserved fishes and aquatic invertebrates (after correcting for the effects of chemical preservation) and by historical studies on fishes and invertebrates in the system (Vander Zanden et al. 2003). Historically, the top predator was the Lahontan Cutthroat Trout (Oncorhynchus clarkii henshawi), which evolved in Pleistocene Lake Lahontan (see Chapter 3 and Figure 3.3). By the late 1930s, this species, which fed on schools of pelagic fishes, was extirpated from Lake Tahoe as a consequence of combined effects of Lake Trout and other nonindigenous fish introductions starting in the late 1800s, overexploitation, dam construction, and loss of spawning habitat. Lake Trout essentially occupied the same place in the food web as Cutthroat Trout. In the 1960s, a nonindigenous, large zooplankter, Mysis relicta, was introduced and became well established a decade later, corresponding with the extirpation of two native cladocerans, Daphnia and Bosmina (Vander Zanden et al. 2003). In spite of the long history of introduction of nonindigenous fishes, the most significant change in the Lake Tahoe food web occurred subsequent to the introduction of Mysis, in concert with the onset of eutrophication. Historically, although the food web was driven by both benthic and planktonic primary production, benthic primary production predominated. The contemporary food web has been significantly shifted toward pelagicbased production (Vander Zanden et al. 2003; Schmidt et al. 2007; Turner et al. 2010).
Competitive Exclusion
As exemplified previously, competitive interactions among or within species can affect fish assemblages on ecological and evolutionary time scales. On ecological time scales, competition can result in changes in resource use, changes in growth rates and condition, and presumed changes in fitness. On evolutionary time scales, competitive interactions can provide strong selective forces resulting in genetically fixed differences in morphology and behavior, leading, at least in some cases, to speciation. However, unequivocal evidence of competitive exclusion of one species by another, other than in laboratory or mesocosm experiments, is difficult to obtain and is uncommon for any taxonomic group (Ricklefs 1990; Begon et al. 1996), including North American native fishes. In fact, various studies suggest that physical variation in flow and temperature, recolonization dynamics, and temporal variation in reproduction have greater impacts on North American fish assemblages than competitive exclusion (Schlosser 1982; Grossman and Freeman 1987; Grossman and Boulé 1991).
A seemingly excellent opportunity to document competitive exclusion in nature is the long-term investigations mentioned previously on Dolly Varden and Cutthroat trout (reviewed by Northcote 1995). However, even though the work provides a convincing example of asymmetric competition, competitive exclusion did not occur. The loss of species due to competition may be more prevalent in terms of impacts of nonnative species on native species (reviewed by Fausch 1988; Ross 1991; Fuller et al. 1999). However, even for the relatively well-studied cases of interactions of native and introduced salmonids, clear evidence of the mechanism involved where interactive segregation in habitat use was demonstrated is generally lacking (Fausch 1988). Where mechanisms of interaction are documented, impacts of direct predation are better supported than competition (Ross 1991).
The strongest support for competitive exclusion comes from a study of the replacement of Rio Grande Silvery Minnow (Hybognathus amarus) by the Plains Minnow (H. placitus) (see also Chapter 15). Rio Grande Silvery Minnow is native to the Rio Grande and Pecos river systems and the Plains Minnow is widespread in midwestern streams. Both use similar habitats and have equivalent life histories (described in Chapter 5, Ontogeny and Movement). The time of introduction of the nonindigenous Plains Minnow into the Pecos River, as determined from museum records, occurred in the early 1960s. Based on genetic data, the colonizers came from two sources, and the total number of original colonizers was around 32–115 (Moyer et al. 2005). Within only 10 years, the native Rio Grande Silvery Minnow was gone from the Pecos River. Given that the Plains Minnow successfully became established in the Pecos River, there are two competing hypotheses for the loss of the Rio Grande Silvery Minnow—hybridization or competition. Genetic assignment tests and phylogenetic analyses have excluded hybridization as a primary factor in species replacement; in fact, there are very few individuals that are potentially of hybrid origin. Population modeling indicates that species replacement could have occurred within 10 years, given the initial number of colonizers and a fitness difference between the native and nonindigenous Hybognathus species. Consequently, given the lack of support for the hybridization hypothesis, the alternative hypothesis of competition, most likely exploitation competition, is probably the cause of the species replacement (Moyer et al. 2005).
SUMMARY
Studies of species’ interactions as a consequence of resource acquisition have long remained a major area of ecological research. One line of evidence for the potential occurrence of competition has been the degree of dietary overlap, traditionally determined by studies of gut contents or by direct observations of feeding. Over the last several decades, the analysis of stable isotopes has proven an additional and effective means of determining the trophic bases of fish populations, especially where a time-integrated approach is needed.
Competition can occur within or among species through active interference that limits access to resources by another individual or species. Competition can also occur passively through the common use of a resource. When competition occurs, it often is asymmetrical, impacting one individual or group more than another. Competition is typically evaluated through observational field studies, such as resource partitioning, through “natural experiments,” including character displacement and release, and through manipulative field and/or laboratory studies, all of which can be useful approaches. Especially with observational studies or “natural experiments,” evidence of competition is often inferred from shifts in niche overlap, as shown by the differentiation of sticklebacks and sunfishes in species-poor lakes into benthic and limnetic forms. Niche concepts are widely used in ecology, although their use is complicated by competing niche definitions. In interpreting any observational study of differential resource use, such as the commonly observed vertical habitat segregation by fishes, it is important to first eliminate the possibility that the pattern is a consequence of the history of the species (e.g., phylogeny), rather than caused by contemporary interactions. Studies of resource use and niche overlap in fishes are further complicated by the great range of body sizes, and perhaps habitats, shown by fishes as they transition from larval, to juvenile, to adult stages.
Trophic interactions in a community are complex, shaped by competitive or predatory interactions. Food webs and food chains (food chains show only a single pathway) can be in the form of connectance (essentially who eats whom) or the amount of energy transferred at each link or level. Interaction webs or chains comprise a third group and show the strengths of interactions, such as the impact of a predator on lower trophic levels. The number of trophic levels is sensitive to environmental disturbance and to the introduction of nonindigenous species. Competitive interactions have varying effects on fish assemblages, both over ecological and evolutionary time scales. Although a long-established concept in ecology, competitive exclusion of one group by another is rarely documented in nature.
SUPPLEMENTAL READING
Bowen, S. H. 1996. Quantitative description of the diet, 513–29. In Fisheries Techniques, 2nd ed. B. R. Murphy and D. W. Willis (eds.). American Fisheries Society, Bethesda, Maryland. An excellent source of information on the methods of dietary analyses in fishes.
Hurlbert, S. H. 1981. A gentle depilation of the niche: Dicean resource sets in resource hyperspace. Evolutionary Theory 5:177–84. An interesting attempt to categorize the multitude of different niche concepts.
Hurlbert, S. H. 1984. Pseudoreplication and the design of ecological field experiments. Ecological Monographs 54(2):187–211. An influential paper on problems of experimental design in ecology—I consider this a “must read” paper for students in any area of ecology.
Pfennig, K. S, and D. W. Pfennig. 2009. Character displacement: Ecological and reproductive responses to a common evolutionary problem. The Quarterly Review of Biology 84:253–76. A thorough current review of the concept of character displacement.
Post, D. M. 2002. The long and short of food-chain length. Trends in Ecology and Evolution 17:269–77. A clear discourse on the types of food webs and food chains and on factors influencing food-chain length.
Schoener, T. W. 2009. Ecological niche, 3–13. In The Princeton Guide to Ecology. S. A. Levin (ed.). Princeton University Press, Princeton, New Jersey. A concise overview on three current niche concepts.
Wiens, J. A. 1977. On competition and variable environments. American Scientist 65:590–97. A timeless paper emphasizing the aperiodicity of competitive interactions in naturally variable environments.