CHAPTER 33
Temperature Management
III
Introduction
Temperature management is an American Society of Anesthesiologists (ASA) standard and required basic monitor. Patients undergoing any anesthetic must have temperature monitored “when changes in body temperature (intended, anticipated or suspected) could be clinically significant , ” and those undergoing general anesthesia must be monitored for temperature in cases greater than 30 minutes. Patients under MAC (monitored anesthesia care) sedation need temperature monitoring available. During regional anesthesia, core temperature should also be measured in patients likely to become hypothermic, such as those undergoing intra-abdominal surgery.
The body produces heat from cellular metabolism. Maintaining homeostasis to provide a consistent body temperature involves a delicate balance between basal metabolic rate, muscle activity, vascular tone, and sympathetic stimulation against outside factors that influence the body to produce heat and/or release heat to the environment. This balance is tightly regulated and can be disturbed by anesthesia. Almost all body functions work best in a very narrow range of temperature, and small disturbances can produce poor patient outcomes after surgery; thus, anesthesia providers focus on monitoring temperature and maintaining it when patients cannot do this for themselves.
Thermoregulation
Thermoregulation is maintained centrally in the brain by the hypothalamus, which distributes impulses via the central nervous system (CNS) throughout the body to maintain temperature between 36.1°C-37.2°C (97°F-99°F) with 37°C (98.6°F) considered normal. Studies have shown that a “normal” body temperature can range widely. A temperature over 38°C (100.4°F) is considered to be a fever. Conversely, hypothermia is defined as a temperature below 35°C (95°F). Thermal receptors distributed throughout the body (skin, abdomen, thoracic tissues, and spinal cord) transmit to the hypothalamus via A-δ and C fibers to influence temperature adjustments from the CNS. Control is mediated by voluntary mechanisms (e.g., adjusting environmental temperature, wearing appropriate clothing, going indoors, etc.) and involuntary reflexes like vasodilation, sweating and shivering, and alteration of metabolic rate via the thyroid and adrenal glands.
Mechanisms to Protect the Body against Cold are Blunted by General Anesthesia
General anesthetics produce a profound dose-dependent reduction in the core temperature, triggering cold defenses that include arteriovenous shunt, vasoconstriction, and shivering. Anesthetic-induced impairment of normal thermoregulatory control, and the resulting core-to-peripheral redistribution of body heat, is the primary cause of hypothermia in most patients (Fig. 33.1).
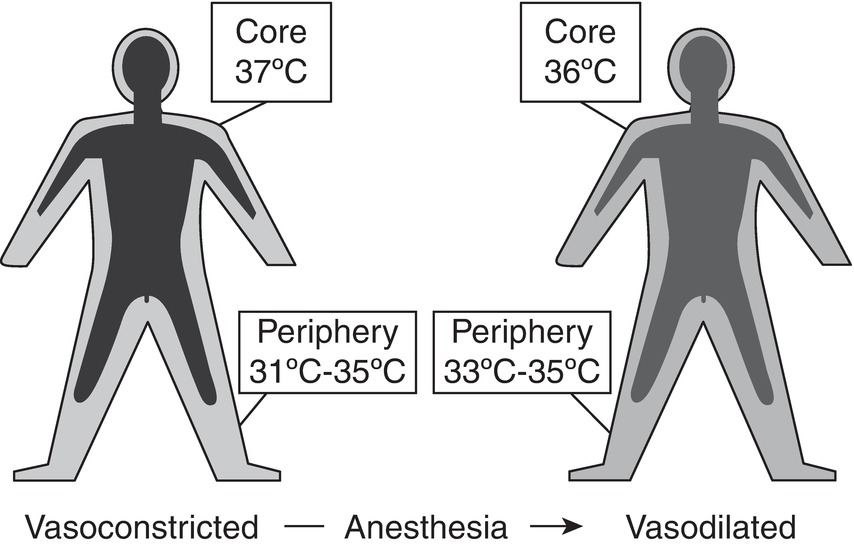
FIGURE 33.1. Redistribution of heat due to volatile anesthetics.
Volatile anesthetics and opioids have little influence on heat release via sweating but reduce vasoconstriction and shivering thresholds profoundly. Regulation of temperature is well maintained in infants and children but may be impaired to an extent in the elderly patient. Neuraxial anesthesia (spinal or epidural anesthesia) also impairs thermoregulatory control, though to a lesser extent than general anesthesia. Patients regionally blocked and insensate may not subjectively feel hypothermic, which is why temperature monitoring is necessary. Prolonged epidural analgesia is associated with hyperthermia; the physiologic cause and significance of this is unknown.
With anesthesia-induced vasodilation, heat loss is rapid due to radiation, convection, conduction, and evaporation in the operating room with its lower set temperatures. The most common mechanism of intraoperative hypothermia is heat loss by radiation, followed by convection, conduction, and evaporation. Since up to 90% of body heat is lost via radiation and convection, these become the major targets for maintaining normothermia perioperatively (Fig. 33.2).

FIGURE 33.2. Four mechanisms of heat loss. A: Radiation. Heat energy radiates directly from the warm body, just as light and heat energy radiate from the sun. B: Convection. A warm person continuously warms the layer of air around him or her. If this air remains still, it insulates the person against further heat loss. (This is how a fluffy jacket works, and why it is no longer warm if it is squished down.) Circulating air continuously blows away the warmed air; the warm person then loses additional heat warming the surrounding cold air. Operating rooms have standards for continuous laminar air flow. C: Conduction. When a warm person is next to a cold surface, heat diffuses lost from the warm person into the cold object. If the cold object conducts heat well and continues to carry it away (like a large, cold operating room table), the heat loss can be great; this is called a “heat sink.” A gel pad, which does not conduct heat well, can help the patient retain heat. D: Evaporation. Water vapor leaving the body leaves at room temperature, carried away by dry, cold gas, and carries heat energy away with it.
Radiation:
- Major contributor to intraoperative heat loss.
- All surfaces above absolute zero (Kelvin) radiate heat.
- Heat transfer is proportional to the 4th power of the absolute temperature difference at an interface.
Convection:
- “Wind chill”—normal air currents disturb the insulating layer of still air next to the skin, allowing heat to escape
- Increased laminar flow in operating rooms
- Proportional to the square root of air speed
Conduction:
- Heat loss is proportional to the difference between two adjacent surfaces and the strength of thermal insulation separating them.
- Foam or gel pad between the patient and operating table reduces conductive loss.
Evaporation:
- Sweating increases skin evaporative loss but is rare under anesthesia.
- Insensible loss from open wound evaporation, varies with size of wound and degree of injury.
- Loss from skin surface (absent sweating) is less than 10% metabolic heat production in adults.
- Infants, especially premature babies, may lose up to 20% of metabolic heat production through transcutaneous evaporation.
- Can be profound with ventilation especially at high gas flows.
Hypothermia has important consequences for patient safety. Severe hypothermia can be life threatening if not corrected. Intraoperative hypothermia can lead to coagulopathies, electrolyte and acid-base abnormalities, as well as increased surgical site infection risk. A thorough list of physiologic effects of hypothermia can be found in Table 33.1.
Table 33.1. Table of Physiologic effects of Hypothermia
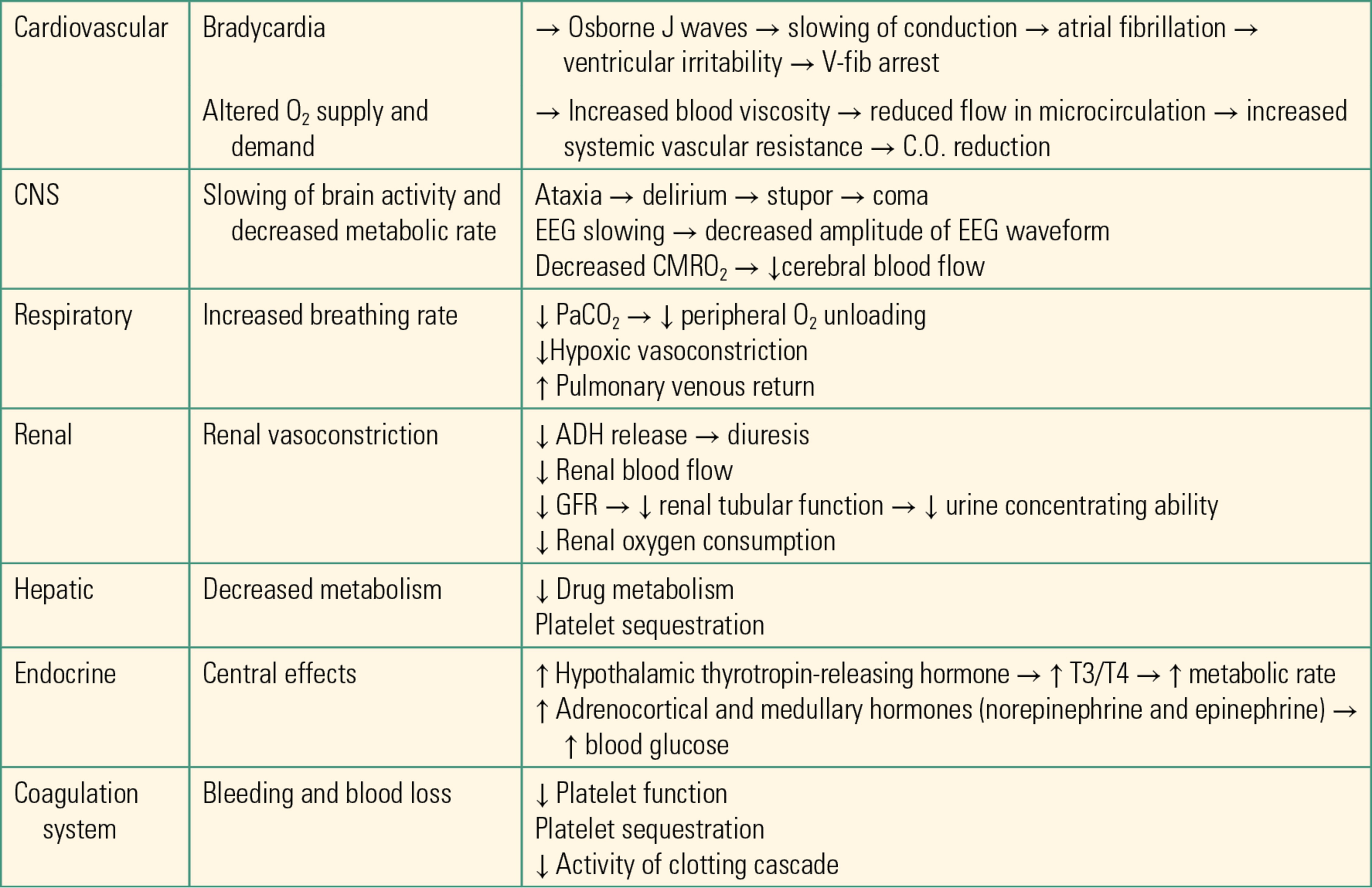
Adverse Effects of Hypothermia in Anesthetized Patients
Increased Blood Loss
Many studies have evaluated increases in blood loss due to hypothermia, including up to 500 cc EBL with a 1.6°C drop in temperature, and a loss of 186 cc with only a 0.5°C drop.
Increased Risk of Surgical Site Infection
Perioperative hypothermia is an established risk factor for surgical site infections in several retrospective studies. A twofold increased infection risk has been demonstrated with a 2°C drop in temperature.
Increased Cardiac Morbidity
Normothermia (maintaining a normal temperature) has shown a greater than 50% reduction in cardiac risk. Hypothermia can lead to dysrhythmias, from ECG changes like bradycardia and Osborne J waves. Mild hypothermia can also cause hypertension, increasing stress on the heart. Lowering the heart’s temperature to less than 28°C begins to induce ventricular fibrillation, due to slowing of transmembrane potential, multiple firing, and reentry. See Figure 33.3 for EKG of Osborn J waves.
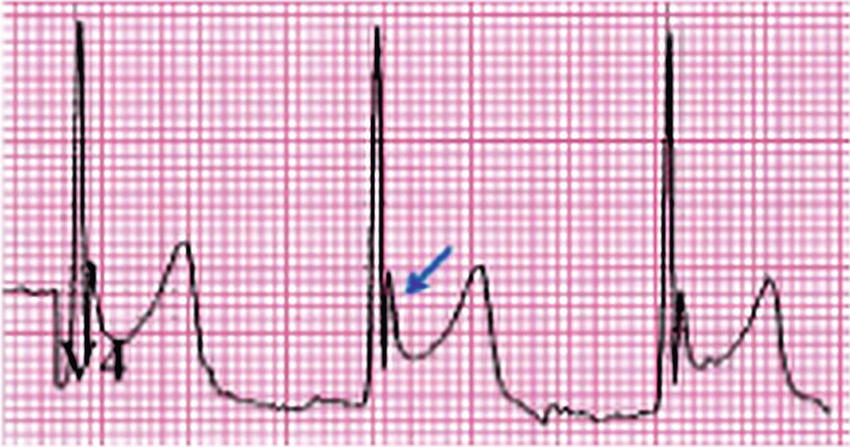
FIGURE 33.3. Osborn J waves.
Increased Anesthetic Potency and Prolonged Metabolism
Hypothermia has been shown to decrease MAC by 15% for every 1°C drop in temperature. Nondepolarizing muscle relaxants may be prolonged as much as 50% under hypothermic conditions.
Increased Recovery Time—PACU and Hospital
Hypothermia has been shown to increase total hospitalization for up to 3 days with a 2°C drop. Emergence and extubation from general anesthesia has been shown to be up to 10 minutes faster when normal temperature is maintained.
Treatment Options
Prewarming Before an Operation
Using a forced-air warming blanket preoperatively is effective at raising core temperature for patients waiting to proceed to the operating room. Some sites use blankets that are warmed in a cabinet, but many hospitals have transitioned to forced air blankets that are built into the patient’s gown so they may be warmed pre- and postoperation.
Forced Air
Intraoperative (forced air) warming devices are a cost-effective means of maintaining and augmenting body temperature in the operating room. These devices use a “blanket” that has many small perforations that allow the warmed air to escape under the drapes. There are many types and sizes of blankets: over body, underbody; some are even built into the patient’s gown. Devices come with multiple available temperature settings, which can be chosen depending on the goal for the patient: 43°C is the standard setting for warming patients, as this is considered the warmest safe setting to avoid burn injury. Cooler temperatures can be chosen if cooling is desired, either for patients with fever or for therapeutic hypothermia (see below). Warming hoses should never be used without the blanket attachment, as blowing undispersed warm air onto the patient can cause burns. Placing a blanket between the patient and the warming blanket is also not recommended.
Warming Fluids
Warming intravenous fluids with appropriately calibrated fluid warmers has been a mainstay in operating rooms for many years. There are several technologies that complete the same task: warming the flowing IV fluid before it reaches the patient. Heated water tubing, inductive heating, and electric elements are some of the options. Warming fluids is more effective at preventing than at treating hypothermia, because fluids cannot be heated beyond 43°C before they run the risk of burning patients. Three liters of fluid at 18°C (a typical operating room temperature) infused into a patient at 37°C can cool patients significantly, even in the absence of other causes of temperature drop, because the temperature difference between 18°C and 37°C is large. Heating blood products is even more important, since blood is stored at 4°C. These large thermal gradients cause large changes in temperature. A fluid warmer should be introduced as soon as transfusion begins. Care must be taken when warming fluids not to introduce air into lines.
Heating Lights
Properly positioned and measured distance-verified warming lights have been used to prevent heat loss, especially in pediatrics. These lights are available from many manufacturers and are available with or without visible light. Oftentimes, these lamps are infrared and used for heating only.
Maintenance
Artificial warming devices must be interrogated and calibrated on a scheduled basis to certify that they meet standards for equipment and do not overheat due to an inaccurate thermostat. Potential for harm, especially with fluid warmers not correctly calibrated, can be devastating. All anesthesia workrooms must engage an independent device reconciliation service, which provides a time stamp for the next interrogation of a device to meet its standard.
Hyperthermia
Hyperthermia is any increase in core temperature above the normal range. Fever is an active, regulated response to increase core temperature that is mediated by circulating endogenous pyrogens. Hyperthermia from fever can result from a variety of etiologies like serious infection, blood transfusion reaction, allergic response to medications, neuroleptic malignant syndrome, and malignant hyperthermia (MH). Serious attention is warranted due to the potential consequences of severe hyperthermia, including increased ICP, seizures, hypermetabolism, and even death. Diagnostic efforts must be taken to ascertain the source of a fever. Hyperthermia is particularly harmful to nervous system tissues in the brain and spinal cord; hyperthermia is aggressively treated in neurosurgical patients, and patients with suspected neurologic injury (such as after cardiac arrest) are occasionally even deliberately cooled for brain protection (see Chapter 58, Cardiac Arrest).
Neuroleptic Malignant Syndrome
This is a rare condition resulting in marked hyperthermia, rigidity, autonomic instability, and altered consciousness, which is caused by central dopamine receptor blockage in the brain. Key agents implicated in this disorder are drugs that interfere with normal dopamine release such as antipsychotics like haloperidol (Haldol), metoclopramide (Reglan), and abrupt stoppage of dopamine agonists like bromocriptine and levodopa as used in Parkinson disease patients. The syndrome, which has a high mortality, can manifest within 24-72 hours of receiving one of the offending agents and is not related to dose or course. Treatment involves discontinuation of the offending agent (or restarting dopamine agonist) along with dantrolene, supportive care, and close observation. A little-known treatment for these patients is electroconvulsive therapy, which releases a profound amount of dopamine to the brain. Neuroleptic malignant syndrome should not be confused with MH: it is not a genetic disease and not caused by anesthetic agents.
Malignant Hyperthermia
Temperature monitoring and management is an extremely important aspect in detection of severe reactions like MH. A sudden unexpected death of a healthy individual undergoing surgery can be a result of this rare but devastating response to volatile anesthetics (not nitrous oxide) or from a known triggering agent like succinylcholine. An MH crisis is an aberrant biochemical response “triggered” by anesthetic agents, which sets off a chain reaction. Although temperature increase is potentially a late sign, it is part of a massive hypermetabolic reaction, which manifests as increased heart rate, massively increased body metabolism with an uncharacteristically high ETCO2, and muscle rigidity. Fever may exceed 43°C (110°F) along with muscle breakdown, derangements of body chemicals, and increased acid content in the blood. Keys to treatment include cooling the patient, stopping triggering medications (succinylcholine and volatile anesthetics), frequent measurement of arterial blood gases, administering dantrolene, and providing full support with aggressive monitoring. Severe complications include cardiac arrest, brain damage, internal bleeding, or failure of other body systems. Mortality is high and primarily due to cardiovascular collapse. MH, even when treated appropriately, can result in survivors with permanent damage to the brain, kidneys, other major organs, and muscle. See Chapter 60 for more information regarding MH.
Temperature Monitoring
Core temperature can be measured in many locations, including by tympanic membrane, pulmonary artery catheter, or distal esophagus. Because these sites are not usually as convenient, a variety of “near-core” sites can be used such as the oropharynx, nasopharynx, bladder, and rectum. More limited sites may be the only ones available, such as the axilla or skin. Limitations are related to undersensing but can be used clinically in appropriate circumstances, with the main focus being to detect extremes of temperature as in malignant hyperthermia or severe hypothermia as well as trends in temperature.
The level of accuracy clinically necessary is not completely set in stone, but a well-studied guideline is that the combined inaccuracy of a site/thermometer combination should not exceed 0.5°C, which is the minimal difference at which temperature complications have been seen.
Summary
For patients on the operating table in an OR, there are numerous ways that they can and will lose heat produced by their body. Many anesthetics blunt normal physiologic responses to cold, exacerbating this problem. It is the job of the anesthesia provider to both monitor and maintain a normal body temperature for the patient. Fortunately, there are many ways to monitor body temperature and myriad methods through which we can help patients stay warm.
Review Questions
1. Thermoregulation is maintained centrally in the brain by which of the following structures?
A) Amygdala
B) Hypothalamus
C) Pineal gland
D) Right frontal cortex
Answer: B
The hypothalamus is responsible for controlling the body’s regulation of its temperature.
2. Patients may lose heat via conduction, convection, evaporation, and radiation, yet some of these heat loss mechanisms are of greater concern than others. Which two mechanisms of heat loss account for approximately 90% of body heat loss in the operating room?
A) Evaporation and conduction
B) Convection and conduction
C) Radiation and conduction
D) Evaporation and convection
E) Radiation and convection
Answer: E
Radiation and convection account for about 90% of body heat loss. In regard to radiation, all surfaces with a temperature greater than absolute zero radiate heat, and heat transfer is proportional to the 4th power of the absolute temperature difference between the two surfaces. Likewise, for convection, heat loss is proportional to the square root of air speed. Conduction and evaporation are less of a concern since a foam pad between the patient and operating room table minimizes heat lost due to conduction, and sweating (which increases skin evaporative heat loss) is rare under anesthesia.
3. Which of these physiologic effects is not a potential result of hypothermia in an anesthetized patient?
A) Ventricular fibrillation
B) Increased systemic vascular resistance
C) Hypoglycemia
D) Delayed emergence
E) Excessive urine production
Answer: C
Hypothermic patients are typically at risk for hyperglycemia. Hypothermic patients are actually at greater risk of bleeding than are normothermic patients, due to poor platelet and clotting factor function. Hypothermia can cause EKG conduction abnormalities including Osborne J waves, prolonged intervals, and, in extreme hypothermia, ventricular fibrillation. Hypothermia impairs nervous system function, delaying emergence from anesthesia. It also impairs renal function, specifically urine concentrating ability, so that patients cannot appropriately regulate their fluid balance and produce too much urine.
4. At what temperature would you most likely expect to see a patient experience temperature-related ventricular fibrillation?
A) 18°C
B) 28°C
C) 32°C
D) 37°C
E) 41°C
Answer: B
Ventricular fibrillation is known to occur with hypothermia and occurs when the patient’s temperature is less than 28°C.
5. According to the ASA Guidelines on Monitoring, which of the following patient scenarios require temperature monitoring intraoperatively?
A) Electroconvulsive therapy
B) Ear tube placement under mask anesthesia
C) A minimally invasive robotic prostatectomy
D) A CT scan of the head with contrast
Answer: C
The ASA standards for basic monitoring state that those undergoing general anesthesia must be monitored for temperature in cases greater than 30 minutes. A robotic prostatectomy will surely take longer than 30 minutes, so that the patient requires temperature monitoring. All the other cases listed are less than 30 minutes.
6. You have a patient with Parkinson disease who is presenting with a high fever, rigidity, and unusual shifts in consciousness. Given this information, what is likely to be the patient’s treatment?
A) Dantrolene, dopamine agonist, supportive care, and close observation
B) Dantrolene, metoclopramide, supportive care, and close observation
C) Dantrolene, discontinuation of dopamine agonist, supportive care, and close observation
D) Dantrolene, discontinuation of metoclopramide, supportive care, and close observation
E) None of the above
Answer: A
The patient likely has neurologic malignant syndrome, a rare condition that is marked by hyperthermia, rigidity, autonomic instability, and altered consciousness. This disorder can be caused by drugs that interfere with normal dopamine release, such as antipsychotics (e.g., metoclopramide), and by abruptly stopping dopamine agonist use. Given that our patient has Parkinson disease, and that dopamine agonists are used in treatment of Parkinson’s, it is likely that our patient has abruptly stopped the course of dopamine agonists, which has induced neuroleptic malignant syndrome.
SUGGESTED READINGS
De Witte J, Sessler DI. Perioperative shivering: physiology and pharmacology. Anesthesiology. 2002;96:467-484.
Heier T, Caldwell JE. Impact of hypothermia on the response to neuromuscular blocking drugs. Anesthesiology. 2006; 104:1070-1080.
Kurz A, Sessler DI, Lenhardt RA. Perioperative normothermia to reduce the incidence of surgical-wound infection and shorten hospitalization. Study of wound infections and temperature group. N Engl J Med. 1996;334:1209-1215.
Mayer SA, Sessler DI, eds. Therapeutic Hypothermia. 1st ed. New York: Marcel Dekker; 2005.
Mercier FJ, Benhamou D. Hyperthermia related to epidural analgesia during labor. Int J Obstet Anesth. 1997;6:19-23.
Rubinstein EH, Sessler DI. Skin-surface temperature gradients correlate with fingertip blood flow in humans. Anesthesiology. 1990;73:541-545.
Schmied H, Kurz A, Sessler DI, Kozek S, Reiter A. Mild intraoperative hypothermia increases blood loss and allogeneic transfusion requirements during total hip arthroplasty. Lancet. 1996;347:289-292.
Sessler DI. Perioperative heat balance. Anesthesiology. 2000;92:578-596.
Sessler DI. Complications and treatment of mild hypothermia. Anesthesiology. 2001;95:531-543.
Sessler DI. Temperature monitoring and perioperative thermoregulation. Anesthesiology. 2008;109(2):318-338.
Winkler M, Akça O, Birkenberg B, et al. Aggressive warming reduces blood loss during hip arthroplasty. Anesth Analg. 2000;91:978-984.