5
The Eye Designs of the Animal Kingdom
If we could put our submersible from chapter 2 into a time machine and take another dive, this time into the oceans of 540 million years ago, we would encounter a world of strange and wonderful creatures—all new to us but somehow reminiscent of the animals around us today. In fact, one of the most spectacular events in the history of the animal kingdom has just occurred. In the space of a just few million years—a blink of an eye in geological terms—many of our familiar modern animal lineages suddenly appeared on the Earth. They typically had well-developed eyes, and they used them in matters of life and death. This explosion of new animal forms ushered in the Cambrian epoch and with it a dangerous new world of fast-moving predators. Vision became the survival sense par excellence. Bigger and sharper eyes not only improved a predator’s chances of spotting its prey but also helped the prey to unmask the predator. This sensory arms race drove the rapid evolution of a sophisticated spectrum of eye types, each formed to outsmart an adversary, possibly by detecting the telltale movements of a predator or by deciphering the subtleties of near-perfectly camouflaged prey. Of course, over time eyes also evolved in response to much gentler—albeit no less urgent—influences. Animals rapidly specialized in shallower and deeper aquatic habitats and eventually became terrestrial. The properties of light and visual scenes experienced by animals thus became ever more diverse, and their eyes evolved accordingly. The featureless vastness of the deep ocean, where faint blue daylight can been seen above and animals signal each other using fleeting points of bioluminescence, or the bright open savannahs of Africa where animals scan the horizon for predators and prey, or the complex three-dimensional world of the dense green entanglement of a tropical rainforest are just three examples of habitats that have had a profound influence on the evolution of eyes. And within these habitats, the search for mates—often involving complex visual signaling—has had a further but no less profound influence. In less than 100 million years, the great variety of eyes we know today arose. In this chapter we look at them in detail.
Today there are 10 generally recognized optical eye types that have evolved in various branches of the animal kingdom. Whereas vertebrates possess only one of them, invertebrates possess all 10, from simple assemblies of photoreceptors that underlie phototaxis to advanced compound and camera eyes that support a sophisticated range of visual behaviors. Some invertebrates even possess several eyes of more than one type. We have already mentioned some of these eye types in the context of sensitivity and resolution, namely pigment-pit eyes (figure 4.1), compound eyes, and camera eyes. The last of these are characteristic of the vertebrates, although they are also commonplace among the invertebrates. The remaining nine eye types are found only within the invertebrates. Of these, several are various subtypes of the compound eye, the eye design that is possessed by the vast majority of species in the animal kingdom.
The account that follows is a brief description of each eye type, including where in the animal kingdom each type can be found and which basic optical principles underlie each. These descriptions are necessarily brief, but full accounts can be found in several marvelous reviews and books on the subject. The most recent book is the highly recommended second edition of Animal Eyes by Michael Land and Dan-Eric Nilsson (2012), which lucidly treats this and many other topics in this chapter in considerably greater depth (they have also authored many classic reviews on the optics of invertebrate eyes, e.g., Land, 1981 and Nilsson, 1989). For a truly excellent overview of camera eyes throughout the vertebrates, nothing surpasses the remarkable The Vertebrate Eye and Its Adaptive Radiation by Gordon Walls from 1942, which is still as insightful and relevant today as it was 70 years ago. For a more optical treatment of vertebrate eyes, Hughes (1977) is also recommended.
We begin here by returning briefly to the eye type we started with in chapter 4: the pigment-pit eye. In one fascinating group of animals—the ancient and beautiful deep-sea cephalopods of the genus Nautilus—this eye type has reached its logical evolutionary endpoint, functioning somewhat like a pinhole camera.
The Pinhole Eye of Nautilus
As we saw earlier, the simplest pigment-pit eyes are nothing more than crude photoreceptor-lined invaginations in the epidermis (figure 4.1). Such an eye was probably ancestral to more advanced pigment-pit eyes (like those of Nautilus) as well as to camera eyes with lenses (Land and Nilsson, 2012). As we mentioned earlier, the higher-performance camera eyes such as those of other cephalopods most likely arose as the result of a pigment-pit eye acquiring a lens (Nilsson and Pelger, 1994).
In the lineage that led to Nautilus, pigment-pit eyes became more and more spherical, and the pupil—the eye’s “pinhole”—became smaller. Each photoreceptor in the retina thus received light from a smaller receptive field, and spatial vision improved. Such an improvement is already evident in the small (0.5-mm diameter) pinhole eyes of the giant clam Tridacna maxima, whose photoreceptors have acceptance angles Δρ of around 17° (Land, 2003). But as this evolution progressed, smaller receptive fields only came at the cost of having a smaller pupil and thus a less sensitive retina (see chapter 4). The obvious solution to this trade-off is a lens—which provides better resolution and better sensitivity—but for unknown reasons this never occurred, as the pigment-pit eye evolved relentlessly along its path.
Although lacking a lens, the eyes of Nautilus are large (about 10 mm across) and well developed (figure 5.1A,B). Remarkably, the pupil is also mobile and varies in size with light level, with a diameter that ranges from 0.4 mm to 2.8 mm (Hurley et al., 1978). Pinhole eyes are thought to work like a pinhole camera: the small pupil creates a dim, inverted, and somewhat blurry image on the retina, and smaller pupils produce sharper (albeit dimmer) images (figure 5.1C). Like a pinhole camera the eye is also likely to have a near-infinite depth of field, meaning that accommodation mechanisms are not required to reach best focus at different distances from the eye (as is needed, for example, in our own eyes). Muntz and Raj (1984) discovered that in the Nautilus retina the interreceptor angle φ is around 0.3°. However, even with a 0.4-mm pupil, the photoreceptor’s acceptance angle Δρ is still eight times greater than Δφ, which indicates that the retina has the potential to resolve much finer spatial detail than the pupil can actually supply (see figure 5.1C). A similarly sized eye in a fish, with a wide pupil and a lens producing sharp diffraction-limited images, would most likely result in a smaller Δρ than Δφ. Moreover, the image would be over two orders of magnitude brighter (Muntz and Raj, 1984). Thus, compared to camera eyes of the same size, the pinhole eye of Nautilus has rather poor sensitivity and resolution, which probably explains the rarity of this eye type in nature.
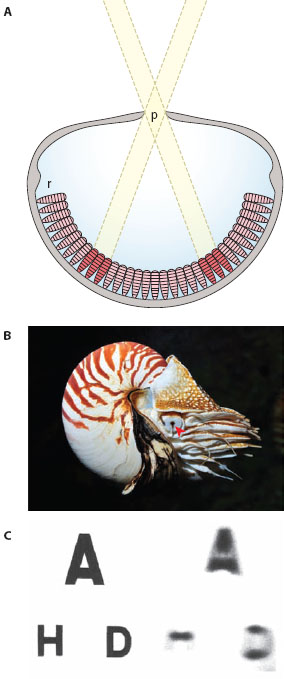
Figure 5.1 The pinhole eye of Nautilus. (A) Light rays entering the pupil (p) from different directions in space form a dim, blurred image on the retina (r). (Image courtesy of Dan-Eric Nilsson) (B) The cephalopod Nautilus showing the position of the right eye and the conspicuous pupil (arrowhead). (Photo credit Visarute Angkatavanich, 123rf.com Photo Agency) (C) Images of a Lizars eyesight test chart (left) photographed at a distance of 12.5 cm through a model Nautilus eye with its smallest pupil, 1 × 0.4 mm (right: pupil-to-image distance 9 mm). Resolution of the chart’s top line represents a visual acuity of 4°, the second line 2.5°. Even with its smallest pupil, the smallest angular detail resolvable by the Nautilus eye is thus likely to be greater than 4°. (Reproduced from Muntz and Raj, 1984, with permission from the Journal of Experimental Biology)
Concave Mirror Eyes
Another curious eye design involves the use of a concave mirror, rather than a lens, to focus an image into the retina. Like pinhole eyes, concave mirror eyes are rare in nature, but they have nonetheless evolved several times (see Land and Nilsson, 2012), notably in the bivalve mollusks and within at least three classes of crustaceans (Maxillopoda, Ostracoda, and Malacostraca). Remarkably, they have also appeared in a fish: the deep-sea spook fish Dolichopteryx longipes (Wagner et al., 2009). Of the crustaceans, the best-known examples are found among the amphipods, ostracods, and copepods, notable among them being the giant deep-sea ostracod Gigantocypris with its huge ocular reflectors covering about a third of its dorsal body surface. These eyes are likely used as highly sensitive light concentrators for detecting bioluminescent prey in the deep (Hardy, 1956; Land, 1981).
The species in which concave mirror eyes were first described in detail—by Michael Land in the mid-1960s—is the scallop Pecten maximus, a bivalve mollusk. The mirror (or “argentea”) of Pecten is a multilayer reflector that most strongly reflects blue-green light (Land, 1966a). Within the cells of the argentea, each reflective layer is formed from flattened membrane-bound vesicles densely packed with shiny guanine crystals (Barber et al., 1967). The mirror so formed is also perfectly spherical, which, as we will see below, is a prerequisite for undistorted image formation.
Scallops possess a large number of little concave mirror eyes evenly spaced along the edge of each shell, on either side of the scallop’s open gape. In some species they look rather like tiny blueberries, with a characteristic glow from the mirror clearly visible through the pupil (figure 5.2B). Each eye possesses a weak jelly-like “lens” (of low, homogeneous refractive index) and an underlying retina that is backed by the mirror (Barber et al., 1967). Unlike in a camera eye, the retina of a concave mirror eye is not separated from the lens by an optically homogeneous aqueous medium, a spacer component that is usually needed to provide the required focal distance between the lens and the image plane. This interesting fact, combined with the weak lens, means that incoming parallel rays of light are brought to a focus a long way below the back of the retina (indeed, a long way below the back of the eye!). Thus, had the eye relied solely on its lens, it would have been badly underfocused (Land, 1965). The mirror is the key to why, in reality, this is not the case.
Like lenses, concave mirrors focus images by bending rays of light, not by refraction (as in a lens) but by reflection (figure 5.2A). The mirror’s image can be upright relative to the object and magnified (as in a shaving mirror), or it can be inverted and minified (as is the case in a concave mirror eye); exactly which combination of image orientation and size results depends on the location of the object relative to the focal plane of the mirror. This focal plane lies halfway between the spherical surface of the mirror and its center of curvature, that is to say the mirror’s focal length f is simply 0.5r where r is the radius of the spherical mirror surface. In the scallop’s concave mirror eye the image of a distant object lies slightly closer to the mirror than this because the incoming light rays are weakly refracted by the lens. Not surprisingly, the retina is optimally located to receive this focused image, being separated from the mirror by a smaller (as in Pecten: Barber et al., 1967) or larger aqueous space (as in the sessile scallop Spondylus americanus: Speiser and Johnsen, 2008). Curiously, even though the image is eventually focused, light must first pass unfocused through the retina to reach the mirror. Because the photoreceptors are incapable of telling the incoming and reflected light apart, the visual contrast of the image will thus be somewhat degraded.
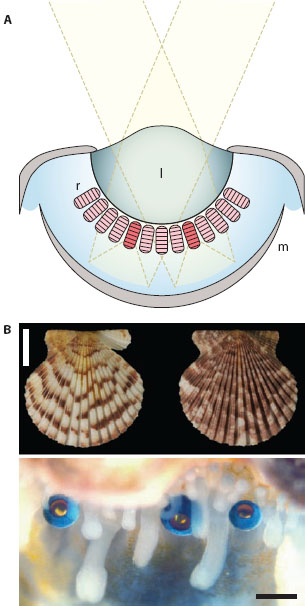
Figure 5.2 The concave mirror eye of the scallop Pecten. (A) Light rays are weakly refracted by a gelatinous lens (l) and are reflected by a spherical concave mirror (m) lining the back of the eye. This mirror creates a focused image in a retina (r) suspended above the mirror. (Image courtesy of Dan-Eric Nilsson) (B) Shells of the scallop Pecten gibbus (above, scale bar 10 mm) and three eyes along the mantle edge of a living but unidentified species of Pecten (below, scale bar 1 mm). (Upper image courtesy of the National Anthropological Archives, Smithsonian Institution, inventory number USNM 605016; lower image courtesy of Dan-Eric Nilsson)
The scallop retina itself is a remarkable structure divided into a proximal layer (closer to the mirror) and a distal layer (closer to the lens). In Pecten the focal plane of the mirror lies within the distal layer—following reflection, light first passes unfocused through the proximal layer before reaching the distal layer. In other species, such as the sessile scallop Spondylus, the aqueous space between the mirror and retina is larger than in Pecten, and it may be the case that the mirror’s focal plane lies in the proximal layer, although this is yet to be determined with certainty (Speiser and Johnsen, 2008). Interestingly, in all species examined by Speiser and Johnsen the interreceptor angle Δφ is quite small in both layers (ranging from 1° to 5°), and in the proximal layer Δφ seems to be correlated with swimming strength: species that are stronger swimmers tend to have a smaller proximal Δφ than species that are sessile, which suggests a role for vision during swimming. In Pecten, which is a reasonably good swimmer, such small interreceptor angles seem well matched to the receptive field sizes of the distal photoreceptors, which are capable of reacting to the images of moving targets as small as 2° in size (Land, 1966b).
Remarkably the two retinal layers also house two entirely different types of photoreceptors. In the distal layer, the light-sensitive parts of the photoreceptors are constructed of cilia and hyperpolarize in response to light (Hartline, 1938), properties reminiscent of those found in the outer segments of vertebrate photoreceptors (chapter 3). In contrast, the light-sensitive parts of proximal-layer photoreceptors are constructed of microvilli and depolarize in response to light—such properties are similar to those found in the majority of invertebrate photoreceptors. Interestingly, each layer also expresses a different visual pigment, with the result that the spectral sensitivity of photoreceptors in the distal layer is shifted to longer wavelengths than the sensitivity of photoreceptors in the proximal layer (Speiser et al., 2011a). In some species this may offset chromatic aberration produced by the weak lens because shorter wave-lengths would be focused closer to the mirror than longer wavelengths.
The curious mix of rhabdomeric and ciliary photoreceptors in the same retina has a particular functional significance. Due to its hyperpolarizing response properties, a distal-layer photoreceptor experiences a dark target that moves into its receptive field as a sudden removal of light and responds accordingly with a brisk volley of action potentials, that is, with a vigorous “OFF response” (Land, 1966b; McReynolds and Gorman, 1970; Wilkens, 2008). Behaviorally, the sudden appearance of a dark target within the visual field of a sedentary scallop could potentially signal the presence of a predator, and with both shells open while filter feeding, scallops are particularly vulnerable. Not surprisingly, in Pecten the intrusion of even a small (~2° wide) dark target is sufficient to influence the animal to close its shells completely (Buddenbrock and Moller-Racke, 1953)—and, as we saw above, this is also sufficient to generate an off response in the distal layer receptors. In Pecten these receptors are thus thought to be specialized for signaling approaching predators, whereas the proximal layer receptors—generating “ON responses” to general changes in light level—are thought instead to be used for guiding scallops to brighter and darker areas of their habitats (Land, 1966b). The large number of concave mirror eyes found in a single scallop, evenly spaced along the edge of each shell, probably act collectively as a highly efficient “burglar alarm” for detecting predators (Nilsson, 1994). In fact, each point in space is viewed by one receptor in each of about 17 eyes, so the likelihood of a predator surprising a scallop unawares is probably very small (Land, 1968)!
Camera Eyes
Even though camera eyes are not the most common eyes in the animal kingdom—a title overwhelmingly held by the compound eyes—they are arguably among the most widespread taxonomically (Land and Nilsson, 2012). Sometimes also referred to as the simple eye or lens eye, the camera eye is the principal eye type of all vertebrates. Camera eyes are also found in a large number of invertebrate taxa, including molluscs, annelids (notably polychaetes), crustaceans (only in pontellid copepods, e.g., Labidocera), cnidarians (e.g., cubozoan jellyfish), arachnids (spiders, scorpions, ticks, and mites), and even in insects (as ocelli and larval eyes).
Per unit mass (and therefore size), camera eyes are capable of supplying more spatial information than any other type of eye (Laughlin, 2001), and this evolutionary advantage is possibly one of the main reasons they have become so widespread. Their various optical and retinal components are also surprisingly malleable to the forces of evolution, and this has led to a remarkable variability in their forms. Some, for instance, have evolved for exquisite spatial vision in bright daylight, others are incredibly sensitive for use at night or in the deep sea, and yet others can undergo drastic but reversible optical changes to optimize vision during a sudden transition between air and water. We explore many of these camera eyes (and their visual ecological functions) in the chapters that follow. Here we describe their optics and basic ground plan.
Optics
The feature that unites all camera eyes is the presence of a single optical unit (consisting of an internal lens and very often an external cornea) that focuses images of the external world onto an underlying retina of visual cells (figure 5.3A). As we saw earlier in chapter 4, in some camera eyes—such as the ocelli of insects or the lens eyes of box jellyfish—the focal plane of this image is well behind the retinal surface, and deliberately so in order to optimize specific visual tasks by reducing the spatial information sampled by the retina. Most other camera eyes, in contrast, have a well-focused image either on or in the retinal layer. In all camera eyes, focused or not, the image is inverted on the retina.
In the camera eye of a terrestrial vertebrate in air there are two main refractive elements that collectively focus light onto the retina (figure 5.3A): the curved outer surface of the cornea and the internal lens. Like a convex glass lens, the curved corneal surface is able to focus light because it separates two optical media of very different refractive indices n: the outside air (n = 1) and the aqueous interior of the eye (which has a refractive index close to that of water, n ≈ 1.34). Without this refractive index difference the considerable refractive power of the cornea, which accounts for around two-thirds of the total refractive power of the eye, would be eliminated, and the lens, which is responsible for the remaining one-third of the eye’s refractive power, would be incapable on its own of focusing an image. This is why we experience an incredibly blurry world if we try to see underwater without a facemask: the watery medium on both sides of the cornea eliminates its refractive power. Only by inserting a layer of air in front of the eyes—the chief role of a diver’s facemask—can underwater vision be restored.
How then do fish, marine mammals, and mollusks all manage to see under water, when the refractive power of their corneas is negligible? The answer lies in the lens, which in aquatic camera eyes is typically spherical and powerful (figure 5.3B)—underwater vision is permitted by a remarkable gradient of refractive index (figure 5.3C). This gradient, first discovered in the early 1880s by the German physicist and zoologist Ludwig Matthiessen (1882), is roughly parabolic and falls radially in every direction from the center of the lens to its edge, the result of a continuous decline in the concentration of proteins (known as crystallins) that make up the lens. The refractive index of the lens falls from a central value of n ≈ 1.55 (the value for a dry protein lens crystallin) to an edge value approaching that of the surrounding aqueous medium (n = 1.34). Such gradients bend light rays continuously within the lens (figure 5.3D), and remarkably they also eliminate spherical aberration in the resulting image (which would have been a major problem had the lens been homogeneous in refractive index—see chapter 4). And because of the continuous bending of light, the graded-index lens ends up bending the light much more than would have occurred in a homogeneous lens (resulting in a much shorter focal length). It is this single feature that accounts for the superior refractive power of the lens in marine camera eyes and overcomes the loss of the cornea. Matthiessen also discovered another curious fact about these lenses: whether from a fish, a cephalopod, or a marine mammal, the focal length (f) is invariably about 2.5 lens radii (r), that is f/r ≈ 2.5, a ratio today known as Matthiessen’s ratio. Interestingly, graded-index lenses are even found in terrestrial camera eyes, their benefits for resolution (reduced spherical aberration) and sensitivity (a short focal length and low F-number) being the major reason. We mentioned earlier that even our own lenses (and indeed the lenses of all other mammals) contain a weak gradient in order to improve resolution (Pierscionek and Chan, 1989). Many spider lenses also have gradients, but instead to improve sensitivity. The chitinous lenses of the nocturnal net-casting spider Dinopis subrufus, whose eyes have among the lowest F-numbers known in the animal kingdom (0.6), are a good example (Blest and Land, 1977). As we see below, graded-index lenses are also found in some groups of compound eyes, but for very different reasons.
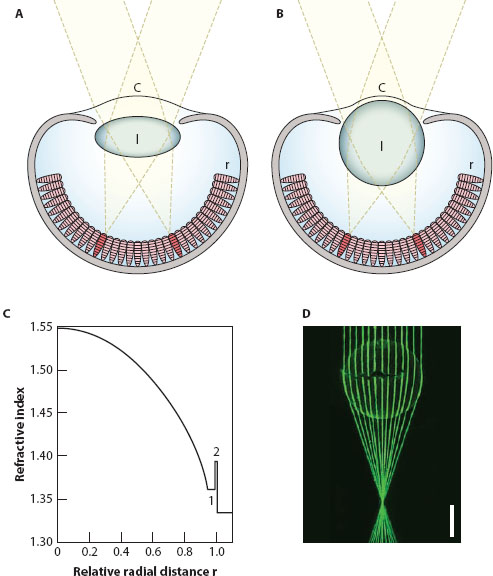
Figure 5.3 Camera eyes. (A) A terrestrial camera eye. The cornea (c) is responsible for a significant (and often major) fraction of the eye’s focal power in air. The flattened lens (l) provides the remaining refractive power, and together both optical elements create a focused image on a retina (r). (B) An aquatic camera eye for use in water. Because the cornea (c) lacks refractive power, the focal power of the eye resides in the spherical lens, whose parabolic gradient of refractive index provides a crisp spherical-aberration-free image on the retina (r). (Images in A and B courtesy of Dan-Eric Nilsson) (C) Gradient of refractive index found in the spherical lens of the cichlid fish Astatotilapia burtoni from the lens center (r = 0) to its edge (r = 1). 1 = the refractive index in the constant index zone of the lens; 2 = the refractive index of the lens capsule. (From Gagnon et al., 2012) (D) Continuously bent light paths of thin laser beams focused by the large spherical lens of a swordfish (Xiphias gladius). Scale bar = 1 cm. (Eric Warrant and Kerstin Fritsches, unpublished data)
Of course, not all animals live permanently in either air or water. Some live sporadically or permanently in both optical media. Marine mammals, diving birds, and even certain kinds of fish spend considerable time both above and below the water surface. These amphibious animals have evolved several remarkable optical tricks to overcome the problems of seeing well in two media.
Amphibious Vision with Camera Eyes
Many aquatic animals—such as flying fish, frogs, and seals—spend a significant fraction of their lives in air. And just as curiously, there are many terrestrial animals that instead spend a large fraction of their lives in water. Several remarkable species of diving birds, many turtles, and even some groups of humans are all notable examples. What these animals all share is a need to see well in both media, and optically this is far from trivial. The major problem for such “amphibious vision” is the presence of the cornea. As we discussed above, the cornea is a powerful lens that is responsible for a substantial fraction of the refractive power of a terrestrial eye. Without its contribution, as would be the case when submerged, the eye becomes poorly focused, and spatial vision badly compromised.
The simplest evolutionary solution to this problem has been to permanently reduce the cornea’s power in both media, allowing reasonably good vision in either air or water. Instead of being curved (and thus optically significant in air), the cornea in many amphibious animals is a flattened window. Seals and penguins are excellent examples of such animals. Of course with a flattened cornea the lens needs to be powerful, and in seals this is indeed the case: with near-spherical multifocal Matthiessen lenses (Sivak et al., 1989; Hanke et al., 2008), seals have a visual acuity in air that is similar to that in water (Hanke and Denhardt, 2009). The downside of having a flattened cornea is that the visual field of the eye is somewhat reduced. Certain fish—notably the flying fish Cypselurus heterurus (Baylor, 1967), the Galápagos four-eyed blenny Dialommus fuscus (Munk, 1980), and the four-eyed rockskipper Mnierpes macrocephalus (Graham and Rosenblatt, 1970)—have overcome this problem by having a raised cornea of flattened windows oriented in different directions (figure 5.4A).
An alternative to having a flattened cornea is to drastically accommodate during the transition from air to water, increasing the eye’s optical power by so much that it actually compensates for the sudden loss of the cornea. This is a common strategy among seals and among diving birds such as cormorants and diving ducks and among amphibious reptiles such as turtles. Optical power is commonly measured in diopters (D) and is equivalent to the reciprocal of the focal length measured in meters: a 1.0-D lens has sufficient optical power to bring parallel light rays to a focus at 1 m, a 2.0-D lens sufficient power to bring them to a focus at ½ m, and so on. The increase in optical power produced by underwater accommodation can approach 50 D in cormorants (Walls, 1942) and around 100 D in the European pond turtle Emys orbicularis (Heine, 1908; Munk, 1996). (As a comparison, the maximum accommodation measured in humans is around 15 D, in infants.) The eyes of these animals are characterized by a heavily muscularized iris supported by a ring of tiny bones called scleral ossicles. During accommodation the ciliary muscles of the iris contract, squeezing the soft lens through the narrow pupil (figure 5.4E). The section of the lens that is forced through the pupil bulges outward, and the anterior surface of this bulge acquires a much more pronounced curvature and thus much greater optical power. Many diving birds chase fish at high speed under water, and for this hunting task the acute underwater vision potentially provided by this powerful accommodation mechanism might be essential. Strangely though, recent measurements in the great cormorant Phalacrocorax carbo reveal that their underwater visual acuity is actually rather poor (White et al., 2007), suggesting that these birds can detect and pursue prey only at close range (less than 1 m).
Another fascinating (although rather less dramatic) example involves our own species. Certain human tribes, such as the Moken people who live along the coasts of Southeast Asia, have for generations relied on their ability to forage for food from the sea floor. This task is usually reserved for children, who can dive to depths of more than 20 m to visually search for mollusks and crayfish without the aid of a facemask (figure 5.4B). Psychophysical measurements have shown that these children have around double the underwater visual acuity of a European child (Gislén et al., 2003). This ability results from a combination of maximal accommodative power (15 D) and the acquisition of an unusually small pupil while underwater. The smaller pupil significantly reduces image blur and thus improves acuity. This ability, however, has not been inherited—even European children after several weeks of training can learn to constrict the pupil and maximally accommodate in the same fashion as the Moken children (Gislén et al., 2006).
And finally, two rather unique examples of how animals have solved the problem of amphibious vision. The first of these is the South American river-dwelling “four-eyed” fish of the genus Anableps, which spend their lives literally at the water surface (figure 5.4C) and have eyes that are constructed to see in air and water simultaneously (figure 5.4D). In the light-adapted state, each eye has two pupils created by two touching “iris flaps” lying exactly at the water surface that divide the original pupil into an upper and lower half, one half admitting light from the air above, the other admitting light from the water below. The light admitted by the two pupils is focused onto two separate retinas—a dorsal “water retina” and a ventral “air retina”—each of which sends information to a separate part of the optic tectum, a primary visual center in the brain (Schwassmann and Kruger, 1965). Interestingly, recent work has shown that although both retinas possess an ultraviolet-light-sensitive opsin, each retina possesses a second unique opsin: the dorsal retina has an additional long-wavelength-sensitive (L) opsin, whereas the ventral retina has a middle-wavelength-sensitive (M) opsin (Owens et al., 2012). This is a nice adaptation to the fish’s habitat because river water, into which the dorsal retina is looking, preferentially transmits light of longer wavelengths than in air due to the presence of dissolved organic matter (known as “gelbstoff”), which tends to color the water green-yellow (chapter 2). The lens too is interesting. It has an odd ellipsoid shape, with its long (and optically more powerful) axis oriented to collect light from the water. The optically weaker short axis instead collects light from the air. This unusual lens thus ensures that a sharp image is formed simultaneously on both retinas, giving the four-eyed fish excellent vision in both air and water.
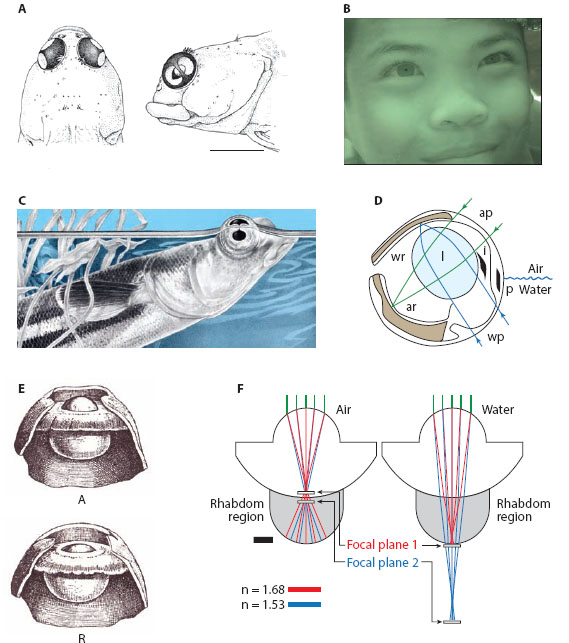
Figure 5.4 Amphibious vision in camera eyes. (A) Two-faced flattened cornea of the Galápagos four-eyed blenny Dialommus fuscus. Scale bar = 5 mm. (Reproduced with permission from Munk, 1980) (B) Under water image of a Moken child from Southeast Asia. (From Gislén et al., 2003) (C) Four-eyed fish Anableps dowi. (Original image by Trudy Nicholson, courtesy of Natus Neurology—Grass Technologies brand products) (D) A schematic cross section through the eye of the four-eyed fish, showing the ellipsoidal lens (l). Light passing through the air pupil (ap) is focused (green rays) on the air retina (ar). Simultaneously, light passing through the water pupil (wp) is focused (blue rays) on the water retina (wr). p = corneal pigment band; i = iris flaps. (Drawn from various sources) (E) Accommodated (A) and relaxed (R) eye of the European pond turtle Emys orbicularis. Note how the lens bulges through the pupil in the accommodated state, providing the eye with an additional 100 D of refractive power when in water. (Reproduced from Walls, 1942) (F) Eyes of the chiton Acanthopleura granulate, whose birefringent aragonite lenses provide a focused image in the retina both in air and water. (Reproduced from Spieser et al., 2011b, with permission from Elsevier)
The second and final example concerns a marine mollusk—Acanthopleura granulata—a chiton that lives on rocks close to the shoreline, which, depending on the tide, can be found above or below the water surface. The shells of these animals are embedded with hundreds of small camera eyes (ocelli), each of which remarkably has a lens made out of aragonite, a feature unknown anywhere else in the animal kingdom (figure 5.4F: see Speiser et al., 2011b). Aragonite is birefringent, and a property of birefringent materials is that they have two refractive indices (nα and nβ) and thus two focal planes: for aragonite nα = 1.68 and nβ = 1.53. If one models the chiton eye in air, the two focal planes are found to lie quite close together near the distal surface of the retina (figure 5.4F). In water (when the power of the external surface of the lens is severely reduced), the two focal planes are quite separated: even though the focal plane for nβ is below the retina (i.e., underfocused), that for nα is located in the proximal retina. Thus, the chiton eye receives a focused image both in air and water, an impressive fact supported by behavioral studies showing that chitons have the same spatial resolution in both media (Speiser et al., 2011b), allowing them to respond defensively to the slight decreases in illumination that are induced by fast-moving targets as small as 9° in size.
The Pupil
In camera eyes, changes in image brightness that result from changes in ambient illumination are partially compensated by a variable pupil whose size depends on light level. In the dark the pupil of our own camera eye has a diameter of 8 mm, reducing to 2 mm in bright daylight. This represents a change in area (and image brightness) of 82/22 or 16 times, which of course is a lot lower than the 108 times change in light level that occurs from night to day. Nevertheless, even though our pupil is clearly incapable of fully compensating for this daily change, it turns out that its diameter at different natural light levels maximizes the eye’s information capacity by optimizing the compromise between image blur induced by a wider pupil (caused by aberrations) and the extra sensitivity that this wider pupil affords (Laughlin, 1992). The camera eyes of nocturnal animals such as cats and geckos often have pupils that are capable of considerably greater changes in area than the pupils of our own eyes. This is simply because they can be much larger in the dark and much smaller in bright light. A tiny pupil has a real advantage because it can allow even a very sensitive nocturnal eye to operate during the day. The slit pupil of the domestic cat is a superb example. In the dark, it is large and round with a diameter of around 13 mm. As light levels increase the pupil constricts, not to a small circle as in our own eyes but to a thin vertical slit that almost completely closes. The change in pupil area is at least 135 times (Wilcox and Barlow, 1975). In nocturnal geckos the change is even greater. In the Tokay gecko Gekko gecko (which has an all-rod retina), the area change is at least 350 times (Denton, 1956), with the pupil constricting from a large ellipse to a curious vertical slit with four pinhole-sized circular holes evenly spaced along its length. A similar pupil shape results in the helmut gecko Tarentola chazaliae, although the area change is smaller, around 100–150 times (Roth et al., 2009; see figure 5.5). The light-adapted pupil shape of vertebrate and cephalopod camera eyes is thus highly variable—in addition to singular and multiple circular pupils and vertical slit pupils, there are horizontal slit pupils (e.g., in horses and reindeer), ring-shaped pupils (e.g., catfish), and W-shaped pupils (e.g., some cephalopods). The functional significance of this great variety is still a matter of conjecture, although it has been suggested that vertical slit pupils allow nocturnal animals with multifocal lenses (chapter 4) to focus images corrected for chromatic aberration (Malmström and Kröger, 2006), and multiple circular pupils in geckos might aid in distance estimations (Murphy and Howland, 1986).
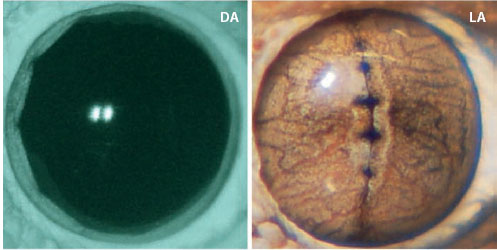
Figure 5.5 The dark-adapted (DA, left) and light-adapted (LA, right) pupil of the nocturnal helmet gecko, Tarentola chazaliae. (Reproduced from Roth et al., 2009, with permission from the Association for Research in Vision and Ophthalmology (ARVO).
The Retina
The retinal morphologies of animals with camera eyes are also quite variable. In invertebrate eyes (of all optical types), the visual cells of the retina are exclusively photoreceptors. In more advanced invertebrate visual systems, such as those of mollusks and arthropods, the next stages of processing occur in the optic lobes, regions of the brain placed adjacent to the eyes and specialized for higher visual processing (see the octopus eye in figure 5.6). For instance, in arthropods, signals carried by the photoreceptors are first analyzed in the lamina, the most peripheral neuropil of the optic lobe, whose role, among other things, is to optimize visual contrast. The massively complex medulla, which receives input from both the lamina and the retina, is responsible for the early processing of color, polarization, and motion information. The analysis of optic flow and the detection of moving targets are essential tasks of the lobula and lobula plate, the most central neuropils of the optic lobe (see chapter 10).
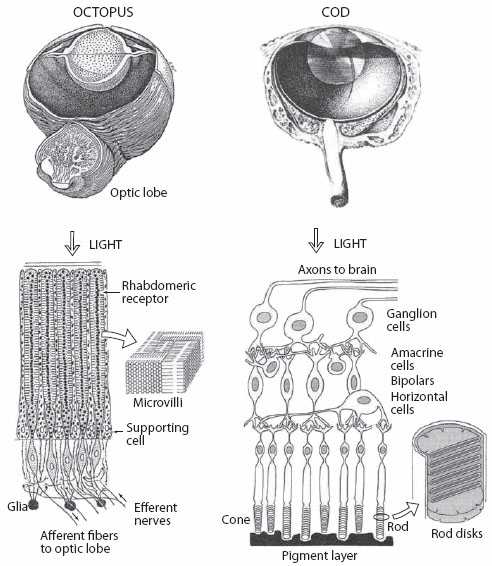
Figure 5.6 Camera eyes of the octopus and the cod (Gadus morhua). Despite a similar size (10 mm diameter) and gross morphology, these eyes have very different retinas. Visual cells of the octopus retina consist exclusively of rhabdomeric microvillar photoreceptors of a single type that point outward toward incoming light. Cod retina consists of many cell types important for the early processing of visual information. Two types of ciliary photoreceptors—rods and cones—point inward away from the incoming light. (Reproduced from Land and Nilsson, 2012, with the permission of Oxford University Press)
Many of these visual processing tasks—which take place outside the retina in invertebrates—are instead performed inside the retinas of vertebrates. This is possible because neurons in the vertebrate retina are not just photoreceptors. Many other nerve cell types—including bipolar cells (to which the photoreceptors connect), amacrine cells, horizontal cells, and ganglion cells—together create a complex and still only partially understood visual processing layer within the eye itself (figure 5.6). To add to this complexity, vertebrate retinas also contain two classical types of photoreceptors (chapter 3): rods, which are responsible for sensitive low-resolution monochromatic vision in dim light; and cones, which are responsible for high-resolution polychromatic vision in bright light. Recently a third type of photoreceptor has also been identified in the vertebrate retina. This is actually a special class of retinal ganglion cell, containing the visual pigment melanopsin, which measures ambient light levels and is responsible for setting the circadian rhythm, regulating pupil dilation, and controlling melatonin levels in the body (e.g., Hattar et al., 2002). In this book, however, we restrict our discussion of vertebrate photoreception to that occurring in the rods and cones.
The neural circuits of the vertebrate retina are responsible for the elementary processing of contrast, color, and motion, visual features first extracted only in the lamina and medulla of the invertebrate optic lobe. Signals that leave the vertebrate retina via the optic nerve (which is dominated by the axons of ganglion cells) to the visual cortex of the brain thus do so in highly processed form.
A final curious difference between photoreceptors in the retinas of vertebrates and invertebrates is that they are oriented in opposite directions with respect to the incoming light. This can be readily seen by comparing the camera eyes of an octopus and a fish (figure 5.6). At a gross morphological level they seem remarkably similar. Both eyes have roughly the same form, and both have spherical lenses (with exquisite gradients of refractive index) that produce crisp aberration-free images on the underlying retina—without doubt one of the most beautiful examples of convergent evolution in the animal kingdom. However, if one looks more closely at the retina an important difference becomes apparent: the photoreceptive layer (the rhabdoms) of the octopus retina point outward toward the incoming light, whereas the corresponding layer of the fish retina (the rod and cone outer segments) points inward away from the incoming light. This difference arises due to significant developmental differences between the two animal groups. Even though the vertebrate construction seems illogical, it nonetheless works, thanks to the fact that the overlying retinal cell layers are very transparent and in the most important part of the retina—the high-resolution fovea—are also thinned out to the bare minimum.
Compound Eyes
Simply on the basis of the sheer numbers of species that possess them, compound eyes are by far the most common eye type in the animal kingdom. For the general public they are most firmly associated with insects, but they are also commonplace among crustaceans. They are even found, often in a quite rudimentary form, in some chelicerates (e.g., the horseshoe crab Limulus), annelids (e.g., sabellid worms), and bivalve mollusks (e.g., ark clams).
Unlike the eye types we have already discussed, compound eyes, as their name suggests, are constructed of many individual optical units. Known as “ommatidia” (figure 5.7A), these units are tubular in shape and consist of one or more lenses—typically an outer transparent cuticular “corneal lens” and an inner “crystalline cone”—that supply light to a number of photoreceptor cells (or “retinular cells”) assembled underneath. The exact number of retinular cells that exist within a single ommatidium is variable across taxa, but a very common number is eight (and these are known as retinular cells R1 to R8). Primary and secondary pigment cells (holding dark granules of screening pigment) are also present within each ommatidium and ensheath the ommatidial tube. The external corneal surfaces of most compound eyes are marked by a crystalline matrix of hexagonal “facets,” each being the curved external surface of a single corneal lens that supplies light to underlying retinular cells. In some compound eyes, notably the reflecting superposition eyes (see below), the facets are instead square, and for good optical reasons.
Each ommatidium receives light from a small region of space—for most compound eyes somewhere between 1° and 10° across—and two neighboring ommatidia receive light from two neighboring such regions. Thus, the greater the number and density of ommatidia in a compound eye, the more finely sampled is visual space. A large dragonfly may have over 30,000 ommatidia in each of its two compound eyes, thus dividing visual space into over 60,000 “visual pixels,” to again borrow the digital camera term (figure 5.7B). Dragonflies, like many insects, have almost complete wrap-around vision, being able to view nearly the entire 360° panoramic sphere of visual space around them, with the exception of a small blind spot immediately behind (where the body is). This ability to see in almost every direction simultaneously is unrivaled by any other eye type and is without doubt one of the major advantages of the compound eye design. Even though dragonflies have many fewer “pixels” than one would wish for in a camera, they nonetheless have formidable visual powers that allow them, among other things, to catch prey on the wing (typically other flying insects—see chapter 10). At the other end of the scale are some ants, which may have fewer than 20 ommatidia. An extreme case is presented by soldiers of the tropical army ant Eciton burchellii, whose compound eyes appear to consist of a single giant ommatidium, although the structure of the retina suggests that this “ommatidium” may instead function as a camera eye (Werringloer, 1932).
Each of the retinular cells possesses a microvillous photosensitive rod-like region known as a “rhabdomere”; this it contributes, together with those of the ommatidium’s other retinular cells, to a collective “rhabdom” (figure 5.7C,D). The rhabdomeres are either fused to create a “fused rhabdom” (figure 5.7D, the most common arrangement) or remain separated to create an “open rhabdom” (figure 5.7C, typical of Dipterans [flies] and most Hemipterans [bugs]). The rhabdom (or an individual rhabdomere) is a light-guiding structure that houses the rhodopsin molecules and receives and absorbs the incoming light. Even though the lenses of the ommatidium focus an image of the outside world on the tip of the rhabdom, the spatial information present in this image is lost as the light propagates through the rhabdom. Thus, an individual rhabdom is capable only of discriminating the mean intensity of the light that forms the image. And because, as we saw in chapter 3, the particular rhodopsin molecule resident in the photoreceptor sets the range of wavelengths that will be absorbed with greatest efficiency, the rhabdom provides the information necessary for determining the color of this light. Each ommatidium thus signals the average intensity and color (and in some cases polarization) of light that is incident from the small region of space (or visual field) that it views. In this way the matrix of ommatidia together create an erect view of the world, formed much like a “mosaic,” to quote the German physiologist Johannes Müller, who in 1826 was the first to recognize this mechanism of imaging in compound eyes (Müller, 1826).
A little more than 60 years after Müller, in 1891, the Austrian physiologist Sigmund Exner published a groundbreaking monograph concerning the optical functions of compound eyes, for the first time recognizing that they fell into two broad subtypes (figure 5.8): apposition eyes and superposition eyes. Despite coming under significant scrutiny during the 1960s, Exner’s brilliant insights endure. Not only do these subtypes exist, but we now know that within each there are several further subtypes. It is to these subtypes we now turn our attention, beginning first with apposition eyes.
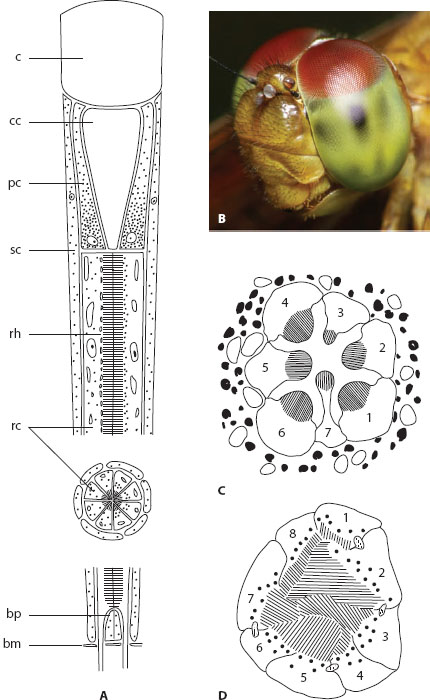
Figure 5.7 Ommatidia. (A) Schematic longitudinal section (and an inset of a transverse section) through a generalized Hymenopteran ommatidium, showing the corneal lens (c), the crystalline cone (cc), the primary pigment cells (pc), the secondary pigment cells (sc), the rhabdom (rh), the retinular cells (rc), the basal pigment cells (bp), and the basement membrane (bm). Lefthalf of the ommatidium shows screening pigment granules in the dark-adapted state; right half shows them in the light-adapted state. (Redrawn from Stavenga and Kuiper, 1977) (B) Compound eyes of an unidentified species of dragonfly. (C) Schematic transverse section through the open rhabdom of a fly, showing the seven distal retinular cells with their separated rhabdomeres. (D) Schematic transverse section through the fused rhabdom of the Collembolan Orchesella, showing the eight retinular cells with their apposed rhabdomeres. (Redrawn from Paulus, 1975)
Apposition Compound Eyes
An essential difference between apposition eyes and superposition eyes lies in the number of lens units that provide light to a single rhabdom. In apposition eyes only one is involved. Light rays entering a corneal facet lens are focused exclusively onto the rhabdom within the same ommatidium. In superposition eyes many lens units are involved: a single rhabdom instead receives light rays that enter a large number of corneal lenses (usually several hundred). A second essential difference between the two subtypes of compound eyes is that in apposition eyes the rhabdoms run the length of the ommatidium to join the proximal tips of the crystalline cones. In superposition eyes the rhabdoms are instead compressed to a proximal layer in the eye: a wide optically homogeneous region called the “clear zone” separates this layer from the crystalline cones.
In apposition eyes (figure 5.8A), rays are prevented from passing to neighboring ommatidia by a sleeve of light-absorbent screening pigments that entirely encases each ommatidium. Because a single-facet lens is only a few tens of micrometers across, the amount of light that each can supply is very limited. Not surprisingly, apposition eyes are typically found in animals active in bright light such as flies, butterflies, bees, dragonflies, and fiddler crabs. But amazing exceptions do exist—there are several species of nocturnal and deep-sea arthropods, some with quite extraordinary visual abilities, that have apposition eyes (see chapter 11). Even though apposition eyes have restricted sensitivity, the ommatidia of day-active species tend to have high F-numbers and narrow rhabdoms and thus the potential for good spatial resolution. In large apposition eyes (such as those of dragonflies and mantises), experimentally measured acceptance angles Δρ (see chapter 4) can be as low as 0.8°, among the lowest values measured in compound eyes. Typically, the quality of the image supplied by a diurnal apposition eye is close to the diffraction limit.
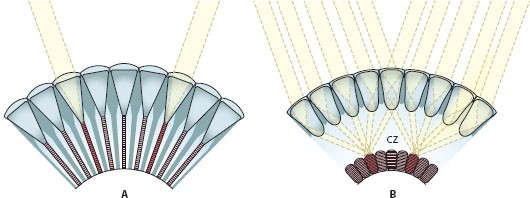
Figure 5.8 The two broad subtypes of compound eyes. (A) Apposition eyes (in this case a focal apposition eye). (B) Superposition eyes (in this case a refracting superposition eye). cz = clear zone. (Images courtesy of Dan-Eric Nilsson)
Apposition eyes come in three main forms, but there are many other rare and interesting variants of these eyes (see Land and Nilsson, 2012). The first of the three most common forms is the “focal apposition eye” (figure 5.9A), a widespread design among insects and crustaceans. They are referred to as focal because the focal point of the optical system lies at the distal tip of the rhabdom. The second form, the “afocal apposition eye,” has distinctive crystalline cones with tapering apical “stalks,” and the distal rhabdom tip is not located in the focal plane (figure 5.9B). So far, afocal apposition eyes are known only from papilionoid butterflies, where they are of general occurrence. The third form is the “neural superposition eye,” and despite its name, it is an apposition eye in which signals from individual retinular cells in seven neighboring ommatidia—which all view exactly the same small region of space—superimpose on the same second-order cell of the lamina, the first optic neuropil behind the eye. This interesting design—which results in a sevenfold boost in sensitivity for no loss in spatial resolution—is characteristic of higher flies (Kirschfeld, 1967).
In the majority of focal apposition eyes the external curved surface of the corneal lens provides the refractive power needed to focus an inverted image on the distal rhabdom tip. The crystalline cone, with its low and homogeneous refractive index, acts merely as a watery spacer (figure 5.9A). This is not the case in all focal apposition eyes, such as those of the horseshoe crab Limulus polyphemus. In Limulus the surface of the corneal lens is flat and thus without optical power, and the lens itself is internally elongated to form an inwardly pointing cone-shaped extension—the crystalline cone is absent. The focal power of this elongated corneal lens is instead provided by a moderate internal gradient of refractive index that brings rays to a focus on the distal rhabdom tip (Exner, 1891; Land, 1979).
The situation is somewhat different in afocal apposition eyes (figure 5.9B): instead of being entirely homogeneous in refractive index, the crystalline cone at its proximal end possesses a cylindrical “cone stalk” containing a powerful radial gradient of refractive index from the axis of the cone stalk to its periphery (Nilsson et al., 1984, 1988; Nilsson, 1989). Parallel rays focused by the external corneal surface are brought to an intermediate focus at the distal entrance to the stalk and are then recollimated by the refractive index gradient. This stalk, which acts as a powerful second lens, plays a key role in the waveguide optics of the rhabdom, acting as a “mode coupler” that significantly improves visual performance (figure 5.9B).
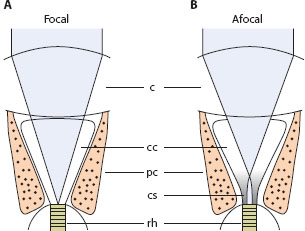
Figure 5.9 Focal and afocal optics in apposition compound eyes. (A) In focal apposition eyes, light (gray) is focused by the corneal facet lens (c) directly onto the distal tip of the rhabdom (rh); the crystalline cone (cc) is watery and acts merely as an optical spacer. (B) In afocal apposition eyes, light is brought to an intermediate focus at the entrance of the powerful cone stalk (cs), which then recollimates the light and directs it to the rhabdom. The gray shading in the cone stalk schematically indicates the presence of the refractive index gradient. pc = pigment cell.
The rhabdoms of butterfly afocal apposition eyes are usually quite thin, some as thin as 1.5 μm, values that begin to approach the wavelength of light (0.5 μm for blue-green light). We mentioned in chapter 4 that when this happens the rhabdom begins to behave as a “waveguide” and to propagate waveguide “modes,” stable patterns of light traveling along the waveguide. The wider the rhabdom, the greater the number of different mode orders that can propagate. The bell-shaped fundamental mode LP01 always propagates, no matter how thin the rhabdom. For rhabdoms between about 1.6 μm and 2.5 μm wide, LP01 is joined by the second-order mode LP11, and for widths between about 2.6 μm and 3.5 μm these are joined by the third-order modes LP02 and LP21. A narrow rhabdom, such as the 1.5-μm rhabdom of the butterfly Argynnis paphia, propagates only the fundamental mode LP01. So too does the equally thin proximal cone stalk joining the rhabdom. However, in the slightly wider conical distal stalk, both LP01 and LP02 can propagate. These two modes are excited to propagate the Airy diffraction pattern of light produced by the corneal facet (see above). As these two modes propagate proximally through the narrowing cone stalk, the power of LP02 is entirely transferred—or coupled—to LP01, which alone is capable of propagation in the rhabdom. This mode coupling greatly improves the efficiency of light transmission to the rhabdom, significantly increasing both spatial resolution and sensitivity (Hateren and Nilsson, 1987).
Superposition Compound Eyes
Unlike the ommatidia of apposition eyes, those of superposition eyes are not optically isolated from each other by screening pigments (except in the extreme light-adapted state in some species): light rays entering many corneal lenses are focused onto a single rhabdom in the retina below. This is facilitated by the presence of a wide optically homogeneous “clear zone” (figure 5.8B), which separates the crystalline cones from the rhabdoms. Exactly how this focus of light is achieved is remarkable in itself, and it is this optical trick that is probably the most important feature that distinguishes superposition eyes from apposition eyes.
In a focal apposition eye, when a bundle of parallel light rays enters the corneal lens at an angle to its optical axis, the rays are brought to a focus (at the distal rhabdom tip) on the opposite side of the axis. This is why the image of a distant scene is inverted at the focal plane of the corneal lens. This image also occurs multiple times, once in each facet of the eye. In superposition eyes, however, the situation is very different. Due to remarkable specializations in the crystalline cones, a bundle of parallel light rays that enters the cornea-cone lens pair at an angle to its common optical axis is instead redirected to the same side of this axis. This redirection is also accompanied by a recollimation of the light beam: rays that enter the lens pair parallel also leave it parallel. The thin beam of parallel rays that results is then fired across the clear zone toward a single rhabdom in the retina far below. In superposition eyes, typically hundreds of such thin pencils of light, leaving equally many crystalline cones, are targeted at one and the same rhabdom. At the focal plane of the eye—which coincides with the targeted rhabdom—these beams superimpose to form a single erect image. That all these beams of light end up at the same place seems almost miraculous, and indeed, the degree to which they superimpose is a major determinant of image quality and spatial resolution in this type of compound eye. But the fact that each single photoreceptor receives light from not one but from hundreds of facets means that sensitivity is boosted tremendously. Not surprisingly, superposition eyes are common in insects and crustaceans active in dim light, such as nocturnal moths and beetles as well as deep-sea crustaceans. But remarkable exceptions do exist, particularly among day-active moths, skipper butterflies, and scarab beetles.
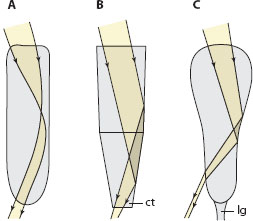
Figure 5.10 The three different types of superposition optics found in the Arthropoda, showing the paths of incident light rays focused by the cornea into the crystalline cones (shown schematically). (A) Refracting superposition optics, in which incident light rays are refracted by powerful gradients of refractive index within the cornea and crystalline cones. (B) Reflecting superposition optics, in which incident light rays are reflected by the flat tapering sides of each homogeneous box-shaped crystalline cone. (C) Parabolic superposition optics, in which incident light rays are reflected and collimated by the parabolic sides of each homogeneous crystalline cone. ct = cone tract, lg = light guide.
How do the crystalline cones manage this marvelous act of superposition? It turns out that they do so in one of three possible ways, each one having evolved independently in one or more branches of the arthropods and each giving rise to a completely unique subtype of superposition eye (figure 5.10). The first—functioning by refraction (figure 5.10A)—was discovered in fireflies by Sigmund Exner in the 1880s, but it took a further century before the other two were discovered. In the mid-1970s Klaus Vogt (1975) discovered that the superposition eyes of crayfish and lobsters function by reflection (figure 5.10B), and over 10 years later Dan-Eric Nilsson (1988) discovered a superposition eye in a crab that functioned by a combination of both refraction and reflection (figure 5.10C). However, by far the most studied superposition eyes are those discovered by Exner, namely refracting superposition eyes, the type found universally in insects, such as dung beetles (see figure 5.11) as well as in some groups of crustaceans, for example, krill (Land et al., 1979).
In refracting superposition eyes—the type of superposition eye shown in figures 5.8B and 5.11C—each ommatidium possesses a corneal lens and a crystalline cone that together function as a Keplerian telescope (figure 5.11D; Exner, 1891; Cleary et al., 1977; Land, 1981; Caveney and McIntyre, 1981; McIntyre and Caveney, 1985). Such telescopes contain two lenses separated by the sum of their focal lengths: a parallel bundle of light rays incident on the first lens will be brought to an intermediate focus between the two lenses and recollimated into a parallel bundle by the second lens (figure 5.12C). In such an optical system the ray bundle will exit on the same side of the optical axis as it entered, thus forming an erect image. To cope with large angles of incidence, the second lens needs to be quite large, but in refracting superposition eyes, the Keplerian optics is preserved in miniature form via a remarkable mechanism. Instead of being homogeneous as in an apposition eye, the corneal lenses and crystalline cones possess powerful gradients of refractive index (figure 5.12A).
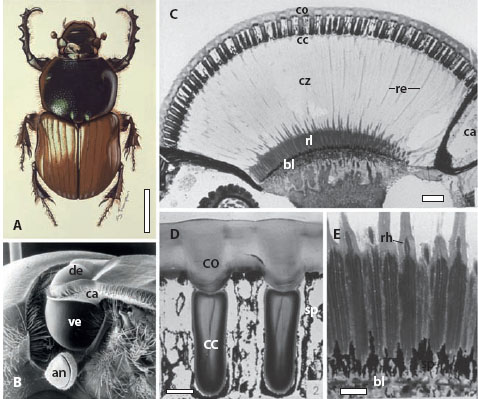
Figure 5.11 Refracting superposition eyes of the nocturnal dung beetle Onitis aygulus (A, scale bar 5 mm), which consist of a smaller dorsal eye (de) and a larger ventral eye (ve) that are separated by a chitinous canthus (ca) on each side of the head (B). The beetle’s right antenna (an) is also visible. (C) A longitudinal section through the ventral eye showing the cornea (co), crystalline cones (cc), clear zone (cz), retinular cell extensions (re), rhabdom layer (rl), basal membrane (bl), and canthus (ca). Scale bar 100 μm. (D) A close-up of the cornea and crystalline cones shown in C. sp = screening pigment, Scale bar = 20 μm. (E) A close-up of the retina from C showing the rhabdoms (rh). Scale bar = 20 μm. (Panels C–E used with permission from McIntyre and Caveney, 1985)
Sigmund Exner predicted these gradients in 1891, and they were finally measured (using interference microscopy) in the meal moth Ephestia kühniella, the animal in which Exner’s superposition theory was finally proved to be correct after a long period of contention (Kunze, 1969, 1972; Kunze and Hausen, 1971; Hausen, 1973; Vogt, 1974; Cleary et al., 1977). Since then refractive index gradients have been measured in the lenses of many arthropod superposition eyes (e.g., dung beetles: figure 5.12A). The corneal lens and distal cone focus an incoming ray bundle into the cone’s waist region. The proximal cone then recollimates the bundle, allowing it to exit from the proximal cone tip as a narrow parallel beam on the same side of the optical axis that it entered (figure 5.12C). Due to the inherent (roughly spherical) curvature of the eye surface, facets further from the central axial facet must accept parallel light rays at larger angles of incidence. Their exit angles are larger too, and in a classical spherically concentric superposition eye, the ratio of the two angles is roughly 1. This would create a best focus (i.e., a superposition) of exiting light beams, from all the ommatidia of the superposition aperture, at about halfway between the layer of lenses and the center of curvature of the eye. In practice this ratio of exit angle to entrance angle—known as the optical system’s angular magnification—is usually a little greater than 1, which places the best focus slightly closer to the layer of lenses. Not surprisingly, the retinal surface lies close to this position (McIntyre and Caveney, 1985).
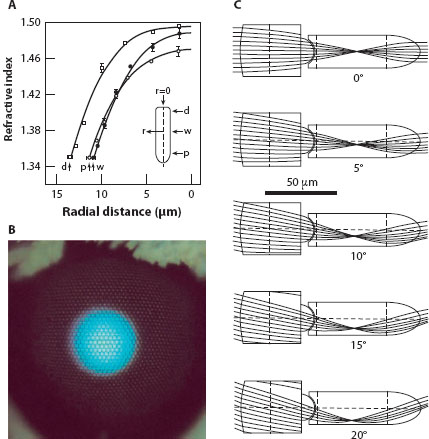
Figure 5.12 Graded refractive index optics in the refracting superposition eyes of the nocturnal dung beetle Onitis aygulus. (A) Refractive index as a function of radial distance r in the distal (d), waist (w), and proximal (p) regions of the crystalline cone (r = 0 denotes the axis of the cone: inset) (B) Superposition aperture of an unidentified moth revealed by its “eye glow.” (C) Theoretical ray traces showing how parallel light rays incident at different angles of incidence (0–20°) on the corneal surface are bent and recollimated by internal gradients of refractive index present in the corneal facet lenses and crystalline cones. In O. aygulus the maximum angle of incidence allowed (before rays escape the side of the crystalline cone to be absorbed by surrounding screening pigment) is around 27°. (Panels A and C modified with permission from McIntyre and Caveney, 1985)
Another consequence of an ever-increasing angle of incidence of parallel light rays at the corneal surface is that beyond a certain maximum angle the cornea and cone can no longer bend the incoming beam sufficiently to allow it to safely exit the cone tip to begin its journey across the clear zone toward the retina (figure 5.12C). Instead, the beam exits the side of the cone, where it is absorbed by screening pigments. Because the angle of incidence of light steadily increases as one moves further from the central axial facet in all directions, the maximum acceptable angle defines the diameter of the region of facets that accepts light for superposition on a single rhabdom. In refracting superposition eyes this region is a large circular aperture of corneal facets known as the “superposition aperture,” and, as we mentioned above, this may contain hundreds or (in extreme cases) thousands of facets. A larger superposition aperture implies greater sensitivity to light, and larger apertures are indeed found in more nocturnal species, as has been nicely documented in dung beetles (McIntyre and Caveney, 1998). The superposition aperture is readily visualized in a nocturnal moth or beetle with a reflective tapetum (see chapter 4): illumination of the eye from the same direction as it is viewed reveals a bright round “eye glow” whose area is approximately equivalent to the superposition aperture (figure 5.12B).
Remarkably, despite the superposition of hundreds of individual beams of light on the retina, refractive superposition eyes usually have quite decent spatial resolution. By using an ophthalmoscope to image the retina, Land (1984) discovered that image quality in the superposition eyes of skipper butterflies and agaristine noctuid moths is only marginally worse than that due to diffraction at the individual corneal facet. This truly impressive result implies that these superposition eyes—with hundreds of imaging facets—can have the same optical resolution as an apposition eye having a single imaging facet of the same size. In an optical model of the superposition eye of the day-active dung beetle Onitis westermanni, McIntyre and Caveney (1985) calculated the destinations of a large number of traced light rays focused by a super position aperture of 109 facets (figure 5.13B) and discovered that nearly all of them fell neatly over a single flower-shaped rhabdom (figure 5.13A); this again demonstrates how sharply focused superposition eyes can be. In the closely related crepuscular O. alexis (figure 5.13C: superposition aperture of 241 facets) and the nocturnal O. aygulus (figure 5.13D: superposition aperture of 647 facets), the image becomes progressively worse but is still quite well focused despite the increasingly larger and more sensitive superposition aperture.
But as we saw in the previous chapter, even an exquisitely well-focused image on the retina is no guarantee of a sharp visual image. This is especially true in eyes of low F-number, such as superposition eyes, where the angles of incidence of individual light rays can exceed 40°. Without some form of shielding between the rhabdoms (see figure 4.8), such rays will simply spread throughout the retina, degrading contrast and spatial resolution. Indeed, in the crepuscular dung beetle O. alexis, a species that lacks rhabdom shielding, the shape and width of the electrophysiologically measured receptive field can be entirely explained on the basis of this light spread (Warrant and McIntyre, 1990). Not surprisingly the actual receptive field is much broader (Δρ = 4°) than the distribution of light on the retina (which has a half-width of ~1°: see figure 5.13C). A rather different situation is found in the remarkable nonspherical superposition eye of the diurnal hawkmoth Macroglosslum stellatarum, a species possessing a reflective tapetal sheath around the full length of each rhabdom (see figure 4.8D; Warrant et al., 1999). Such a sheath fully contains the cone of focused light by total internal reflection and prevents light from spreading to neighboring rhabdoms. The resulting receptive field is the narrowest ever measured from a photoreceptor in a superposition eye, with Δρ just 1.3° (Warrant et al., 2003). Many apposition eyes, such as those of flies and bees, have values of Δρ that are close to double this.
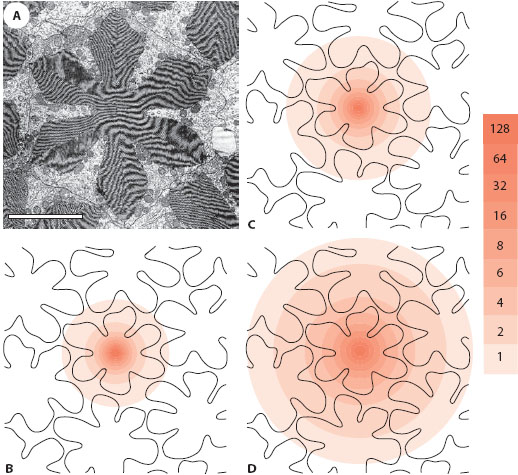
Figure 5.13 Theoretical retinal image quality in the refracting superposition eyes of three species of dung beetles from the genus Onitis, the diurnal O. westermanni (B), the crepuscular O. alexis (C), and the nocturnal O. aygulus (D). After an optical model of the eye of each species had been constructed, parallel light rays (incident on the surface of the eye in a square grid with spacing 1.5 μm) were traced until their destination on the retina. In transverse section the rhabdoms of dung beetles are flower shaped (electron micrograph in A); in all three species a density contour map (pink) showing the destinations of rays targeted at a single rhabdom reveals that most rays fall on their target, although neighboring rhabdoms may also be intercepted (color scale shows rays per 1 μm2). Image is sharpest in the diurnal species (109 contributing facets), followed by the crepuscular species (241 facets), and then the nocturnal species (647 facets). The rhabdom diameter in all three species is 14 ± 1 μm. Scale bar in A = 5 μm. (Panels B–D redrawn from McIntyre and Caveney, 1985)
Thus, considering their formidable sensitivity to light, superposition eyes can have impressive spatial resolution. It is no doubt this combination that has allowed many nocturnal insects with superposition eyes to have spectacular visual behaviors at night, including the abilities to see color, to orient using the faint pattern of polarized light formed around the moon, and to avoid obstacles during high-speed flight through a forest. These, however, are topics for a later chapter (chapter 11).












