CHAPTER 1
Matter and Energy
Matter
Chemistry is the study of matter, that is, anything that takes up space and has mass. Mass (a measure of the number of particles in an object) should not be confused with weight, which is the influence of gravity on mass. Because gravitational forces can differ, an object that has the same mass on Earth as it does on the moon will have a lower weight on the moon because there is less of a gravitational pull on the moon. Because matter has mass and takes up a certain volume (space), the density for any variety of matter can be calculated using the equation: D = m/V. Units typically used to calculate density are grams (for mass) and milliliters (mL for volume). Sometimes the unit cubic centimeters (cm3) is used instead of milliliters. These volumes are equivalent. You will see an exception to the general rule when calculating the density for gases later in this book.
Problem:
What is the density of a solid cube that has a length of 2.0 cm on each side and weighs 6.0 grams?
Solution: Because the length of each side of this cube is 2.0 cm, the volume will be the length × width × height. This is (2.0 cm)(2.0 cm)(2.0 cm), or 8 cm3. The mass is 6.0 grams. Substitute into the equation D = m/V and you get D = 6.0 grams/8.0 cm3. The density of this solid is 0.75 grams/cm3.
THINK ABOUT THIS 
Substances
A substance can be defined as any variety of matter with identical properties and composition. Substances are classified as either elements or compounds. Elements cannot be broken down chemically, whereas compounds can be broken down chemically. An element is made up of a particular atom, the basic building block of matter. Compounds are formed from the bonding of two or more elements. Consider the reaction: CH4 + 2O2 → 2H2O + CO2. The reaction shows the elements carbon, hydrogen, and oxygen in different compounds. The chemical equation also shows how the compounds change over the course of the reaction. Although the compounds on the left of the arrow are not the same as those to the right of the arrow, the elements in the reaction are still carbon, hydrogen, and oxygen.
Mixtures are the results of the combination of elements and/or compounds. In a mixture:
• The substances are not chemically combined (each substance retains its properties).
• The ratios of substances can vary.
• The substances can be separated into the original elements and/or compounds.
Mixtures can be classified as homogeneous (the same throughout) or heterogeneous (not the same throughout). An example of a homogeneous mixture is homogenized milk. You do not have to shake milk before using it because all samples of homogenized milk will be the same. Solutions are homogeneous mixtures of a solute dissolved in a solvent and can be represented by a substance followed by the symbol (aq) to show that the substance has formed a homogeneous mixture with water (an aqueous solution). An example of a heterogeneous mixture is a mixture of sand in water. The sand is sure to settle to the bottom of the container no matter how much you stir the mixture.
THINK ABOUT THIS 
Problem:
Classify the following as elements, compounds, or mixtures: salt water, water, argon, methane, and iron.
Solution: Salt water is a homogeneous mixture of water and NaCl. Water is a compound made up of hydrogen and oxygen. Argon is an element. Methane is a compound made up of hydrogen and carbon. Iron is an element.
Chemical and Physical Properties
All substances have physical and chemical properties. Physical properties are the observable and measurable properties of substances. These include phase (solid, liquid, or gas), color, odor, density, boiling or melting point. Chemical properties are the properties observed when a substance reacts with other substances. Chemical changes result in substances with different physical properties. For example, when iron (a gray, solid metal) reacts with oxygen gas (an odorless, colorless gas) the result is iron oxide or rust (a solid that is orange-red in color). You could also note the changes in the density, melting points, and boiling points of the iron, oxygen, and iron oxide.
Problem:
Classify the following as physical or chemical changes: burning a piece of paper, smashing a piece of chalk, melting an ice cube, and the rusting of an iron nail.
Solution: Burning paper changes the paper’s chemical composition. Smashing chalk is a physical change; the chalk is still the same chalk, only in smaller pieces. Melting an ice cube does not change the composition of the water; it’s a physical change. Rusting an iron nail is a chemical change; the iron is now an iron oxide.
Energy
Chemistry is defined as the study of matter, but energy plays an important role in chemistry. Energy is defined as the ability to do work. Energy is conserved, that is, it is not created or destroyed. This means that the amount of energy lost by one system is always equal to the amount of energy gained by another. Energy can also be converted from one form to another. For example, a toaster or a hairdryer converts electrical energy into heat energy.
The units used for measuring amounts of energy are the joule or the calorie. Most people are more familiar with the term calorie. This should not be a problem because the simple relationship between the two units is that one calorie is equal to 4.18 joules. This ratio is helpful in setting up problems that ask for a conversion of one unit to another.
Types of Energy
Energy is found in many forms. As mentioned above, energy can exist as heat or electricity. Other forms of energy are sound, light, chemical energy, and nuclear energy. Probably the two most important forms of energy in chemistry are potential energy and kinetic energy. Potential energy is stored energy. A good example is someone holding up a hammer, ready to strike a nail. The hammer has the potential of falling onto the head of the nail. As the hammer is swung downward and moves through the air, the potential energy is converted to kinetic energy, or moving energy. Because all of the energy is conserved, the potential energy stored in the hammer is turned into kinetic energy. As the hammer is lifted to strike the nail again, the movement of the hammer upward becomes the potential energy stored for the next strike on the head of the nail. One thing to remember in chemistry is that nature prefers a lower energy state. This rule will be noted again and again in this chemistry review.
Endothermic and Exothermic Reactions
Energy may also be absorbed or released in a reaction. When more energy is released than absorbed, the reaction is said to be exothermic. When more energy is absorbed than released, the reaction is said to be endothermic. A potential energy diagram can be used to graph these changes as in Figure 1.1.
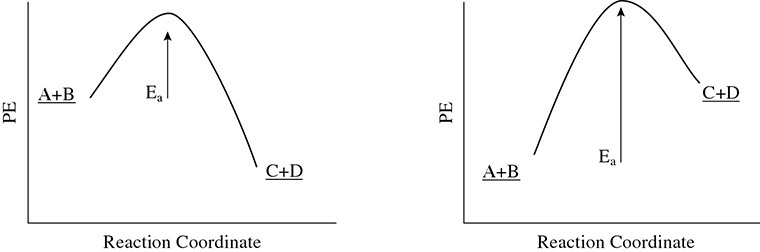
Figure 1.1 Two Potential Energy Diagrams
Notice that energy is always absorbed and released in a reaction. The relative amounts are what cause the reaction to be endothermic or exothermic. Also, it takes energy to start the reaction. This is called the activation energy (Ea).
Finally, take note of the difference in the energy of the reactants and products. The change in the energy of the reactants or products is called the heat of reaction. This is designated by the symbol ΔH. This symbol stands for the change in heat energy, or enthalpy. The simple way to remember how to calculate the change in enthalpy is to use this mnemonic device:

When ΔH has a positive value, the reaction is said to be endothermic (enters heat). When ΔH has a negative value, the reaction is said to be exothermic (exits heat).
Problem:
In a reaction the potential energy of the reactants is 150 joules/mole and the potential energy of the products is 400 joules/mole. What is the heat of reaction for this reaction? Does this demonstrate an endothermic or exothermic process?
Solution: Use PEP − PER! ΔH = 400 joules/mole − 150 joules/mole = +250 joules/mole. Because the sign is positive, the reaction is endothermic.
REVIEW QUESTIONS
1. Which substance can be decomposed chemically?
(A) Ammonia
(B) Iron
(C) Neon
(D) Hydrogen
(E) Fluorine
2. Which units could be used to express the amount of energy absorbed or released during a chemical reaction?
(A) Degree and gram
(B) Torr and mmHg
(C) Gram and liter
(D) Calorie and joule
(E) Meter and cm3
3. Which sample represents a homogeneous mixture?
(A) CH3OH(l)
(B) CH3OH(aq)
(C) CH3OH(g)
(D) CH3OH(s)
(E) None of the above
4. A book is lifted off of the floor and placed on a table that is one meter above the floor. The book has
(A) gained sound energy
(B) lost chemical energy
(C) gained potential energy
(D) gained kinetic energy
(E) lost nuclear energy
5. Which statement is incorrect regarding energy?
(A) Energy can be given off in a reaction.
(B) Energy can be gained in a reaction.
(C) Energy cannot be created or destroyed.
(D) Energy can take various forms.
(E) Energy has mass and takes up space.
6. What is the mass of an object that has a density of 13 g/mL and a volume of 10 mL?
(A) 1.3 g/mL
(B) 0.77 g/mL
(C) 1.3 g/L
(D) 130 g
(E) 130 g/L
7. Which sentence below is incorrect?
(A) Salads are heterogeneous mixtures.
(B) NaCl(aq) is a homogeneous mixture.
(C) Milk is a homogeneous mixture.
(D) Sand and water make a heterogeneous mixture.
(E) Pure iron is a heterogeneous mixture.
8. Which type of change is different from the other four?
(A) Baking a potato
(B) Rusting of an iron nail
(C) Burning a piece of paper
(D) Melting an ice cube
(E) Ignition of propane
9. Which of the following is not a physical property?
(A) Color
(B) Phase
(C) Odor
(D) Boiling point
(E) Reactivity with oxygen
10. The difference between the potential energy of the products and the potential energy of the reactants in a reaction is called
(A) The Temperature of Reaction
(B) The Heat of Reaction
(C) The Change of Reaction
(D) The Exothermic Reaction
(E) The Endothermic Reaction
11. The study of matter is called
(A) Chemistry
(B) Biology
(C) Geology
(D) Physics
(E) Psychology
12. Refer to the following choices:
I. solid to liquid
II. liquid to gas
III. solid to gas
Which phase change above is endothermic?
(A) I only
(B) II only
(C) III only
(D) II and III only
(E) I, II, and III
13. The energy needed to start a reaction is called
(A) potential energy of the reactants
(B) potential energy of the products
(C) activation energy
(D) heat of reaction
(E) sound energy

ANSWERS AND EXPLANATIONS
1. (A) Compounds can be broken down chemically, while elements cannot. Ammonia is the only compound while the other choices are all elements.
2. (D) Heat is measured in calories or joules. Another unit that you might encounter is kilojoules, where 1 kilojoule is 1,000 joules.
3. (B) All solutions must be homogeneous. The notation (aq) is used to designate this.
4. (C) The lifting of something to a higher level or height provides it with more potential energy.
5. (E) Matter, not energy, is the term used for something that has mass and takes up space.
6. (D) Be careful of the units! Density is mass/volume. So, mass = volume × density. 10 mL × 13 g/mL = 130 grams.
7. (E) All elements and compounds are pure substances. Iron, an element, falls into this category.
8. (D) The melting of an ice cube is a physical change. The other choices are all chemical changes.
9. (E) The reaction of any elements results in a chemical change.
10. (B) The difference in potential energies of the reactants and products of a reaction is called the Heat of Reaction, ΔH.
11. (A) The study of matter is called Chemistry.
12. (E) It takes heat energy to be added to a substance to melt, evaporate, or sublime it. All of the phases changes listed are described by melting, evaporating, or sublimation of it, respectively.
13. (C) The activation energy is the energy needed to reach the activated complex, the point where reactants become products.
14. (T, F) An exothermic reaction does release more energy that it absorbs. However, that means that the potential energy of the products will be lower than that of the reactants.
15. (F, T) As the book falls, the potential energy is transformed into kinetic energy. The second statement is true and summarizes the concept of conservation of energy.