13
Signals and Camouflage
A juvenile swordfish is slowly cruising the depths of the Sargasso Sea when it spots a small object silhouetted against the dim and blue downwelling light. It is hard to see clearly, but the object resembles the body of a small fish drifting sideways, as if it were injured, sick, or dying. The swordfish, which has been traveling the empty sea for over a day without seeing any suitable food, turns upward and begins to approach this easy prey with ever-increasing speed. Just as it is about to strike, the swordfish briefly notices a much larger and blue cylindrical object surrounding the silhouette of the fish. Suddenly, everything vanishes and the swordfish feels a stabbing pain in its right flank, where a circular plug of flesh has been neatly extracted. The small sickly fish and the blue cylindrical object are now nowhere to be seen. The swordfish has just been outwitted by the cookie-cutter shark (Isistius brasiliensis), a 50-cm-long and relatively sluggish animal with a unique feeding strategy that uses a form of bioluminescent camouflage known as counterillumination to hide the bulk of its silhouette while leaving a small portion of its ventral surface visible to be used as a lure (Widder, 1998; figure 13.1). This allows it to prey on animals that are much larger and faster.
As we have seen in this book, vision serves many purposes, but two of the most important are predation and reproduction. Therefore, it is not surprising that a diverse assemblage of species have evolved clever ways to hide themselves and equally clever ways to signal information to both conspecifics and heterospecifics. Camouflage is usually used either to avoid being eaten or to improve the chances of getting within striking range before being detected, although it can also be seen in conspecific interactions (e.g., “sneaker” males). Signals are used to communicate with conspecifics, most often for reproductive and social purposes, and to either lure heterospecifics or warn them of chemical or other defenses. Both camouflage and signals often need to function in a number of different optical environments and against visual systems with varying abilities, many of which are under strong selective pressure to either break the camouflage or eavesdrop on the signal. In certain cases, an individual needs to signal clearly to a conspecific while remaining inconspicuous to other species. This evolutionary arms race between hider and seeker, the potential need to send private signals, and the intimate connection with the optical environment make camouflage and signaling exciting areas of research for the visual ecologist.

Figure 13.1 (Top) The silhouette of the cookie-cutter shark (Isistius brasiliensis), as viewed from below without counterillumination. (Bottom) The silhouette of the same animal with its many small ventral photophores turned on. Note that a small patch near the mouth still casts a silhouette that is thought to act as a lure.
The study of animal camouflage and signaling has grown tremendously since Hugh Cott’s and Abbot Thayer’s pioneering work during the first half of the twentieth century (Thayer and Thayer, 1909; Cott, 1940). A complete review of the subject would extend beyond optics, physiology, and morphology into perceptual and cognitive psychology and easily fill several large volumes (e.g., Fox and Vevers, 1960; Ruxton et al., 2004; Stevens and Merilaita, 2011). Because this book has primarily focused on the connection between visual systems and the optical environment, this chapter primarily concerns itself with the various mechanisms of camouflage and signaling and their success in different habitats, leaving the perceptual, cognitive, and evolutionary aspects for others to review (e.g., Stevens, 2013). It also focuses more on the principles underlying the various mechanisms rather than on an attempt to review all the biological instances of each.
Detection versus Recognition
Before we discuss the mechanisms of camouflage, there are a few terms that need to be defined. The fields of camouflage and signaling are famously dense with conflicting and complex definitions that we will not reproduce here (but see review by Stevens and Merilaita, 2011). However, it is important to distinguish detection from recognition. A signal or animal is detected if it can be visually separated from the background; it is recognized if it can be identified as belonging to a particular category relevant to the viewer (in most cases, as being either a conspecific or a potential competitor, predator, or prey). In relatively featureless habitats such as the open ocean and the sky, reducing detection is often the central goal of camouflage, since most detected objects will be approached and investigated in more detail, regardless of their initial familiarity. In these habitats, we see camouflage strategies that reduce the contrast of the object as much as possible, such as transparency, mirroring, countershading, and counterillumination (figure 13.2, left). We also see signals that are relatively simple in optical terms, such as bioluminescence.
However, in more complex habitats such as forests and coral reefs, an animal’s camouflage may make it detectable but not recognizable. The primary camouflage strategy in these habitats involves patterning of the surface via pigments and structural colors. These patterns either break up boundaries and three-dimensional form, making the animal difficult to recognize as a single object, or they disguise the animal as some component of the habitat that is innocuous to the relevant viewers (e.g., algae, rocks: see figure 13.2, right). Thus, the camouflaged animal is seen but not identified as important, or possibly identified as being important but in the wrong category. The perceptual mechanisms underlying recognition are exceedingly complex, and—as is well known by biologists searching for animals in native habitats—experience greatly improves the odds of finding previously unrecognizable animals. Thus, this area of camouflage, which is related to the field of machine vision, is one of the great unsolved problems of visual cognition. Complex optical habitats also make signaling more challenging, and signals must often be multimodal to be clearly separated from the background. Thus, the steady glow of a deep-sea bioluminescent octopus is replaced with the flashing color patterns found on Heliconius butterflies in flight.

Figure 13.2 (A) Animals in featureless environments, such as this snub-nosed dart (Trachinotus blochii), tend to employ strategies that reduce contrast and thus detectability. (B) In contrast, animals in complex environments, such as the giant Australian cuttlefish (Sepia apama), tend to employ strategies that reduce recognition, usually via color patterns and in some cases texture.
No discussion of camouflage and signaling is simple, however, and one could argue that a fish hiding in the open ocean is not reducing detectability but instead mimicking water, or that a leafy sea dragon (Phycodurus eques) is not mimicking algae but rendering itself undetectable on a noisy background. However, we feel that the distinction between strategies that (generally) reduce detection in simple environments and those that reduce or enhance recognition in complex habitats is sharp enough to guide a discussion of the visual ecology of camouflage and signals.
The remainder of this chapter is broken up by mechanism based on the four ways that matter can interact with light: (1) emission (bioluminescence), (2) transmission (transparency), (3) reflection (mirroring), and (4) absorption (pigmentary colors) (figure 13.3). The first three mechanisms, at least when used as camouflage, are primarily found in pelagic species. The last is found in nearly all habitats and has the most varied expression. This division is not perfect because pigments require reflective tissues below them that reflect the light, and some mirrors are colored. Nevertheless, it serves as a useful way to organize a discussion of camouflage and signaling mechanisms.
Camouflage via Emitted Light—Counterillumination
In both terrestrial and aquatic habitats, fewer photons are going up than going down. On land this is because most natural surfaces reflect less than half the light that strikes them. In aquatic habitats this is due to the fact that most down-traveling photons are absorbed before they have a chance to be scattered back upward. The latter effect is much stronger than the former, so while the ground is about 1/10 to 1/2 as bright as the sky on land, the upward radiance in natural waters is only 1/100 to 1/10,000 the value of the downward radiance (Mobley, 1994; Bohren and Clothiaux, 2006). Because it is this upward radiance, either from the ground or from the watery depths, that illuminates the ventral surface of an animal, its low value relative to the downward background radiance means that animals viewed from below are nearly always seen as black silhouettes. This is true whether the ventral surface is colored black or white (Johnsen, 2002). For example, birds, unless they reflect direct sunlight down to the viewer, nearly always appear black when flying far overhead, and scuba divers swimming above are black regardless of the color of their wetsuits (again, unless they reflect direct sunlight to the viewer). Calculations of the contrasts of ventral surfaces in underwater light fields typical of ocean and coastal water have shown that they are essentially independent of the color of the ventral surface, always appearing black (Johnsen, 2002; Johnsen and Sosik, 2003).
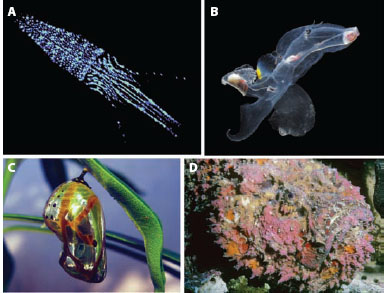
Figure 13.3 The four primary mechanisms of camouflage and signaling are based on the four ways in which light can interact with matter. (A) Emission: counterillumination in the squid Abralia veranyi. (Courtesy of Edith Widder) (B) Transmission: transparency in the heteropods mollusk Carinaria sp. (C) Reflection: the reflective chrysalis of a common crow butterfly (Euploea core). (Courtesy of Pon Malar) (D) Absorption: colored patterns due to pigmentary colors in the reef stone fish (Synanceia verrucosa).
This black silhouette is especially problematic for aquatic animals because water is an attenuating medium, and—as discussed in chapter 9—contrast is attenuated slowly when viewed from below, so the silhouette can be visible from a large distance. Add to this the fact that the upward viewing direction provides by far the most light, and that many deep-sea species have eyes that look directly upward (figure 13.4), and this ventral silhouette becomes a substantial vulnerability.
For opaque animals, there appear to be only two solutions to this problem. The first, which is discussed in the section on mirroring, is to have no ventral surface, replacing it with a keel-like silvery structure that reflects horizontal light downward. The second is for the ventral surface to emit light via bioluminescence (see chapter 2). This solution, named counterillumination to parallel the concept of countershading, has been adopted by a number of species from a diverse array of taxa. It is particularly common and well developed in mesopelagic squid, fish, and crustaceans (Young and Roper, 1976; Herring, 1976, 1985; Widder, 1999; Johnsen et al., 2004), but counterillumination is also a hypothesized function for the ventral photophores found in shallow-water and benthopelagic taxa such as the midshipman fish Porichthys (Harper and Case, 1999), leiognathid fish (McFall-Ngai and Morin, 1991), and sepiolid squid (e.g., Euprymna scolopes) (Jones and Nichigushi, 2004). Interestingly, although counterillumination would also be useful for crepuscular and nocturnal insects, it has not been documented. Nor has it been hypothesized for any other terrestrial animal.

Figure 13.4 The barreleye spookfish (Opisthoproctus soleatus), one of many deep-sea fish with eyes that look directly upward, presumably to catch the most light and thus improve vision.
Although the central concept of counterillumination—matching the downwelling light to hide one’s silhouette—is straightforward, the strategy is poorly studied and involves more subtleties and limitations than is often appreciated. The first limitation is intensity. Bioluminescence requires ATP at a rate of roughly 60 kcal per mole of photons emitted (for fireflies; 180 kcal/mole for the ostracod Cypridina), and Young and Roper (1977) estimated that counterillumination during the day by the squid Histioteuthis heteropsis at a depth of 400 m in clear oceanic water accounted for 0.3–0.9% of the animal’s resting metabolic rate. Because the downward radiance they need to match goes up by a factor of about 30 for every 100-m decrease in depth, this percentage of resting metabolic rate would rise to 9–27% at 300 m and 270–810% at 200 m. Thus, Young and Roper concluded that daytime counterillumination is limited to animals deeper than 350–400 m in clear water.
In addition to producing enough light, the counterilluminating animals also have to match the brightness of the downwelling light. The visual feedback mechanism by which this is accomplished is poorly understood and is likely to be interesting because many counterilluminators cannot see their own ventral photophores (although some have photophores that emit light into their own eyes). A further complication is that—ideally—the emitted light has to match the background light from a number of viewing directions, not just from directly below. In other words, the animal has to reproduce the angular distribution of the underwater light field. This has been tested only in the deep-sea hatchetfish (Argyropelecus affinis) and the viperfish (Chauliodus sloani), both of which use an ingenious combination of mirrors within the photophores to match the shape of the light field (Denton, 1971; Denton et al., 1972). Whether they can change this distribution to match different distributions of light (for example at shallower depths) is unknown.
A third limitation is the acuity of the viewer’s visual system. With the exception of a few species (mostly squid), the ventral surface is not evenly lit but instead studded with a relatively small number of discrete light organs (figure 13.5). Because oceanic water is so clear (see chapter 9), the light from these separate organs is not blurred together, even at large distances (see Johnsen et al., 2004). Therefore, viewers with acute vision can break the camouflage. Acute vision is likely relatively rare in the nocturnal and deep waters where counterillumination occurs due to spatial summation (see chapter 11) but may nevertheless occur and be used to break this form of camouflage.
The final limitation is that the counterilluminator ideally needs to match the spectrum of the downwelling light. This is advantageous even though many of the animals in the deep sea have monochromatic visual systems. If the spectrum of the bioluminescence does not match the spectrum of the downwelling light, then the perceived brightness may not match either. This is because even monochromatic visual systems vary in their spectral sensitivity. Therefore, their perception of the brightness of the background and the photophores also varies (see chapter 3) unless the underlying spectra themselves match. In addition, some deep-sea animals have yellow filters in their lenses that can enhance the difference between the background and the emitted light. Indeed, the spectrum of counterillumination is often broader than that of the downwelling light, and Muntz (1976) showed that the filters in the eyes of many mesopelagic fish are well suited to maximize this difference.
A final issue is that the spectrum of the downwelling light is not constant—it is broader near the surface and in coastal waters than it is in the depths of the open ocean. Thus, an animal that moves from one water type to another, for example via diel vertical migration, needs to adjust the spectrum of its counterillumination. This has been studied in only a handful of cephalopod genera (Abraliopsis and Abralia), which were found to employ a clever solution (Young and Mencher, 1980). When tested in cold water, these squid emitted blue light that matched that found in the deep sea. When tested in warm water, however, they emitted light with a broader spectrum, better matching that found near the surface. The conclusion of the authors was that these squid were using water temperature as a proxy for depth and adjusting the emission of their ventral photophores accordingly.

Figure 13.5 (Left) The euphausiid shrimp Meganyctiphanes norvegica. (Right) The deep-sea hatchetfish Argyropelecus aculeatus. Each animal is directly above an image of the emissions of its ventral photophores. Note their relatively wide spacing compared to that found in the squid Abralia (figure 13.3). (Courtesy of Edith A. Widder)
In sum, counterillumination is a marvelously developed camouflage mechanism. However, much of our knowledge comes from only a small fraction of the species that employ it, and many central questions remain unanswered.
Signaling via Emitted Light—More Bioluminescence
With the exception of its use in counterillumination, illumination, and certain startle responses, bioluminescence is used as signal. Its hypothesized signaling functions are diverse and range from luring to warning to sexual signaling (figure 13.6). Some of these functions, such as sexual signaling in fireflies, are well established (reviewed by Lewis and Cratsley, 2008). Others, such as luring in deep-sea anglerfish, are considered intuitively obvious but have not been experimentally verified. Some, such as the burglar alarm hypothesis—where light is emitted to attract higher-order predators that then deter (or eat) the animal that is preying on the bioluminescing organism—are one step above fantasy (though see Mensinger and Case, 1992, for an experimental test). The primary difficulty in verifying most hypothesized functions is that most bioluminescing organisms are deep-sea species that are not amenable to behavioral assays. Thus, we know a great deal about sexual signaling in fireflies and ostracods but next to nothing about the possible signaling functions of the complex photophores found on many deep-sea squid.
It is known, however, that bioluminescence is a highly efficient optical signal. The primary reason for this is that it is nearly always emitted against a black background, which has two advantages. First, this means that the contrast of the signal is effectively infinite (see chapter 9). Thus, the distance at which it can be detected is limited only by the absolute sensitivity of the viewer (see chapter 11). This is why stars can be seen at night but a sparrow flying at 300 m during the day cannot, even though the former are inconceivably more distant than the latter. The light from photophores is, of course, much dimmer than that of stars, but even in an attenuating medium such as water, a typical flash can be seen by a typical deep-sea fish at distances up to 100 m (Warrant and Locket, 2004). The second advantage of emitting light in the dark is that the background is simple. Even the complex geometry of a tropical forest is simplified in the dark, allowing firefly signals to be seen from a substantial distance as long as no structure is directly between the beetle and the viewer (figure 13.7). In contrast, the yellow light organ of a firefly sitting in the canopy during the day would likely remain undetectable at distances greater than a meter, likely even closer for animals with less acute vision than humans.
Another advantage of bioluminescent signals is that they can be turned on and off. Certain signals based on pigmentary and structural colors can be covered and uncovered, but this requires movement and overlapping structures. In contrast, the ease with which most bioluminescence can be activated and deactivated not only helps ameliorate the conflict between sending a conspicuous signal and remaining safe from visual predation but also allows the creation of signals in both time and space. These patterns seem to be primarily used for courtships displays. The flashing courtship displays among fireflies are well studied and even include an example of aggressive mimicry, where Photuris females imitate the flash patterns of Photinus females in order to attract and eat Photinus males (Lloyd, 1965, 1975). In non-firefly species, less is known. The best-studied bioluminescent courtship displays in marine organisms are found in the ostracods (Morin, 1986; Morin and Cohen, 1991; Rivers and Morin, 2008). The males of these small crustaceans essentially vomit small packets of the components of the bioluminescence reaction into the water as they swim, creating species-specific displays in both time and space (figure 13.8). There is even documentation of nonbioluminescent sneaker males waiting near the displays to mate with the females that are attracted to the light (Rivers and Morin, 2008).
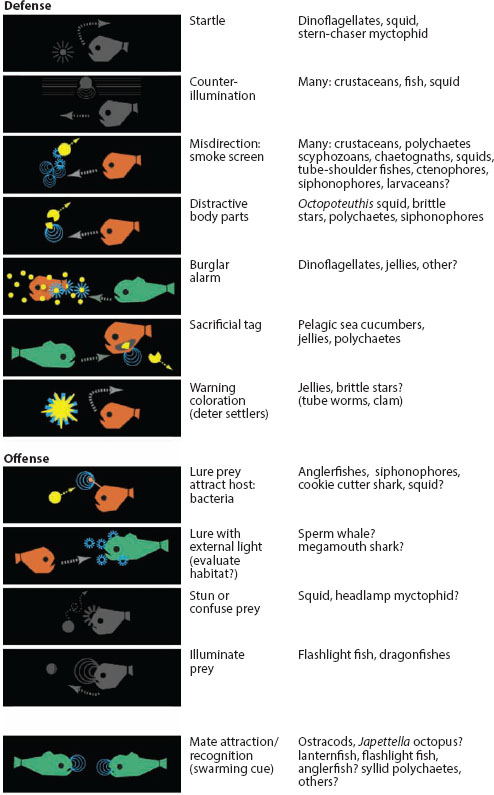
Figure 13.6 Various established and hypothesized functions of bioluminescence in marine species. Those that are not signals are left in gray. (From Haddock et al., 2010)

Figure 13.7 Fireflies at night. Note that the dark and simple background makes the signal easy to discern. (Courtesy of Judd Patterson)
Complex temporal patterns of bioluminescence are also observed in species that do not appear to use bioluminescence for courtship. For example, in many ctenophores and pennatulaceans (sea pens), the light is emitted in slow waves down the length of the body, and certain bioluminescent coral and pelagic octopods appear to twinkle with large numbers of photophores turning on and offasynchronously. However, the most startling display is found in coronate medusae of the genus Atolla. Repeated mechanical stimulation of these jellyfish leads to a rotating “pinwheel” display in which photophores are activated and deactivated in turn (figure 13.9). It is thought that these highly visible displays have evolved to attract higher-order predators via the process described earlier, but this has not yet been demonstrated (although, as mentioned in chapter 2, an Atolla mimic built from blue LEDs was used to attract a giant squid).

Figure 13.8 The bioluminescent courtship displays of various species of ostracod. The arrows denote the direction in which the individual puffs of light were produced. (Modified from Cohen and Morin, 2003, by Todd Oakley)
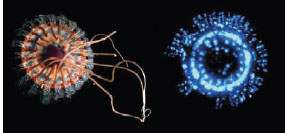
Figure 13.9 (Left) The coronate medusa Atolla wyvillei. (Right) One moment in the animal’s bioluminescent “pinwheel” display. (Courtesy of Edith Widder)
The primary disadvantage of bioluminescence relative to other forms of signals is of course that it requires energy and reactants. Pigmentary and structural colors also require energy (and possibly specific dietary requirements) to construct and maintain, but bioluminescence is the only form of signal (and camouflage) that consumes energy and reactants as it is being used. The amount of required energy is far lower than might be expected—as mentioned above, only about 50–200 kcal/mole of photons (to provide perspective, it takes about 8 minutes for a square meter of sidewalk to intercept a mole of visible solar photons on a sunny day at noon). Even the brightest and largest bioluminescent displays only emit at most 10-10 moles of photons per second (Widder, 1999) and thus consume very little energy (though even this small amount may be significant for small deep-sea animals with low metabolic rates). The real limiting factors are the reactants, in particular the substrate known as the luciferin, which is dietarily derived (see Haddock et al., 2010). Because each photon produced requires the oxidation of one molecule of luciferin, a bright signal uses up stores of this substrate quickly.
The other disadvantage of bioluminescence as a signal is connected with its central advantage—it is highly visible. In dark featureless habitats, any source of light may be investigated. Thus, signaling, particularly for extended periods of time, is hazardous. Indeed, surveys of spontaneous bioluminescence in the ocean find that it is nearly nonexistent. Interestingly, the converse is found in terrestrial habitats, where a summer field can be filled with the flashing of fireflies (figure 13.7).
Camouflage via Transmitted Light—Transparency
Transparency is perhaps the most conceptually straightforward camouflage mechanism—the animal matches the background because the background is seen through the animal. It is also a surprisingly common tactic, with significant amounts of transparent tissue found in representatives from nearly all phyla (figure 13.10). As with most camouflage mechanisms, transparency appears to have evolved multiple times, in this case restricting itself almost entirely to marine pelagic species. The majority of transparent species are found in the following 10 groups, all of which are almost exclusively marine and pelagic: cubozoans, hydromedusae, ctenophores, hyperiid amphipods, tomopterid polychaetes, pterotracheid and carinarid heteropods, pseudothecosomatous pteropods, cranchiid squid, thaliaceans, and chaetognaths (figure 13.11). In contrast, transparency is found in only a few freshwater pelagic and marine benthic species, and limited to the wings of insects on land (reviewed by Johnsen, 2001). The main hypothesized reasons for the lack of transparency in terrestrial species are (1) the larger refractive index difference between tissue and air, which leads to surface reflections, (2) the greater need for support structures on land, which are often mineralized and thus opaque, and (3) the greater need for UV-protective pigments on land, which may also absorb in the visible range. The lack of transparency in benthic species is less well understood but may be related to the delicate nature of many transparent animals and thus their greater likelihood of being damaged by contact with the substrate. Another possibility is that benthic transparent animals may be revealed by the shadows that result from the lensing of light by their tissues.
Although it is in theory a perfect camouflage solution, transparency suffers from a number of limitations. The foremost of these is that it is difficult to achieve. Unlike other camouflage mechanisms that only require a change of the animal’s surface, transparency involves the entire volume of the animal, throughout which the animal must minimize both the absorption and scattering of light. Minimizing absorption is relatively simple, since few biological molecules absorb visible light unless they have evolved to do so (hemoglobin and myoglobin being major exceptions). Minimizing light scattering is far more difficult, because it requires that the animal have a nearly constant density on all size scales greater than half a wavelength of light (e.g., ~200 nm for visible light: see Johnsen and Widder, 1999). Given that many intracellular components are larger than this and that most animals of any significant size have nerves, internal vasculature, connective tissue, and digestive systems, constant density is a challenging requirement. All the ways in which transparent animals solve this problem are not yet known, but potential methods include extreme flattening (either of the whole animal or of the cellular tissue alone, which then covers a larger transparent tissue such as mesoglea), using clearing agents to increase the density of the cytoplasm and interstitial fluids to match that of protein, adjusting the size and spacing of organelles and other ultrastructural components, and having highly hydrated tissue. Most of these solutions limit the physiology and complexity of the affected tissues, which is at least one reason why transparent species tend to be slower and less reactive than related opaque taxa (reviewed by Johnsen, 2001).

Figure 13.10 Assemblage of transparent animals: (A) Amphogona apicata (hydromedusa), (B) Corolla calceola (pteropod), (C) Iasis zonaria (salp), (D) Vogtia sp. (siphonophore), (E) Naiades cantrainii (polychaete), (F) Japetella diaphana (pelagic octopus), (G) Beroë forskalii (ctenophore), (H) Greta oto (nymphalid butterfly), (I) Bathochordeus charon (larvacean), (J) Periclimenes holthuisi (anemone shrimp), (K) Leptocephalus eel larva, (L) Phylliroë bucephala (pelagic nudibranch). (Images courtesy of Laurence Madin, Steve Haddock, Jeff Jeffords, and David Tiller)
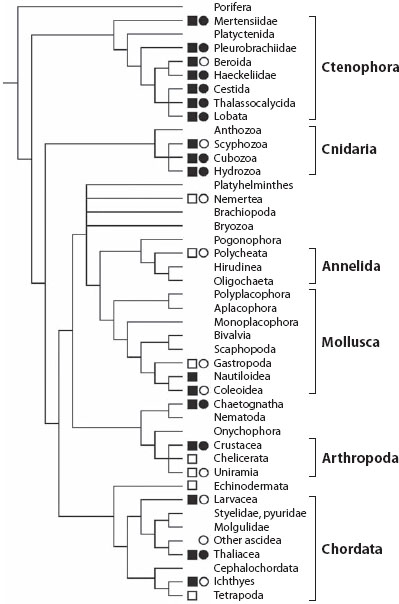
Figure 13.11 Transparency and pelagic existence mapped onto a phylogeny of the major phyla in the Animalia. Open square indicates pelagic existence is rare within adults of the group; filled square indicates pelagic existence is common. Open circle indicates transparency is rare within adults of the group; filled circle indicates transparency is common. (See Johnsen, 2001, for details)
Other limitations of transparency as a form of camouflage are visual in nature. First, even transparent substances reflect and refract light if they have a different refractive index than their surroundings. Because it is impossible for animals to have the same refractive index as water (or air), they can potentially reflect and refract light toward a viewer and thus be detected. This effect would be most pronounced in air, where the index difference is greatest, and—fascinatingly enough—some of the large transparent wings of certain moths have antireflection coatings, presumably to reduce the chances of being detected via reflected light (Yoshida et al., 1997). In water, the problem is less severe but still a concern in two habitats. Near the surface the underwater light is still direct enough to create significant reflections from transparent animals. Many tissues, such as the comb rows of ctenophores, become obvious and even iridescent when viewed near the surface on a sunny day. The other problematic habitat begins at a depth of about 600 m (or at any depth at night), where many fish and crustaceans begin to use bioluminescent searchlights mounted under their eyes. Because the background water reflects essentially none of this light, even the smallest reflection from a transparent animal can be enough to reveal it. In response, many transparent species develop a layer of pigment that strongly absorbs the blue bioluminescence of these searchlights (reviewed by Johnsen, 2005). This change can be seen between closely related species that inhabit different depths or within an individual over its life history or during diel vertical migration. Certain cephalopods, ever the masters of dynamic camouflage, can rapidly alternate between transparency and heavy pigmentation as the light conditions alternate between bright directed light, characteristic of searchlights, and dim diffuse light, characteristic of the ambient illumination at depth (Zylinski and Johnsen, 2011; figure 13.12).
The other limitation of transparency camouflage is that it can—in theory at least—be broken by viewers with UV or polarization sensitivity. Certain transparent animals are vulnerable to detection at UV wavelengths because they absorb light in this portion of the spectrum. These visibly transparent but UV-opaque species are found in oceanic waters at depths where significant UV radiation still penetrates, so it is hypothesized that the lower transparency is due to the presence of UV-protective compounds (Johnsen and Widder, 2001; figure 13.13). Because many zooplanktivorous fish in these same near-surface habitats have UV-sensitive visual pigments, this UV opacity potentially increases the vulnerability of the plankton to visually mediated predation. Johnsen and Widder (2001) analyzed this conflict between UV damage and UV predation in multiple species of transparent zooplankton and found that the animals minimized their risk of predation by primarily restricting their opacity to the UVB portion of the spectrum (280–320 nm), where UV-visual pigments are less sensitive and there is less light overall.
Transparency camouflage may also be broken by species with polarization sensitivity because certain transparent tissues are birefringent (see chapter 8), and the light in clear oceanic waters is polarized, especially in the horizontal viewing direction. Because birefringent materials change the polarization of light transmitted through them, they can become visible against a polarized background. Thus, it has been hypothesized that one function for the polarization sensitivity found in crustaceans, cephalopods, and certain fish is to detect transparent prey. This hypothesis is especially attractive because birefringent organic tissues (e.g., muscle, connective tissue) tend to be protein-rich and thus nutritious. It has also been shown that certain squid preferentially strike at birefringent versus nonbirefringent transparent beads when viewed against a polarizing background (Shashar et al., 1998).

Figure 13.12 The bolitaenid octopus Japetella heathi in its transparent and pigmented forms. It rapidly switches from the former form of camouflage to the latter if illuminated by a directed beam of light. (Courtesy of Sarah Zylinski)
Johnsen et al. (2011) used lab-based and in situ polarization imaging to test whether tissue birefringence increased visibility. Many groups, particularly the cephalopods, pelagic snails, salps, and ctenophores, were found to have ciliary, muscular, or connective tissues with striking birefringence when viewed between crossed polarizers (figure 13.14). In situ polarization imagery of the same species showed that although the degree of underwater polarization was fairly high (~30% in horizontal lines of sight), tissue birefringence played little to no role in increasing visibility. This is most likely due to the low radiance of the horizontal background light compared to the downwelling irradiance. In fact, the dominant radiance and polarization contrasts of the animals turned out to be due to unpolarized downwelling light scattered from high-index tissues (e.g., comb rows on ctenophores) viewed against the darker and polarized horizontal background light. This is a classic example of how lab-based imagery can be misleading because the illumination does not match what is found in nature.

Figure 13.13 (A) The pelagic tunicate Salpa cylindrica. (Courtesy of Edith Widder) (B) Its transmittance of visible and ultra violet radiation. Note that, despite being highly transparent, it is nearly opaque in the UV portion of the spectrum, especially the UVB portion (280–320 nm).
Signaling via Transmitted Light—Transparency as a Signal Component
Because transparent tissue is by definition inconspicuous, it cannot directly be used for signaling. However, as with the cookie-cutter shark described at the start of this chapter, a partial camouflage strategy can also be used as signal. In the case of transparency an animal can use this strategy to hide the bulk of its form while leaving a few portions visible. These remaining visible tissues are then often brightly colored, creating a signal that is isolated from the animal producing it. Possible examples of this combination of transparency camouflage and color signals can be found among the anemone shrimp (genus Periclimenes). These animals, despite being benthic, are extraordinarily transparent. However, they also have distinct and conspicuous color patterns. The function of the markings is not known, but both conspecific and interspecific signaling are possible because anemone shrimp are “cleaners” (species that enjoy a symbiotic relationship with large fish that involves eating their ectoparasites). Most cleaner species are highly colorful and some perform dances that are thought to be part of their communication with client fish (e.g., Chapuis and Bshary, 2010). Thus, conspicuous color patterns on a transparent body may be a way for anemone shrimp to signal to client fish without being recognizable as a prey item.
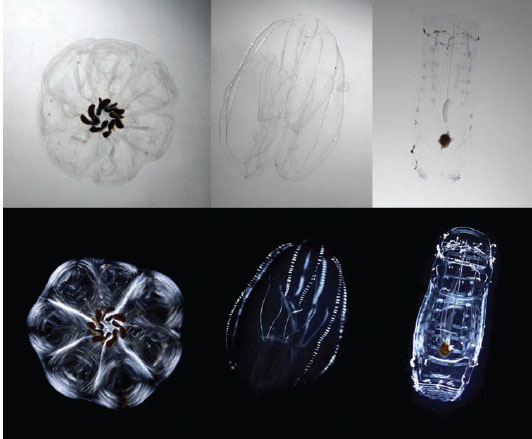
Figure 13.14 Selected images of transparent plankton viewed between parallel polarizer filters (top panels) and crossed polarizers (bottom panels). (Left) A chain of the salp Cyclosalpa floridana. (Middle) The lobate ctenophore Bolinopsis sp. (Right) The salp Salpa cylindrica (solitary form). Note that these three species all contain highly birefringent tissues, as evidenced by their high visibility when viewed between crossed polarizers. (Courtesy of Edith Widder)
Transparency has also been shown to be a component of a luring system in siphonophores (e.g., Purcell, 1980; Haddock et al., 2005). Certain siphonophores (e.g., Agalma okeni) have highly transparent bodies with pigmentation only on the tips of the stinging tentacles, which appear to have evolved to mimic small prey items such as copepods and larval fish (figure 13.16). We have seen these animals while scuba diving, and they appear as innocuous schools of small animals. The main bulk of the animal is invisible except at close range, and then only by animals with acute vision.
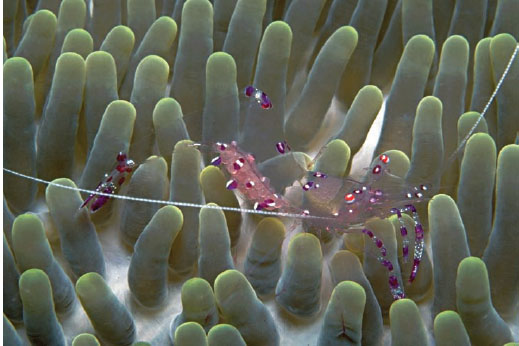
Figure 13.15 The anemone cleaner shrimp Periclimenes holthuisi. (Courtesy of Jeff Jeffords)
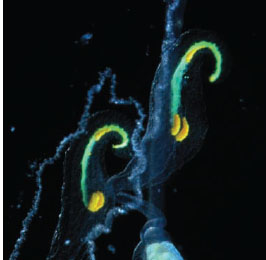
Figure 13.16 Strongly colored lures on the tips of the feeding tentacles of the siphonophore Resomia ornicephala, an example of aggressive mimicry. (Courtesy of Steve Haddock)
Camouflage via Reflected Light—Mirroring
Another way for an animal to resemble its surroundings is for it to mirror them. This works best in relatively featureless environments that also exhibit some degree of symmetry. So, as with transparency, this camouflage tactic is primarily found in pelagic species but has been hypothesized for the metallic chrysalises of certain butter flies (see figure 13.3). As discussed in chapter 2, the underwater light field becomes increasingly symmetrical around the vertical axis as the sun (or moon) nears the zenith and also with increasing depth regardless of the distribution of light in the sky. In this situation, a vertical mirror can be highly cryptic because it reflects light from a region of the underwater light field that has roughly the same spectral radiance as the region that is directly behind the animal, thus significantly lowering the contrast of the animal (figure 13.17).
This camouflage strategy is common in both freshwater and marine habitats but has an odd phylogenetic distribution. It is a common camouflage strategy in multiple families of bony fish (Carangidae, Characidae, Clupeidae, Megalopidae, Myctophidae, Sternoptychidae, and many others) but nearly absent in all other species—with the exception of the vertically oriented guts of certain transparent heteropods, squid, and pelagic octopuses. This does not appear to be due to an inability of other species to make the required reflective structures because multiple taxa use mirrors of similar optical design to camouflage individual organs (e.g., eyes and guts in cephalopods, ventral surfaces of certain pontellid copepods, guts of heteropods). Also, many taxa use mirrors in their eyes, either as tapeta or focusing elements, and even more use colored mirrors as signals (described below). One possible explanation is bony fish are one of the few aquatic taxa that both are laterally flattened and maintain a vertical posture in the water. Other species are either more cylindrical (e.g., sharks, cephalopods) or, if flattened, do not have a fixed orientation (e.g., cestid ctenophores, certain crustacean larvae). The importance of maintaining a vertical orientation can be seen in figure 13.18. Even a small tilt from the vertical can make the animal more apparent.
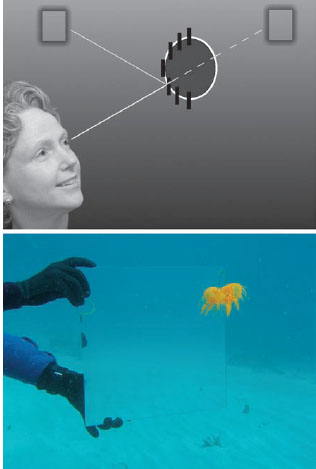
Figure 13.17 (Top) Reflection from vertical mirrors underwater (black lines) can look just like the view behind the animal because the light field is symmetric around the vertical axis. (Bottom) A vertical mirror photographed at 10-m depth shows that mirroring also work as crypsis in shallow waters if the sun is high in the sky.

Figure 13.18 (Left) The snub-nosed dart (Trachinotus blochii) well camouflaged by reflection from its silvery sides. (Right) The same fish a moment later, after it has tilted slightly, reflecting downwelling light to the viewer and becoming more visible.
However, fish do not have perfectly flat lateral surfaces either, so orientation of the reflecting structures requires some care. As was first shown by Eric Denton in the 1960s (reviewed by Denton, 1970), the mirrors in fish (which are structural reflectors composed of thin layers of high-index guanine and low-index cytoplasm) do not have a fixed orientation relative to the scales but instead are oriented within each scale so that they are roughly vertical (figure 13.19). This allows the fish to act as a flat vertical mirror even with a curved lateral surface. In addition, the tissue between the reflectors on the dorsal surface of the fish is heavily pigmented, allowing the fish to match both the dim upwelling light when viewed from above and the brighter horizontal light when viewed from the side. A further refinement found in some fish is that the reflectors are not perfectly vertical but angled slightly upward. This allows them to reflect light from a slightly higher angle of elevation. This incident light is slightly brighter than the background behind the fish, but the reflected light ends up matching because the reflectors do not reflect 100% of the light. In other words, the slight tilt of the reflectors compensates for the less-than-perfect efficiency of the reflectors. A final adjustment found in certain deep-sea fish is that the reflectors are optimized to reflect blue light from above to viewers that are below the fish. This is optimal because deep-sea light is primarily blue (see chapter 2). Because the color of the light reflected from structural reflectors of this sort depends on the angle of the incident light, these fish appear bronze when looked at in a dish, which can be misleading.

Figure 13.19 (A) The common bleak fish Alburnus alburnus. (Courtesy of Piet Spaans) (B) Cross section of the fish showing the vertical orientation of most of the reflecting structures (red) in the scales. (From Denton, 1970)
In general, though, the structural reflectors on mirrored fish reflect nearly all wave-lengths equally and thus appear silvery rather than colored. In essence, these animals have made a biological analogue of polished metal. The details of this are reviewed in Johnsen (2012), but briefly, most seem to use one of two mechanisms to achieve this. Certain fish, such as herring, have three banks of reflectors that each reflect a different portion of the spectrum and together act to reflect the whole visible portion (Denton and Nicol, 1965a,b). Other species appear to have reflectors composed of many layers with differing thicknesses (McKenzie et al., 1995). These, termed chaotic reflectors, again strongly reflect the entire visible spectrum.
Regardless of the mechanism, mirroring is an excellent form of camouflage and has been shown to be more robust to changes in the optical conditions (e.g., water color, time of day, depth, viewing direction) than camouflage based on pigmentary colors (Johnsen and Sosik, 2003). The primary disadvantages—aside from the need to maintain a vertical posture and the reliance on a symmetric light field—are that the camouflage can potentially be broken by visual systems sensitive to UV wavelengths and can definitely be broken by visual systems that can detect the polarization of light. The former occurs when the mirrors do not reflect UV radiation (in habitats where UV radiation is present), and the latter because mirrors change the polarization of the incident light as it is reflected.
The potential for UV vision to break mirror camouflage has not yet been explored in detail. This is because most measurements of the reflectance of mirrored species have not extended into the ultraviolet and also because no behavioral tests have been performed. However, Shashar et al. (2000) have examined the potential for mirror camouflage in fish to be broken by animals with polarization vision and recent in situ polarization imagery and modeling by three of the authors of this book (Johnsen, Cronin, and Marshall) have shown that it would be difficult for a mirrored fish to be cryptic to polarization-sensitive viewers and that many species of pelagic silvery fish from the Great Barrier Reef are highly visible when viewed with polarization-sensitive cameras (e.g., figure 13.20).
Signaling via Reflected Light—Structural Colors
Reflective structures are also used by a myriad of taxa for signals. These are referred to as structural colors and are particularly diverse and well developed in insects (especially beetles and butterflies), birds (e.g., figure 13.21), mollusks, (especially cephalopods, but also a few gastropods), fish, and certain primates (reviewed by Kinoshita, 2008). They are also found in most ctenophores and many annelids, but these structures are not usually thought to serve a visual function. Interestingly, structural colors appear to always be colored. In other words, they reflect only a portion of the visual spectrum, usually a small portion. To our knowledge, broadband mirror-like reflectance has not been proven to serve a signaling function in any animal.
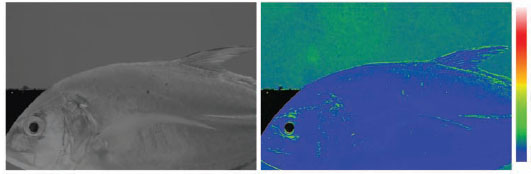
Figure 13.20 (Left) Intensity image of the bluefin trevally (Caranx melampygus), showing that it matches the background light well. (Right) Image of the same fish showing the degree of polarization of the fish and the background. The color bar to the right shows the degree of linear polarization, with blue being 0% and white being 100%. Note that the polarization of the light reflected from the fish is far lower than the polarization of the background.

Figure 13.21 Structural colors in the plumage of the male of the Indian peafowl (Pavo cristatus). (© Jebulon/Wikimedia Commons)
In fact, the narrow spectrum of nearly all structural colors, when compared to those based on absorption by pigments, is one of their primary advantages. As we discussed in chapter 2, the color of an object depends both on its reflectance and on the color of the light illuminating it. More precisely, the radiance is proportional to the product of the illuminating spectrum and spectral reflectance. Color constancy (see chapter 7), which appears to be found in many taxa, helps moderate, but does not completely eliminate, the effects of changing illumination on the perceived color of an object. And, as we also discussed in chapter 2, certain habitats (e.g., aquatic and forest) have particularly variable illumination, and the illumination in all terrestrial habitats changes dramatically during crepuscular periods. Thus, colors based on pigments, with their broad reflectance spectra, can look quite different under different illuminants (e.g., figure 2.1). In contrast, the narrow spectra of light reflected from most structural colors do not change shape as the illuminating spectra change shape. The reflected radiance may change in intensity as the illumination changes, but hue will remain fairly constant. This relative stability of structural colors under varying illumination may explain why they are often found in optically dynamic habitats such as forests.
Offsetting this ability to create a highly saturated signal that is constant under varying illumination is the fact that both the intensity and hue of many structural colors depend on the relative orientations of the illuminant, the viewer, and the reflective structures. This is because most structural colors are mediated by the stacks of alternating refractive indices described above, and thus, the intensity and hue of the reflected light are orientation dependent. In certain cases this orientation dependence can be advantageous because motion of the reflective structure can create a flashing display, as has been hypothesized for various species of butterflies (see Pirih et al., 2011). In many other cases, though, it may be a distinct limitation. To address this, some species have evolved two- and three-dimensional structures that strongly reflect light of the same hue from all directions. In cases where these structures are highly periodic, they are known as photonic crystals (e.g., the colorful spines of the polychaete Aphrodite; reviewed by Vukusic and Sambles, 2003). However, even less periodic structures can reflect the same color over a wide range of viewing angles, although the color tends to be less pure (e.g., the wings of the Morpho butterfly; reviewed by Kinoshita and Yoshioka, 2005).
Camouflage via Absorbed Light—Color Patterns
By far the best-studied camouflage mechanism is the use of pigments—molecules that selectively absorb light of certain wavelengths. Camouflage involving pigments ranges from the simple red or black pigmentation of deep-sea organisms that has evolved to foil bioluminescent searchlights, to the stark black-and-white countershading of penguins and killer whales, to the highly complex and, in some cases, dynamic patterns of certain vertebrates and cephalopods. Even an optical environment as simple as the pelagic ocean contains a remarkable variety of pigmentation strategies (figure 13.22). In more complex habitats, such as forests and coral reefs, the variety of colors and patterns is dazzling. Entire books can and have been written about camouflage via pigmentation (e.g., Thayer and Thayer, 1909; Cott, 1940; Fox and Vevers, 1960; Ruxton et al., 2004; Stevens and Merilaita, 2011) in addition to an extensive record in scientific journals. We cannot possibly hope to review this entire subject within this chapter, and so, as before, we confine ourselves to a discussion of the basic principles and the relative advantages and disadvantages of pigmented camouflage versus other forms.

Figure 13.22 The coloration of oceanic species as a function of depth. (From Johnsen, 2007)
First and foremost, it is important to remember that pigments can only absorb light. It is only our human preference for things we can see over those that we cannot that lead us to name pigments by the colors they do not absorb rather than by those they do. This has three major implications. First, it can cause us to misinterpret certain situations. For example, many deep-sea benthic species display a pale-orange coloration that is much less saturated than the deep-red coloration found in the animals swimming just above them (figure 13.23). The function of the pigment responsible (usually a carotenoid), however, is not to give the animal an orange appearance but to absorb enough blue light so that it matches the reflectance of the substrate for the wavelengths of bioluminescence and the remaining solar radiation. For the animals swimming above in the water column, the reflectance of the surrounding water is very low for all wavelengths, so a higher concentration of blue-absorbing “red” pigment is required. Thus, deep-sea pelagic animals are often deep red, and deep-sea benthic animals are often pale orange because they are regulating light absorption at blue wavelengths to match the background.

Figure 13.23 (Left) The red-eye gaper (Chaunax stigmaeus) photographed at depth under broad-spectrum lighting. The blue channel of the same image (Right) shows how the animal would appear under the natural blue illumination found at that depth. Note that the reflectance of the fish at blue wavelengths (lowered by the presence of the red pigment) matches that of the surrounding substrate. Note also how apparent the unpigmented ventral fins are under blue light.
The second major implication of the fact that pigments can only absorb light is that any pigment must have a reflective layer underneath it or its color will not be visible. The only exceptions to this are transparent animals viewed under transmitted light, for example, certain colored jellyfish viewed against the downwelling light in shallow waters. All remaining animals require some sort of underlying reflective layer. In certain cases the reflection is due to a dedicated structure that efficiently reflects much of the incident light. Examples of these are the leucophores of cephalopods (Hanlon and Messenger, 1996) and the highly scattering nanospheres on the white wings of certain Pierid butterflies (Stavenga et al., 2004). However, for most animals, the light is reflected by scattering within the subsurface tissues (e.g., dermal connective tissue in vertebrates). Thus, the default color of many organisms, particularly large and complex ones that scatter a great deal of light, is white. This in turn has two implications for the visual ecologist. First, in countershaded animals, it is not the ventral surface that is made white but the dorsal surface that is made dark. This difference can be seen clearly in surface and cave populations of the Mexican blind cavefish (figure 13.24). Second, and more importantly, the increased reflectance one sees in the UV portion of the spectrum for many animals may not have an adaptive function in most if not nearly all cases. Instead, it is often a simple consequence of the lack of UV absorption by the overlying pigment, allowing the structures underneath to scatter and reflect the light. Many carotenoid pigments, for example, do not absorb UV radiation, and thus many yellow, orange, and red animals have a UV peak in their reflectance spectrum that may have no biological relevance.
A final major implication for those visual ecologists interested in the chemistry and genetics of coloration is that a reflectance spectrum with two peaks may be due to only one pigment that absorbs the light of the intermediate wavelengths, and, conversely, that a reflectance spectrum with one peak may be due to two pigments that absorb light of wavelengths above and below the peak. This may appear obvious in retrospect, but it is surprisingly easy to confuse the peaks of a reflectance spectrum with the action of the pigments when it is in fact the valleys that are truly relevant.
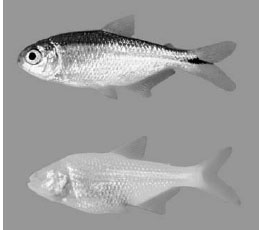
Figure 13.24 The Mexican blind cavefish Astyanax mexicanus. (Top) Individual from surface population. (Bottom) Individual from cave population. Note that although the dorsal coloration differs between the two, the ventral coloration remains the same. (From Johnsen, 2003)
As mentioned above, pigmentation in camouflaged animals ranges from completely unpatterned to exceedingly complex patterns. Although there are, of course, exceptions, in general the complexity of body coloration in camouflaged animals mirrors the complexity of the background. When the background is featureless (snow, ocean, sky), the camouflaged animal is usually one color (or possibly countershaded) in an attempt to match the spectral radiance of the background. When the background is complex (forest, coral reef), most animals use complex patterns that obscure distinctive body features (e.g., eyes), the outline of the body, and in some cases provide false depth and shading cues that disrupt the general three-dimensional impression of the body (e.g., the cuttlefish in figure 13.2 and the stone fish in figure 13.3).
Interestingly, although structural colors could in theory provide the same level of pattern complexity, nearly all complex patterns in organisms are due to pigments. In addition, although counterillumination is a dynamic form of camouflage, and certain structural colors in fish and cephalopods are also changeable, most cases of dynamic camouflage are due to pigments. In fish and a few other vertebrates, this ability to change color is usually due to the migration of pigments. In cephalopods, it is due to the muscle-mediated expansion and contraction of small sacs containing pigments (reviewed by Hanlon and Messenger, 1996). This last example is the only case in which pigmented camouflage can change as rapidly as counterillumination, and in general, pigmented camouflaged animals are more vulnerable to rapidly changing optical conditions than are counterilluminating, transparent, and mirrored animals.
Signaling via Absorbed Light—More Color Patterns
As impressive as the diversity of pigment-based camouflage can be, the diversity of pigment-based color signals is truly astonishing. In addition to incredible variation among species, especially among tropical birds, coral reef fish, beetles, moths, and butterflies, there is also impressive diversity within different populations of certain species, even those from the same geographic region (e.g., figure 13.25). In addition, many species, especially among coral reef fish, have completely different color patterns as juveniles and adults, and of course many species have sexually dimorphic color patterns. The diversity of these colors, the pigments underlying them, and their various aposematic and reproductive functions have been extensively reviewed (Cott, 1940; Fox and Vevers, 1960; Stevens, 2013). Thus, as before, we confine ourselves to a few central optical and visual points.

Figure 13.25 Twelve color morphs of the strawberry poison dart frog (Oophaga pumilio), all from the Bocas del Toro region of Panama. (From Siddiqi et al., 2004)
In general, a color signal is expected to be conspicuous, at least to the intended viewer. However, conspicuousness is difficult to define. It is (relatively) easy to estimate whether a color signal is detectable or not, using either behavioral assays or visual models (e.g., the noise-limited color model of Vorobyev and Osorio, 1998). The more complicated issue is whether one can say that one color signal is more conspicuous than another when both are well above the detection threshold for a given visual system and situation. For example, consider a 440-nm blue laser pointer and 520-nm green laser pointer, both shining on a sheet of white paper and being viewed by color-normal humans. According to perceptually uniform color models such as CIE 1976, the chromatic distance between the blue laser and the paper is far larger than the chromatic distance between the green laser and the paper. However, people in general would likely be divided as to whether the green or the blue laser pointer was more conspicuous, especially if their perceived brightnesses were set to be equal.
In fact, it is difficult to clearly define what is meant by a more conspicuous signal. One possibility is that a more conspicuous signal may be detected more rapidly or selectively attended to in a field of less conspicuous signals. To the best of our knowledge, the first has not been tested and the second has not been correlated with color distances. Of course it is well documented that certain species prefer signals to have a greater chromatic distance from the background, but this is generally thought to occur because this increased distance is possibly an honest indicator of the quality of the individual (Zahavi et al., 1999). This makes it difficult to determine whether preferential responses to a more conspicuous signal are due to increased detectability or simply to underlying motivation.
All this is relevant because it is tempting to use chromatic distance (especially the just-noticeable-differences calculated from the noise-limited color model of Vorobyev and Osorio, 1998) to compare the conspicuousness of different signals and then use this information to make ecological and evolutionary inferences. Until more is known about how animals compare color signals that are well above the detection threshold, this approach is suspect.
There are, however, two cases in which larger chromatic distances between the signal and the background do imply increased detectability. The first is when the background is noisy (e.g., a coral head encrusted with algae and many differently colored invertebrates). In this case, the detection threshold is higher than would be the case for a simple background, and a signal with a greater chromatic distance from the background has a greater chance of being above this threshold. The second is when signals are viewed through an attenuating medium such as water or fog (see chapter 9). Because scattering and absorption by the medium makes any viewed object look more and more like the background with increasing viewing distance, everything eventually becomes indistinguishable (figure 13.26). However, colors that are separated by a larger chromatic distance will remain distinct at a greater distance. Aside from these two cases, however, one must be careful about comparing the conspicuousness of highly visible color signals.
An important implication of this is that a wide diversity of colors can be chosen for color signals as long as they are sufficiently distinguishable from the background. Nevertheless, we do see that some colors are chosen more than others for signaling in certain habitats. In the case of dense forests, it has been noted that red and blue coloration is more common than in other terrestrial habitats. For aquatic species, it is known that fish on coral reefs are often violet and yellow, and fish in greenish coastal and fresh waters are often orange and red (Lythgoe, 1979; Marshall and Johnsen, 2011; figure 13.27). Although there are, of course, many nonoptical reasons why certain colors are chosen, the three patterns just mentioned may at least partially be explained by optical and visual arguments. In the case of forests it is important to remember that the light environment within them is highly variable due to spectral filtering of light by leaves and the relationship of gaps to the location of the sun, clouds, and blue sky (see chapter 2). Thus, as we discussed in the section on structural colors, signals with narrow reflectance spectra provide a more constant signal. Natural pigments usually have broad reflectance spectra, but they can appear narrower to a viewer if they primarily reflect light at the upper and lower edges of the viewer’s spectral sensitivity range. Blue/violet and red signals do this and also contrast with green foliage, which may partially explain why these colors are chosen by certain forest insects and birds.
The situation is different in aquatic habitats. Here the illumination is relatively constant, but with a far narrower spectral range (see chapter 2). Although initial intuition might suggest that the best color for a signal viewed under relatively monochromatic illumination is one that reflects strongly in that limited spectral region (i.e., choose a blue signal for blue illumination), this only creates a bright signal, not one that contrasts chromatically with the background. Instead, to generate a distinguishable color signal under illumination with a narrow spectral range, one must use a pigment whose reflectance changes sharply over that region. In the case of blue coral reef waters, red and orange pigments look black and blue, and many green pigments look light blue. Only violet, yellow, and some green pigments, which have reflectance spectra that change rapidly in the spectral region of the blue illumination, appear to have a color separate from blue (figure 13.28). Analogously, in green coastal and fresh waters, it is primarily the orange and red pigments that have reflectance spectra that change over the green portion of the spectrum and thus appear to have a color distinct from background. Fluorescent pigments, however, are not subject to this restriction because their reflected light does not have to be a subset of the spectrum of the illumination. Therefore, one can have red coloration in blue waters at depth, as is seen in certain fluorescent corals (e.g., Read et al., 1968).
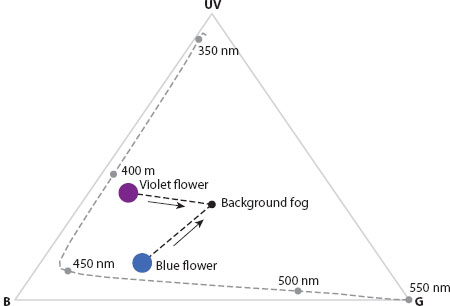
Figure 13.26 Maxwell triangle for a honeybee (Apis mellifera) viewing a violet flower and a blue flower through fog. At close viewing distances, the two flowers are readily distinguishable, but as viewing distance increases (indicated by arrows), the apparent colors of both flowers approach that of the fog background. At a certain distance, the chromatic contrast between the two flowers will be too low to detect, and they will be indistinguishable. In general, the greater the original chromatic contrast between the two flowers, the greater the distance at which they can be distinguished from each other. This is one of the cases where increased chromatic distance is definitely known to affect detectability.

Figure 13.27 (Left) The dottyback (Pseudochromis paccagnellae) photographed at depth on a coral reef. (Right) Head of male three-spined stickleback (Gasterosteus aculeatus) photographed in the greenish water that it usually inhabits. (Right photo courtesy of Piet Spaans)

Figure 13.28 (Top) The GretagMacbeth ColorChecker™ as it appears in daylight. (Bottom left) The same colorchecker as it would appear if it were photographed at 40-m depth in clear ocean water. Note that the only squares that appear to have a color distinct from the blue water are those that were originally violet or yellow. (Bottom right) The same colorchecker as it would appear if it were photographed at 20-m depth in green coastal water. Note that the only squares that appear to have a color distinct from the green water are those that were originally red or orange. In both cases the squares that still have a color separate from the background are those whose reflectance spectrum changes over the wavelength range of the illumination.
A final issue to discuss in this all-too-brief introduction to camouflage and signaling is the potential for private channels. As we have seen, animal visual systems vary in their abilities, including differences in absolute sensitivity, color perception, spectral range, spatial and temporal resolution, and their ability to detect various aspects of polarization. Some of these parameters tend to be roughly equal among animals in a given environment—for example, high absolute sensitivity in the deep sea—but most vary substantially within a group of interacting species. Thus, it is possible for an animal to send a signal that is readily apparent to a certain set of animals (usually, but not always, conspecifics) but inconspicuous to others. Indeed, any analysis of a colored visual scene will show that certain organismal features are more apparent in certain wavebands and using certain opponencies than when using others (e.g., figure 13.29).
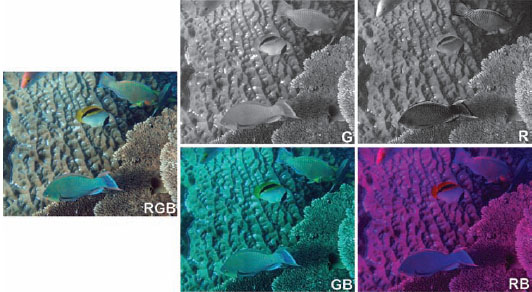
Figure 13.29 (Left) Full-color image of two surf parrotfish (Scarus rivulatus: top right), a lined butterflyfish (Chaetodon lienolatus: middle), a minifin parrotfish (Scarus altipinnis: bottom), and the head of a Hussar (Lutjanus adetii: upper left). (Top middle) The same image viewed by an animal with only a medium-wavelength (i.e., green) visual pigment. Note that the patterns on the three parrotfish are no longer visible. (Top right) The image now viewed by an animal with only a long-wavelength (i.e., red) visual pigment, which increases the achromatic contrast of the details on the parrotfish. (Bottom two panels) A dichromatic viewer can distinguish more than a monochromat. The ability to perceive hue differences in this case is better for a dichromat with a short- and a long-wavelength pigment than for a dichromat with a short- and a medium-wavelength pigment. However, neither dichromat distinguishes all the hues as well as a trichromat. In particular, note that the parrotfish in the upper right has many hue shifts that have no achromatic or dichromatic contrast. Also note that the butterflyfish in the center is conspicuous to all the modeled visual systems, suggesting that its signal is not private.
Hypothesized cases of what have been termed private channels include, but are not limited to, the red patches of the female crab-spider Misumena vatia (Hinton, 1976); the red bioluminescence produced by three genera of deep-sea dragonfish (Partridge and Douglas, 1995); the ultraviolet patterning of certain Heliconius butterflies (By-bee et al., 2012), fish (Cummings et al., 2003; Siebeck et al., 2006, 2010), and passerine birds (reviewed in Stevens and Cuthill, 2007); the polarized reflectance of scarab beetles (Brady and Cummings, 2010), butterflies (Sweeney et al., 2003), cephalopods (Mäthger et al., 2009), and stomatopods (Chiou et al., 2008); and the contrasting stripes of the coral reef fish Pygoplites diacanthus and Thalassoma lunare (Marshall, 2000a). In general, these studies are based on optical measurements and subsequent calculations that demonstrate that a signal is more detectable for one species than for another, although a few have demonstrated this difference via behavioral assays (e.g., Cummings et al., 2003). However, as is noted by Brandley et al. (2013), to date no study of private channels shows that the signaler benefits from the signal being cryptic to potential eavesdroppers or that the potential eavesdropper would benefit from detecting the signal. In other words, it remains to be shown that the privacy of certain signals is actually adaptive. Demonstrating this conclusively will likely be challenging because it ideally involves measures of fitness and at a minimum involves carefully controlled behavioral tests, but is a necessary piece of this fascinating and rapidly developing subfield of visual ecology.
In Conclusion
And so we come to the end of this brief introduction to visual ecology. As we hope we have made clear, it is a highly multidisciplinary field that demands many different approaches. In fact, it may seem that to succeed, one requires the mind of a physicist, the hands of a surgeon, and the intuition of a first-class dog trainer! Seemingly simple behavioral experiments are fraught with issues of interpretation, and even the hairiest mathematical models of vision and optics just barely scratch the surface of the true underlying complexity. Few other subjects ask us to understand so many different concepts and to be familiar with so many different organisms. Perhaps this is why visual ecologists are so happy to collaborate and why the field operates as a relatively collegial superorganism. We need each other.
However, we feel that visual ecology returns to us far more than it asks. Perhaps more than most, the subject truly lives up to the promise of science as art. It combines rigor and intellectual surprise with both beauty and humor. Who is not awed by the bioluminescence of the deep sea or not tickled at the thought of African beetles rolling dung under the polarized moonlight? It is perhaps not surprising that many visual ecologists are also photographers and painters and that nearly all simply must tell you just one more funny story about an odd animal and its visual pursuits.
We, the authors, could not have asked for a more rewarding way to spend our days and for better colleagues to spend them with. We hope the same for you and that this book serves you well.




























