CHAPTER 22
Blood Transfusion
Introduction
Transfusion of blood products is a common occurrence during surgery. Anesthesia technicians may be called upon to retrieve blood products, help check them in, and help administer them. Transfusion of incompatible blood products to a patient can cause serious patient injury, and anesthesia technicians should be familiar with basic transfusion medicine. This chapter provides an introduction to the different types of blood products, what makes them compatible or incompatible with a patient, how they should be administered, and the potential complications or adverse reactions from the transfusion of blood products.
Blood Types
The different blood types (blood groups) and their relationship to the immune system are the basis of transfusion science. Blood types are inherited and represent contributions from both parents. A total of 30 human blood group systems are now recognized by the International Society of Blood Transfusion (ISBT). A blood type is a classification of blood based on the presence or absence of inherited antigenic substances on the surface of red blood cells (RBCs). Antigens, which may be proteins, carbohydrates, glycoproteins, or glycolipids, are present on the cellular membrane of RBCs and are also secreted to plasma and body fluids. Antigens determine the blood group type. In 1900, Karl Landsteiner, Austrian biologist and physician, discovered the ABO blood groups for which he received the 1930 Nobel Prize in Medicine and Physiology. The ABO antigen system is the most important determinant of blood type grouping in transfusion medicine. The two major RBC antigens are known as A and B. The blood groups are A, B, AB, and O, where O is when the RBCs lack both A and B antigens. People with AB blood type have RBCs that have both antigens. People with RBCs that only have the A or B antigen are blood types A and B, respectively.
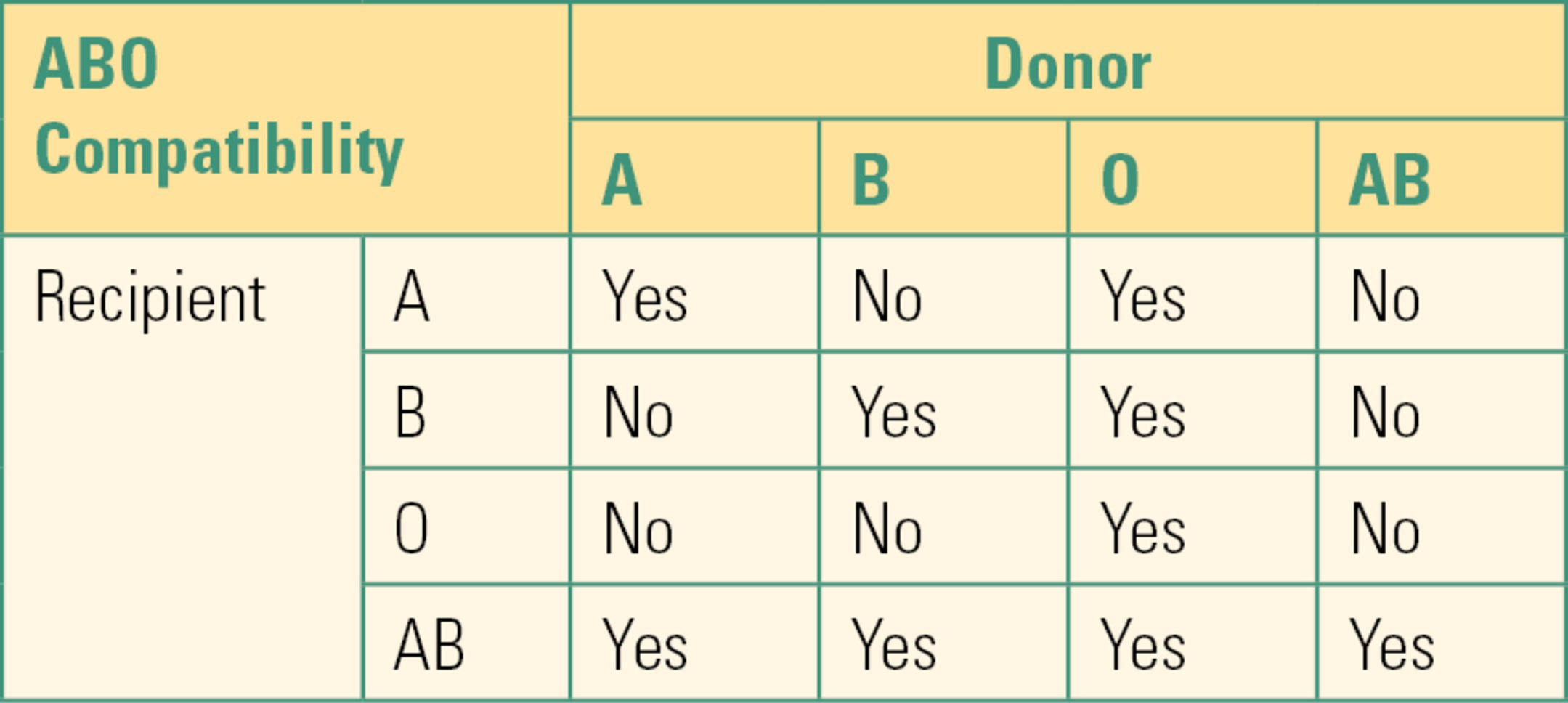
ABO compatibility remains the major safety consideration of blood product transfusions (Table 22.1). Compatibility means that the recipient does not recognize the blood transfusion as foreign. Immune systems of virtually all individuals produce antibodies directed against antigens they do not have (anti-B antibodies in type A individuals, anti-A antibodies in type B individuals, and anti-A and anti-B antibodies in type O individuals). The process whereby foreign antigens from blood groups cause production of antibodies directed against them in the recipient is called alloimmunization. This concept is extremely important to the understanding of transfusion medicine. If a recipient patient receives blood that has antigens foreign to the recipient, the recipient can mount a massive immune reaction (allergic reaction) against the foreign blood. These reactions are particularly severe if the recipient has preformed antibodies (a primed immune system) against the foreign antigen. This type of reaction is akin to an anaphylactic reaction except that the foreign antigen is the transfused blood. Humans form antibodies to A or B antigens in the first years of life if they do not have them on their own RBCs. This is thought to be triggered by the exposure to environmental antigens (food, bacteria, virus, etc.). Thus, humans are usually “primed” against ABO-incompatible blood. A reaction to ABO-incompatible blood is called an acute hemolytic transfusion reaction and is fatal in about 10% of cases. The recipient may not only manifest symptoms of an anaphylactic reaction (low blood pressure, fever, bronchospasm) from the immune mediators released but also suffer because his or her immune system attacks the foreign blood cells, causing them to hemolyze (rupture) and release free hemoglobin into the bloodstream. Free hemoglobin is a large protein and is very toxic to the kidney. It is not surprising that many of the first recipients of blood transfusions, before there was an understanding of blood group antigens, died from a severe transfusion reaction. Other types of transfusion reactions will be discussed below. The primary cause of transfusing ABO-incompatible units (incorrect blood type) is clerical errors in patient identification or errors in sample labeling.
Table 22.1. ABO Compatibility Chart
The second most important blood group system is the rhesus (Rh) system. Rh positivity is indicated by the presence of D antigen in the membrane of the RBC; D antigen is absent in Rh (D)-negative individuals. About 15% of people are Rh (D)-negative. Unlike the ABO system, Rh (D)-negative individuals do not produce anti-Rh (D) antibodies until they are exposed to Rh (D)-positive blood. When an Rh (D)-negative individual is exposed to Rh (D)-positive cells, sensitization occurs and the immune system can produce anti-D alloantibody. Any subsequent exposure to Rh (D)-positive blood can result in a severe adverse reaction. Sensitization can occur by transfusion or during pregnancy. If an Rh (D)-negative mother is pregnant with an Rh (D)-positive baby (the baby inherited the Rh (D) antigen from the father), the mother will become sensitized to the Rh (D) antigen. This happens because there can be some mixing of the baby’s blood with the mother’s blood at delivery. Once a mother is sensitized to the Rh (D) antigen, she can form antibodies that can attack the blood of a subsequent fetus if the fetus is Rh (D)-positive. Even in emergencies, Rh (D)-positive blood should not be given to Rh (D)-negative patients to avoid sensitization. Typically, type O Rh (D)–negative blood (O neg) is stored by hospitals for emergency transfusion because of its near-universal safety for patients with untyped blood due to its lack of AB or Rh (D) antigens.
About 30 other blood group systems exist in addition to the ABO and Rh (D) systems: Lewis, I system, P system, MNScU system, Kell protein, and the Duffy and Kidd antigens. These antigens can be present on RBCs and result in incompatibility, but these are not necessarily tested for in every patient because they are extremely rare, extremely common, or compatibility can be ensured by providing warmed blood (above 30°C).
Patients who have received multiple transfusions over the course of their life are at higher risk of developing antibodies, and it may be more difficult to find a compatible unit. Procuring a compatible unit can take extra time and may result in a surgical delay.
Compatibility Testing
Before any RBC unit is given to a patient, it undergoes several different tests to ensure that it is compatible with the recipient. The tests are separate from the testing done for diseases such as hepatitis and human immunodeficiency virus (HIV). Screening of potential donors and rigorous testing of donated units have largely eliminated the risk of units containing HIV, hepatitis, and other infections. The first test, known as the type and screen, is done on donated blood before releasing the unit. This test determines the blood type—A, B, AB, O, and Rh (D)-positive/Rh (D)-negative. This testing is also done on the patients to determine their blood type as well as screen for the presence of antibodies to A or B and antibodies against other antigens known to cause hemolytic reactions. The test is performed by mixing the sample blood (the patient’s blood or the donated blood) with a solution containing antibodies against the antigen being screened. For example, if one wants to determine if a patient has A antigen on his or her RBCs (he or she is blood type A or AB), a blood sample from him or her is mixed with a solution containing anti-A antibodies. If the resulting mixture agglutinates (clumps), it is because the antibodies have bound to cells with the A antigen. A second phase of the test involves mixing the patient’s plasma with commercially available O-negative RBCs that have approximately 20 different antigens that can cause a hemolytic reaction. If the patient’s plasma does not agglutinate these cells, the screen is negative.
If the patient’s plasma does react to the cells, the patient possesses at least one antibody to a significant antigen and the screen is positive. If the screen is positive, further testing must be done to identify the antibody and to locate blood units that lack the antigen.
The second test, the crossmatch, checks the patient’s blood against a specific donor unit for errors in ABO type. If the screen portion of the type and screen test was negative, the crossmatch consists of matching the patient with compatible donor blood. No real “test” is performed. The crossmatch involves matching the paperwork for the donor unit and the recipient. This step can even be performed by automated vending style machines that scan barcodes from the patient ID and the donor unit. If the patient’s screen was positive, a serologic crossmatch test is performed. This test is conducted by mixing the patient’s serum with a specific donor unit that has been selected because it lacks the antigens that the patient has antibodies against identified during the screen. A crossmatch is only necessary when the patient receives RBCs, as opposed to plasma or other blood derivatives.
Compatibility of plasma is different from that of whole blood or RBCs. Plasma from an AB blood type donor can be transfused to a recipient of any blood group. This is because the AB donor plasma lacks A or B antibodies and will not react with the recipient’s RBCs even if he or she has the A or B antigen. Type O recipients already have A and B antibodies and can receive plasma from any blood type. The only problem is plasma from a type O donor. This donor has A and B antibodies in the plasma, and it cannot be given to a type A, B, or AB recipient.
In emergency situations when RBC transfusion is immediately needed, abbreviated testing methods are necessary. Type-specific blood is always essential (unless the donor unit is O), but there are abridged versions of the crossmatch: partial crossmatch checks for the most severe errors (ABO-Rh (D) blood type) and takes less than 10 minutes; uncrossmatched blood is less risky in previously untransfused patients and those who have not born children. The risk of complication from uncrossmatched blood varies between 1 in 1,000 and 1 in 100 patients depending on the history of previous exposure to donated blood. Trauma centers may also keep type O, Rh (D)-negative (“universal donor”) blood on hand for immediate use. Ideally, it should be completely compatible, but it is always possible that there are anti-A or anti-B antibodies in the donor unit that could cause a reaction.
Indications for Transfusion
According to the 2015 guidelines of the American Society of Anesthesiologists (ASA), a restrictive RBC transfusion strategy may be used to reduce transfusion requirements. The definitions for a restrictive vs liberal strategy for blood transfusion vary in the literature, although hemoglobin criteria for transfusion less than 8 g/dL and hematocrit values less than 25% are typically considered as restrictive.
However, the hemoglobin level should not be the sole consideration, as there are other patient-related and surgical factors that help determine the “trigger” for transfusion. Patients can either receive their own previously donated blood (autologous blood) or someone else’s donated blood (allogenic, homologous blood). Autologous blood, which needs to be donated ahead of time, is considered safer than allogenic blood mainly due to lower risk of infection and is preferred over allogenic transfusion. Potential complications related to autologous transfusion include anemia from the donation itself, the need for more frequent transfusions, and even febrile and allergic reactions. Autologous units are typed and crossmatched just as all allogenic units are. When autologous blood is donated by patient, sufficient time should be allowed for the patient to make more RBCs again before surgery.
Per ASA guidelines, other strategies for reducing intraoperative allogeneic transfusion include blood management protocols, reversal of anticoagulants, antifibrinolytics for prophylaxis of excessive blood loss, and acute normovolemic hemodilution (ANH) with crystalloids.
Complications and Adverse Reactions
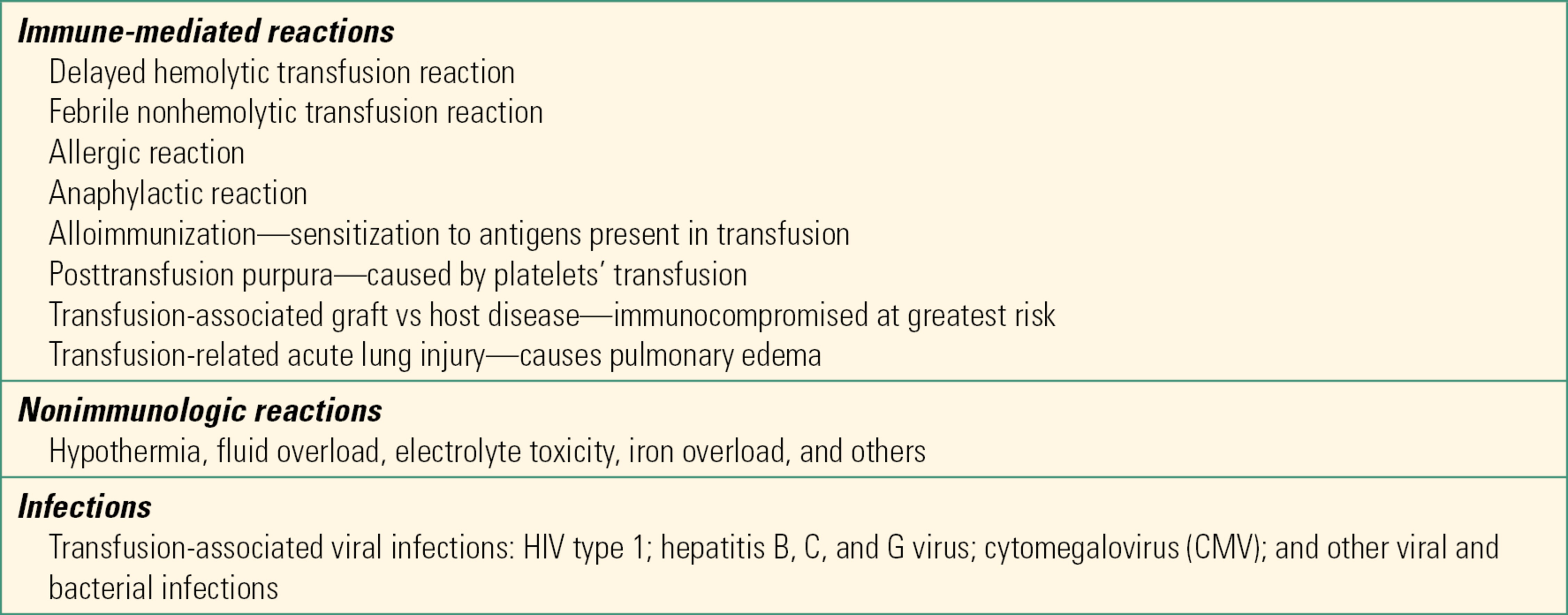
There are a variety of adverse reactions that come from a few basic sources: contaminated or infected blood (e.g., HIV or hepatitis), incompatible blood, and problems related to the infusion of RBCs (e.g., transfusion-related lung injury, volume overload, electrolyte disturbances, and coagulopathy). Table 22.2 summarizes some of the possible complications of transfusions. As soon as an acute adverse reaction to a transfusion is suspected, the first step is to stop the transfusion and call the blood bank. The three most common causes of transfusion-related deaths are hemolytic transfusion reactions, septic transfusions, and transfusion-related lung injury. Acute hemolytic transfusion reaction is caused by type incompatible blood, typically due to human error at some point between issuing the unit and transfusion. The body mounts an immune response to the offending blood, which can lead to severe coagulation issues, kidney failure, and even death.
Table 22.2. Other Adverse Reactions
Other complications are more common during a massive transfusion (loss of at least one times the patient’s blood volume):
- Hypocalcemia: The citrate used to preserve blood can bind calcium in the patient, leading to hypocalcemia. This is more common when the units are transfused quickly (>1 unit in 5 minutes), it is a massive transfusion, or the patient has liver dysfunction and has difficulty in metabolizing citrate. Monitoring of blood calcium levels will guide treatment with calcium.
- Coagulopathy: Packed RBC units contain minimal platelets or plasma clotting factors. Patients who receive large transfusions will require replacement of platelets and clotting factors to avoid a dilutional coagulopathy.
- Hyperkalemia: Typical blood units contain less potassium than normal blood; as the blood is stored, the RBCs release potassium. Rapid or large transfusions of RBC units, particularly older units, can lead to a significant potassium increase in the recipient.
- TRALI: transfusion-related lung injury is characterized by the acute onset of the noncardiogenic pulmonary edema following administration of the blood products. It is typically associated with the transfusion of plasma components like thrombocytes and fresh frozen plasma, less likely with RBC transfusion.
Handling, Verification, and Storage
Retaining the oxygen-carrying capacity of blood throughout its shelf life is one of the primary problems of blood storage. Over time, red cells lose their capacity to carry the same amount of oxygen as they could when fresh. It is hard to standardize the capacity of each unit because each starts from a different level and degrades at different rates. Different products are added to each unit to prolong the shelf life. One of the most common is citrate-phosphate-dextrose-adenine (CPDA-1): citrate is an anticoagulant, phosphate is added as a buffer, dextrose provides an energy source for the red cells, and adenine allows the cells to make adenosine triphosphate (ATP), a common cellular energy source. AS-1 (Adsol), AS-3 (Nutricel), and AS-5 (Optisol) are similar to CPDA-1 with slight variations.
Storing the units between 1°C and 6°C slows down the metabolic processes of the red cells, but glycolysis (RBCs do not use the citric acid cycle because they lack mitochondria) still converts glucose to lactate. This accumulation of lactate lowers the pH of the unit and alters the intra- and intercellular concentrations of sodium and potassium. The lower pH also contributes to the decreased oxygen-carrying capacity of the RBCs. If several units of plasma or packed red cells are needed in the operating room, they are often kept in a cooler or bucket with ice; the ice and blood product should be separated by a towel or other barrier to prevent the blood from freezing. The formation of ice crystals damages the RBCs. Another important reason to keep the blood cold in the operating room is to be able to return it to the blood bank if it is not used.
Administration
Each unit must be checked at the bedside before transfusing. The check consists of double checking the information on the unit, its accompanying paperwork, and the patient information. This procedure will vary from institution to institution, but typically requires two people, and often one of them must be licensed (e.g., a physician or a nurse). Commonly, the unit number, expiration date, blood type, and some type of patient identifier are used (Fig. 22.1). This is a role that the anesthesia technician frequently participates in.
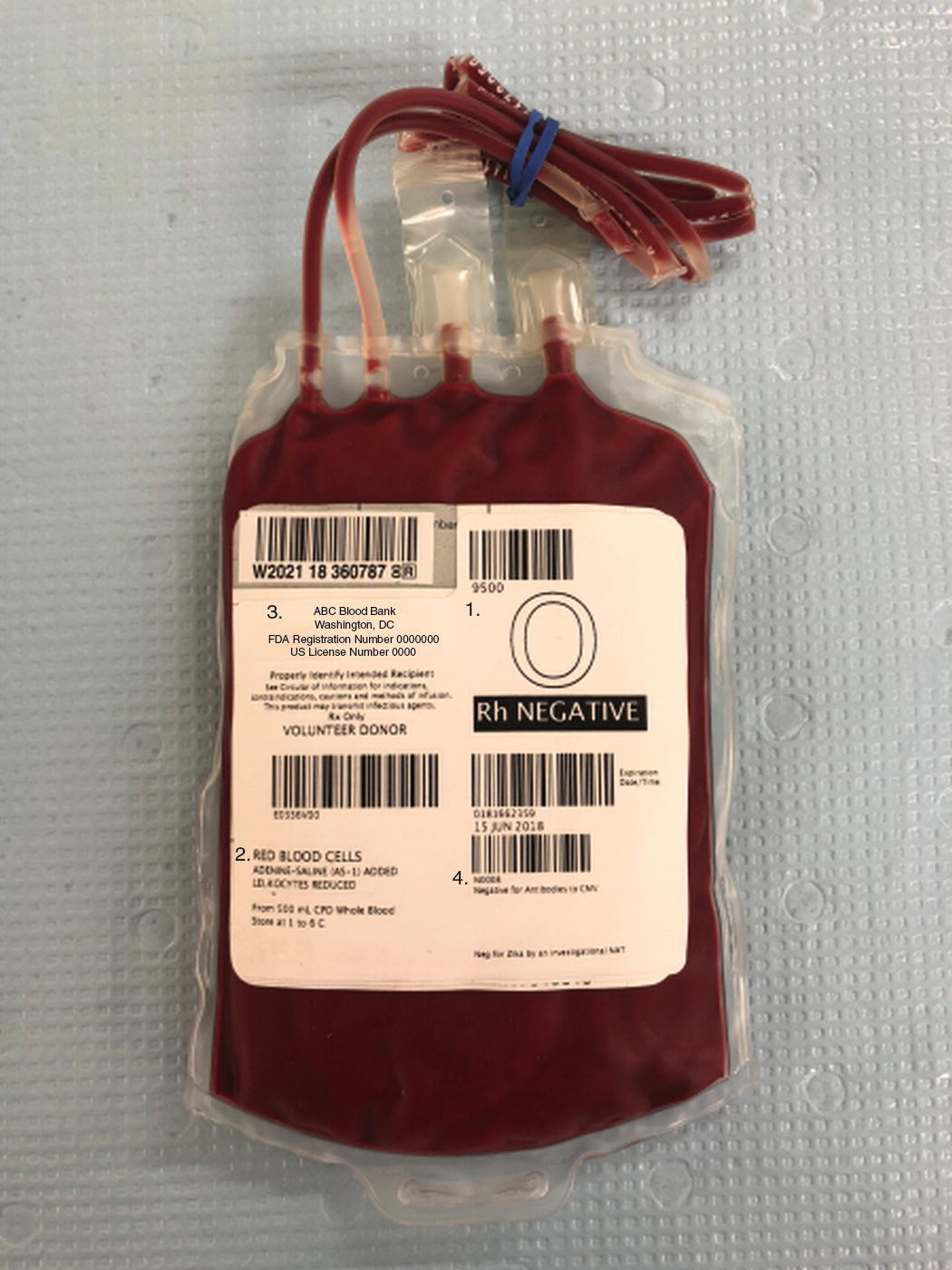
FIGURE 22.1. Transfusion unit of red blood cells (RBCs). 1. Blood type: 0 Rh (D)-negative, 2. RBCs transfusion unit—leukoreduced, 3. Unit number, 4. Expiration date.
Blood products are typically administered through special blood administration tubing (Fig. 22.2). The vast majority of blood administration sets have an in-line filter to remove cellular debris and coagulated proteins. Most filters are designed for transfusion of two to four RBC units before they should be changed. Refer to the product information for specific guidelines. Some practitioners prefer to attach a separate blood filter to the spike. After multiple units have been transfused, the filter can be replaced without having to replace the entire tubing setup. Again, refer to the product information for the number of RBC units that can be transfused before filter replacement is recommended, as some filters allow up to 10 units. The tubing used in operating rooms usually has a Y connector with two separate “spikes” so that a unit or fluid can be prepared on one spike while the other is being actively used for transfusion or fluid administration. Typical blood administration sets used in the operating room also include a squeezable chamber that allows pumping the fluid or blood products to speed administration. Pediatric blood sets can come equipped with a chamber (buretrol) in which the provider can measure out a specific amount of blood product or fluid. This allows more precise administration of fluids or blood products, which can be critical in pediatric patients.
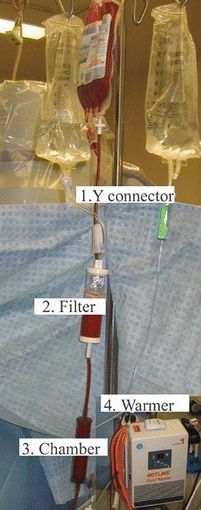
FIGURE 22.2. Blood administration tubing. 1. Y connector. 2. Filter—holds debris and particles. 3. Chamber—blood can be pumped by squeezing the chamber to speed delivery. 4. Warmer—blood is warmed by passing through the hot-line system. (Courtesy of Smiths Medical.)
Blood products are typically warmed before being given to a patient. This is often achieved with in-line heated tubing. The majority of these units utilize heated water, which is circulated outside an inner set of tubing through which the blood product or fluid is administered. As mentioned earlier, heating units above 30°C can reduce the risk of some complications. Additionally, blood is typically stored at 4°C, and transfusing cold units to a patient could rapidly lower his or her body temperature. Platelets are the exception and are stored at room temperature. There are many other transfusion devices that are mainly used during emergency situations, such as the Level One, Belmont, and pressure bags. These devices are covered in more detail in Chapter 62, Massive Hemorrhage. They provide heating, filtration, and the ability to rapidly administer blood products and fluid. It is routine practice to draw blood samples for laboratory testing of the patient’s blood before and after transfusion. Testing includes hematocrit and other values like electrolytes, glucose level, coagulation studies, etc., and it is often the anesthesia technician’s responsibility to run these samples if the operating room suites have their own blood gas analyzer machine.
Different Blood Products
While blood donations typically consist of whole blood, or blood containing all of its normal components (RBCs, plasma, etc.), they are routinely processed into several different products for clinical use. Today, whole blood is not readily available for transfusion and instead serves as the basis for further processing. Because the different components in blood differ in density, blood banks use centrifugation to separate the different parts, resulting in different blood products (see Table 22.3).
Table 22.3. Different Blood Products
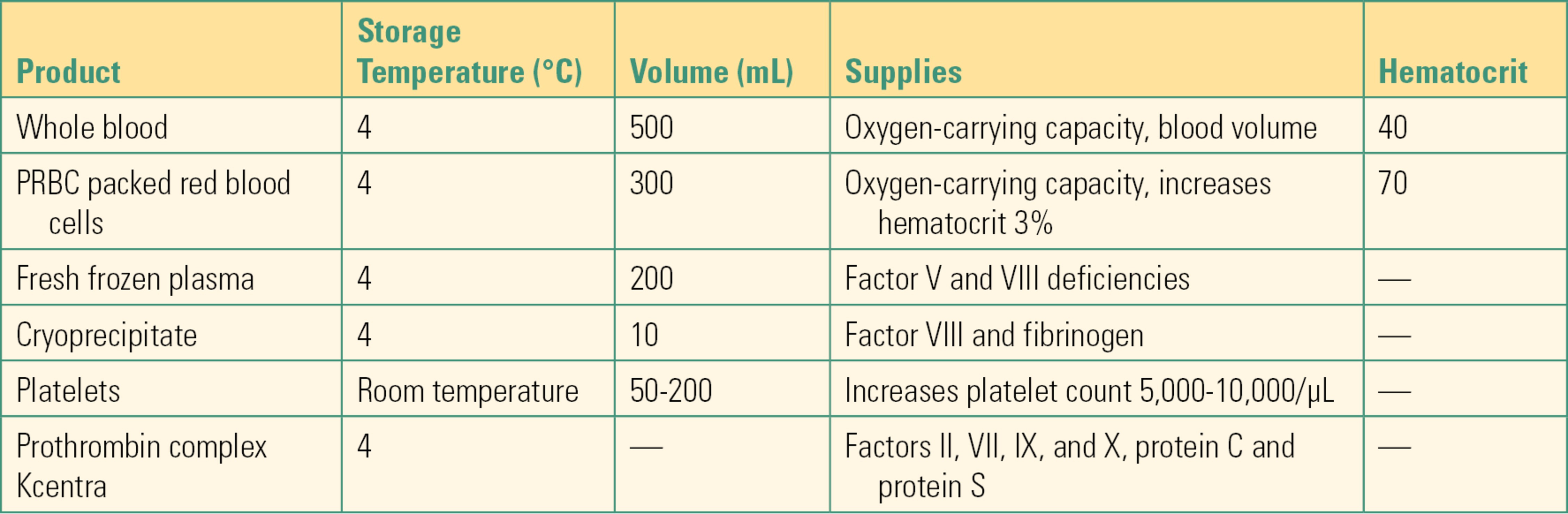
- Packed red blood cells (PRBCs): PRBCs provide additional oxygen-carrying capacity because of the hemoglobin they contain. Transfusion of white blood cells (leukocytes) increases the risk of infection because they suppress the immune system of the recipient. Transfusion of leukocytes can also sensitize the recipient to leukocyte antigens. For these reasons, PRBC units have the vast majority of leukocytes removed by filtration (leukoreduction). The hematocrit value of a PRBC is 70%. When RBCs are separated from whole blood, plasma and other blood components are retained for other use.
- Fresh frozen plasma (FFP): FFP contains plasma proteins including factors V and VIII, which are needed for effective blood clotting. FFP replaces the coagulation factors lost with bleeding and can also be used as a reversal agent for anticoagulation drugs like warfarin, in treatment of immunodeficiencies, in antithrombin III deficiency, in massive blood transfusion, and in other coagulation system deficiencies. FFP does increase the circulating volume but should never be used as primary volume expander. FFP is stored frozen and after thawing it must be used between 24 hours and 5 days, which will be indicated on the expiration date printed on the bag. Because of the time it takes to thaw stored FFP, there can be a delay in receiving FFP after it has been requested. As mentioned above, FFP must be compatible with the recipient’s ABO-Rh (D) type.
- Platelets: Platelets play a vital role in how the body forms blood clots. Platelets can be collected either from whole blood donations or separately from platelet-specific donations. Because they are stored at room temperature (never place them in the refrigerator with other blood products), they present a higher risk of bacterial contamination. Indications for platelet transfusion vary but are guided by the patient’s platelet count (provided by the laboratory) and the extent of surgical bleeding. It is not necessary to provide ABO-compatible platelets.
- Cryoprecipitate: Cryoprecipitate contains high concentrations of clotting factor VIII and fibrinogen and is used to treat clotting factor deficiencies including hemophilia A. Cryoprecipitate also contains other clotting factors and plasma proteins. Cryoprecipitate should be filtered when administered, and it must be used within 6 hours of thawing. Cryoprecipitate is usually administered as ABO compatible, but it is not too important since the concentration of antibodies in cryoprecipitate is extremely low.
- Prothrombin complex: Prothrombin complex concentrate contains vitamin K–dependent coagulation factors II, VII, IX, and X and antithrombotic protein C and protein S. Administration of the prothrombin complex concentrate increases plasma levels of all components. It is used to reverse the effect of vitamin K antagonist warfarin, to treat factor IX deficiency, hemophilia B, and other bleeding disorders.
Summary
Blood cells are intimately related to the immune system and carry multiple antigens. Because of the presence of these antigens, the transfusion of foreign blood products may activate the immune system of the host, resulting in severe reactions, even death. Both patients and blood products prepared by the blood bank undergo extensive testing to ensure compatibility between the donor and the patient receiving the transfusion. This chapter introduced the basic concepts of transfusion medicine including blood types, compatibility testing, indications for transfusion, and potential adverse reactions to transfusions. Familiarity of these concepts is essential for all operating room personnel, including anesthesia technicians, who are involved with the transfusion process.
Review Questions
1. What is an antigen?
A) A unique cellular marker on cell surfaces
B) A molecule made up from amino acids
C) A part of the cell’s nucleus
D) The oxygen-carrying part of a red cell
Answer: A
Antigens are markers that are used by the body to identify different cells.
2. Why is compatibility testing important?
A) To avoid losing blood units at the blood bank
B) To avoid adverse reactions, even death, from transfusion
C) To screen for HIV-1
D) To prolong unit shelf life
Answer: B
Compatibility testing ensures that patients will not receive incompatible transfusions, for this could cause death.
3. Which of these is not a major cause of transfusion-related death?
A) Septic transfusions.
B) Transfusion-related hyperkalemia.
C) Transfusion-related lung injuries.
D) Hemolytic transfusion reactions.
E) All of these are major causes of transfusion-related death.
Answer: B
Hyperkalemia can be a common complication during massive transfusion; however, it is not considered a major cause of transfusion-related death. The three most common causes of transfusion-related death are septic transfusions, transfusion-related lung injuries, and hemolytic transfusion reactions.
4. What are the primary causes of noncompatible transfusions?
A) Bacterial contamination
B) Excessively clumped platelets
C) Clerical errors
D) Expired crossmatch test tubes
Answer: C
Compatibility testing is very sophisticated, but clerical errors can cause an incompatible transfusion.
5. Cross-matching is the process by which the paperwork for the donor unit and the recipient are matched and is subject to clerical error. When is cross-matching required?
A) Whenever a patient receives a transfusion
B) Whenever the patient receives blood from a donor (as opposed to blood they had previously donated)
C) When the patient receives red blood cells
D) When the patient receives plasma
E) Only in nonemergency situations
Answer: C
Cross-matching is only required when the patient receives red blood cells. In emergency situations, there is an abridged crossmatch that tests only for the most severe errors, such as ABO and Rh-D compatibility.
6. What is FFP used for?
A) Raising hematocrit
B) Expanding blood volume
C) Replacing clotting factors
D) Raising concentration of fibrinogen
E) To treat factor IX deficiency
Answer: C
FFP contains plasma factors like factors V and VIII, which are needed for effective blood clotting. FFP replaces coagulation factors lost with bleeding and is used as a reversal agent for anticoagulation drugs like warfarin, in the treatment of immunodeficiencies, in antithrombin III deficiencies, in massive blood transfusion, and in other coagulation system deficiencies; however, it should never be used as a primary volume expander. Other blood products include packed red blood cells (which increase the hematocrit levels), platelets (helps the body form blood clots), cryoprecipitate (contains high concentrations of clotting factor VIII and fibrinogen and is used to treat clotting factor deficiencies like hemophilia A), and prothrombin complex (contains vitamin K–dependent coagulation factors II, VIII, IX, and X and antithrombotic protein C and protein S and is used to reverse the effect of vitamin K antagonist warfarin, to treat factor IX deficiency, hemophilia B, and other bleeding disorders).
7. Why are O-negative patients considered universal donors?
A) They have a lower hematocrit.
B) They lack Rh (D) antigen.
C) They lack antigens on their cell surfaces.
D) They provide extra factor VII.
Answer: C
Type O red blood cells do not carry antigens on their surfaces that the recipient could recognize as foreign. When red cells are transfused, it is the transfused red cell antigens into a patient with an anti-A or anti-B antibody that can cause a hemolytic reaction. Patients who have type A blood, for example, have anti-B antibodies that recognize B antigens as incompatible and cannot receive type B or AB blood cells. Type O blood cells carry no antigens and cannot cause an ABO reaction in any patient.
8. Which of these regarding transfusion tubing is false?
A) Most tubing filters are designed to transfuse 2-4 RBC units before they need changed.
B) The tubing in the OR typically has a Y connector with two separate “spikes” so that a unit or fluid can be prepared on one spike while the other is being actively used for transfusion or fluid administration.
C) Some pediatrics sets come with buretrol in which the provider can measure out a specific amount of blood product or fluid.
D) After multiple RBC units have been transfused, the entire tubing setup should be replaced so as to prevent possible machine malfunctions.
E) In-line heated tubing is used to warm the blood products during transfusion.
Answer: D
After multiple RBC units have been transfused, only the filters need to be replaced; the tubing setup can remain in place. Most tubing filters are designed to transfuse 2-4 RBC units before they need changed, but since specifications can change by manufacturer, you should know how much RBC units can be transfused before the filters you’re using need changed.
9. How is the shelf life of stored blood extended?
A) The addition of nutrient supplements
B) Repeated freezing and thawing
C) Inverted storage freezers
D) Constant centrifugation
Answer: A
Nutrients help keep red cells alive while they are outside of the body. Blood cells cannot be frozen, though storing them between 1°C and 6°C slows their aging and metabolic processes. FFP is frozen once; when it has been thawed, it must be used or discarded and cannot be refrozen.
10. What is the rhesus blood group?
A) The markers of the Kell blood group system
B) What makes AB patients universal recipients
C) The determinant of cryoprecipitate effectiveness
D) Blood group with D antigens in cell membranes
Answer: D
The rhesus blood group is identified by the D antigen.
SUGGESTED READINGS
Apfelbaum JL, Nuttall GA, Connis TR, et al. Practice guidelines for perioperative blood management. Anesthesiology. 2015;122:241-275.
Dzieczkowski JS, Anderson KC. Transfusion biology and therapy. In: Kasper DL, Fauci AS, Hauser SL, eds. Harrison’s Principles of Internal Medicine. 19th ed. New York, NY: The McGraw-Hill Education; 2015.
Hess JR. Conventional blood banking and blood component storage regulation: opportunities for improvement. Blood Transfus. 2010;8(suppl 3):s9-s15.
Miller RD, ed. Patient blood management: transfusion therapy. In: Miller RD, Erikson LI, Fleisher LA, eds. Miller’s Anesthesia. 8th ed. Philadelphia, PA: Elsevier Saunders; 2015:1830-1867.
Welsbay IJ, Hathaway JA. Blood and blood component therapy. In: Longnecker DE, Braown DL, Newman MF, eds. Anesthesiology. 2nd ed. China: The McGraw-Hill Companies; 2012.