CHAPTER 4
The Periodic Table and Periodic Trends
History of the Periodic Table
The periodic table has been developed and perfected over many years. Although there are many scientists who have contributed to the periodic table, the two scientists who are given the most credit are Dmitry Mendeleyev and Henry Moseley. Mendeleyev, even though his periodic table had elements missing from it, is given the most credit for the periodic table and periodic trends. Later on, Moseley used a technique called x-ray crystallography and discovered the idea of the atomic number. This discovery is the basis for the arrangement of the modern periodic table. There were proposed periodic tables based upon atomic mass, but these arrangements did not suffice because of isotopes that can exist for an element.
Arrangement of the Periodic Table
The periodic table contains a number of periods and groups. The periods are the horizontal rows. They are numbered 1 through 7. The groups (or families) are the vertical columns. They are numbered 1 through 18. As we move through the families or periods, we can discover many trends that occur across the periodic table. This is known as the Periodic Law. You will be provided with a periodic table when you take the SAT II: Chemistry test. NOTE: A complete Periodic Table is provided in Appendix 3 at the back of this book.
Metals, Nonmetals, and Semimetals
Two categories of elements on the periodic table are the metals and the nonmetals. Their properties are summarized in the chart below:
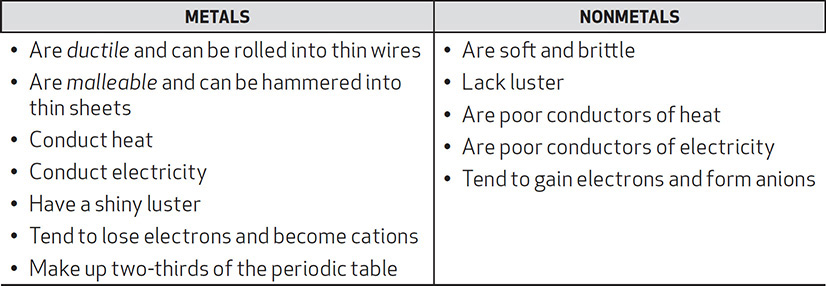
The semimetals, or metalloids, are known to exhibit some of the properties of metals and some of those of nonmetals. The semimetals are B, Si, Ge, As, Sb, Te, and At. They are highlighted in bold in the partial periodic table in Figure 4.1. The elements located to the left of the semimetals are the metals; those to the right of the semimetals are the nonmetals. Identifying an element as a metal, nonmetal, or semimetal is important in identifying periodic trends and in identifying the types of bonds that atoms will form with each other.
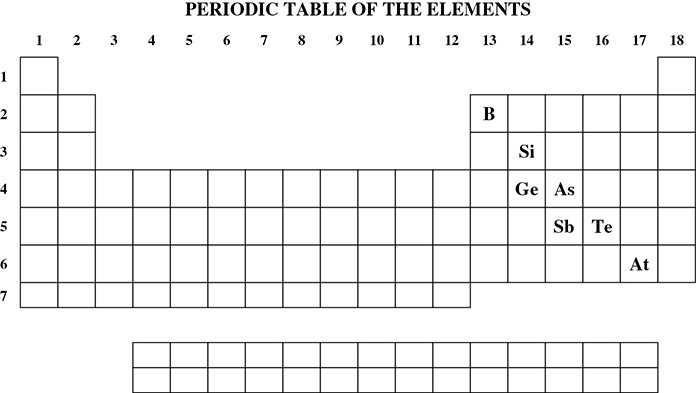
Figure 4.1 The Semimetals
Problem:
Identify the following elements as metals, nonmetals, or semimetals: potassium, calcium, bromine, hydrogen, and neon.
Solution: K and Ca are located on the left side of the periodic table and are metals. Br and Ne are on the right side of the semimetals and are nonmetals. Hydrogen, although on the left side of the periodic table, is a nonmetal. If you’re still not convinced about hydrogen, ask yourself about the properties of hydrogen gas and see where those properties fit in the comparison chart on p. 77.
The Families
Some groups or families are given special names and have certain properties that should be addressed. But first you must understand why elements are put into the same group. Think about a family you know, not a chemical family, but a human family. Children look like their parents. They learn to do things from their parents and do them in the same way. The same holds true for the elements in the families of the periodic table; they react the same way (for the most part). As you learned in the last chapter, each element has a certain number of valence electrons. As you will learn in the next chapter, it is the number of valence electrons of an atom that determines its chemical reactivity. Because the elements in a family have the same number of valence electrons, they will have a similar chemical reactivity. For example, Na and K can be compared in electron configuration and ions formed:
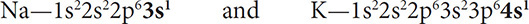
Both atoms have 1 valence electron and will lose this one electron to form ions with charges of 1+. This similar charge will mean that both elements have a similar chemical reactivity.
The important families and groups are listed below followed by their important characteristics. These characteristics will become more familiar to you as you study the chapter on bonding.
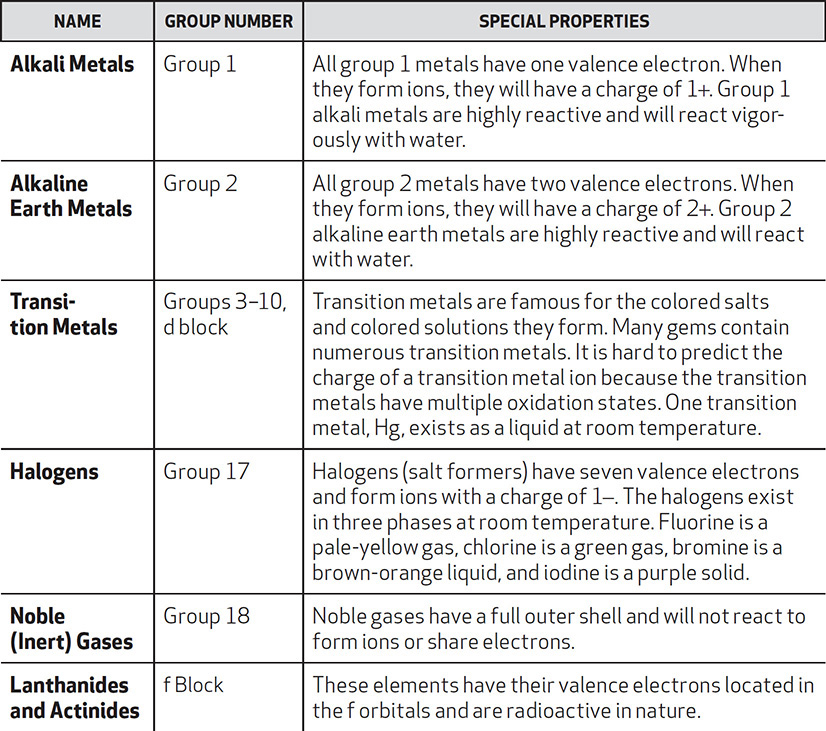
There are important periodic trends that occur across the periods and up and down the groups. It is best to remember the trends of just a few elements. This will simplify the trends greatly and make the periodic trend questions the easiest to answer on the test.
Electronegativity
Electronegativity is a measure of an atom’s ability to attract electrons. The electronegativities of the elements are given a value of between 0.0 and 4.0. The greatest electronegativity value goes to fluorine, 4.0. So where is the element with the lowest electronegativity? Look furthest from fluorine and across to the bottom left of the periodic table. Francium, Fr, has an electronegativity of 0.7. This should make sense because nonmetals tend to gain electrons and have a higher electronegativity value, whereas metals tend to lose electrons and have a lower electronegativity value. Because they don’t react, the noble gases do not have a value for electronegativity.
Problem:
Which is expected to have a lower electronegativity, Na or S?
Solution: Na has a lower electronegativity because it is further from fluorine on the periodic table.
Ionization Energy
Ionization energy, as its name suggests, is the energy needed to remove an electron from an atom and form an ion. This concept should be easy to recognize in the periodic table once you have grasped the idea of electronegativity. It takes a lot of energy to remove electrons from the very stable octets of the noble gases. For example, for helium the first ionization energy is 2372 kJ/mol, whereas neon has a first ionization energy of 2081 kJ/mol. Fluorine, with the highest electronegativity and the ability to “hold onto” electrons, has a first ionization energy of 1681 kJ/mol.
You might have guessed by now that the opposite holds true for the metals as you move further away from fluorine and the noble gases. The proof lies in the first ionization energies for iron (762 kJ/mol) and potassium (419 kJ/mol). These values are just a fraction of the first ionization energies for certain nonmetals.
Problem:
Which is expected to have a greater ionization energy, Ca or Br?
Solution: Br is located closer to F and will have a higher ionization energy.
Atomic Radius
The atomic radius of an atom can be defined as the distance from its nucleus to the outermost electron of that atom. As you go down a group, the radius of the atoms will increase as the atoms fill more principal energy levels with electrons. The proof for this trend can be seen in lithium, which has an atomic radius of 155 picometers (10−12 meters), and cesium, which has an atomic radius of 267 picometers. You might expect the same to happen as you examine the elements from left to right across a period. If lithium has fewer electrons than fluorine, then lithium should have a smaller radius than fluorine, right? Wrong! Fluorine has nine electrons and lithium has just three, yet fluorine has an atomic radius of 57 picometers and lithium a radius 155 picometers. Why the difference? Fluorine has more protons and positive charge in its nucleus than does lithium. It turns out that when looking at atomic radii across a period, it is the nuclear charge (and not the number of electrons) that determines the radius of the atom.
Ionic Radius
As covered in the previous chapter, atoms can gain or lose electrons. The resulting ions can be expected to be of a different radius than that of the original atom. When a nonmetal gains an electron, the ionic radius of the anion will be bigger than that of the nonmetal atom. This is shown in Figure 4.2.
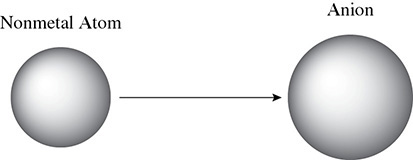
Figure 4.2 Relative Sizes of Anions
The opposite holds true for metal atoms and cations. Metals lose electrons and will experience a decrease in their radius as shown in Figure 4.3.
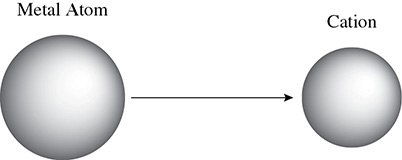
Figure 4.3 Relative Sizes of Cations
The s, p, d, and f Blocks
The location of an element on the periodic table can tell a lot about the number of valence electrons the element has and in which subshell these valence electrons can be located. These blocks are outlined in Figure 4.4.
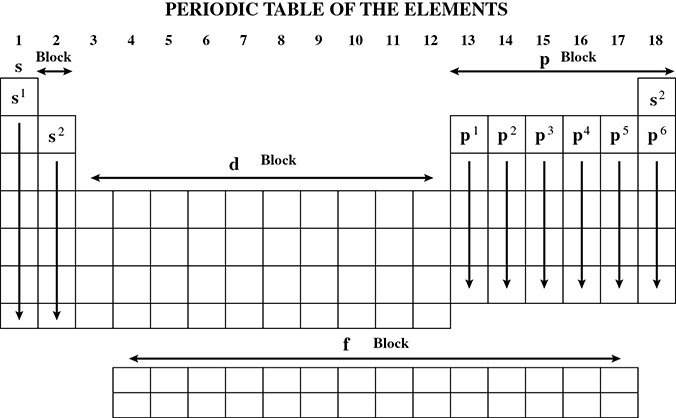
Figure 4.4 s, p, d, and f Blocks of the Periodic Table
The alkali and alkaline earth metals have their valence electrons in the s subshells. Groups 13 through 18 have their valence electrons located in the p subshells. The transition elements have their valence electrons in the d subshells, and finally, the lanthanides and actinides have their valence electrons in the f sublevel.
Finally, as we move to the right of the periodic table, we find that the elements can react to form acidic compounds. Some examples are HF, HCl, HBr, HI, and H3O+. As we move to the left of the periodic table, the elements can react to form basic compounds such as NaOH, KOH, and Ca(OH)2.
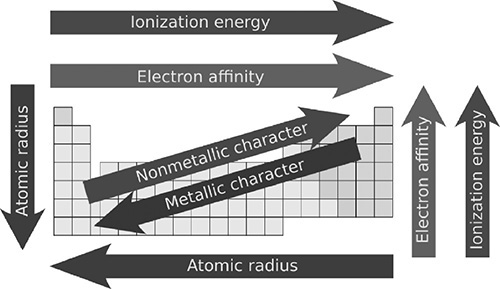
Figure 4.5 A summary of some periodic trends. The arrows indicate an increase in the trend.
REVIEW QUESTIONS
1. The modern periodic table is arranged based upon atomic
(A) isotopes
(B) number
(C) density
(D) radius
(E) mass
2. In period 3 of the periodic table the atom with the largest atomic radius is located in group
(A) 1
(B) 3
(C) 13
(D) 17
(E) 18
3. The elements that display the greatest nonmetallic character are located toward which corner of the periodic table?
(A) Upper left
(B) Dead center
(C) Lower right
(D) Lower left
(E) Upper right
4. Which two elements will display the most similar chemical properties?
(A) Aluminum and calcium
(B) Nickel and phosphorus
(C) Chlorine and sulfur
(D) Carbon and sulfur
(E) Lithium and potassium
5. Assuming the ground state, all of the elements located in group 13 of the periodic table will have the same number of
(A) nuclear particles
(B) occupied principal energy levels
(C) electrons
(D) valence electrons
(E) neutrons
6. Which group contains elements in the solid, liquid, and gas phases at 298 K and 1 atm?
(A) 1
(B) 2
(C) 16
(D) 17
(E) 18
7. An element that has a high first ionization energy and is chemically inactive would most likely be
(A) a noble gas
(B) a transition element
(C) an alkali metal
(D) a halogen
(E) an alkaline earth metal
8. Which salt solution is most likely to be colored?
(A) KClO3 (aq)
(B) KNO3 (aq)
(C) K2CrO4 (aq)
(D) K2SO4 (aq)
(E) KCl (aq)
9. As the elements of period 2 are considered from left to right, there is generally a decrease in
(A) ionization energy
(B) electronegativity
(C) metallic character
(D) nonmetallic character
(E) none of the above
10. Which element is a liquid at room temperature?
(A) K
(B) Hg
(C) I2
(D) Mg
(E) Kr
11. At STP, which element is most expected to exist as a monatomic gas?
(A) calcium
(B) hydrogen
(C) nitrogen
(D) neon
(E) bromine
12. Nonmetals are poor conductors of heat and they also tend to
(A) be brittle
(B) conduct an electrical current
(C) have a shiny luster
(D) be malleable
(E) lose electrons
13. Which statement does not explain why elements in a group are placed together?
(A) They tend to have the same number of valence electrons.
(B) They tend to have a similar oxidation number.
(C) They tend to have the same electronegativities.
(D) They tend to have the same chemical reactivity.
(E) They tend to have the same charge when they form ions.
14. Refer to the following:
I. F, C
II. Na, Mg
III. Fe, Co
Which of the above have multiple oxidation states, colored salts, and valence electrons in the d orbitals?
(A) I only
(B) II only
(C) III only
(D) II and III only
(E) I, II, and III
15. Which metal is not correctly paired with its color when put into a flame?
(A) Lithium—Red
(B) Potassium—Lilac
(C) Sodium—Yellow
(D) Copper—Orange
(E) Magnesium—White

ANSWERS AND EXPLANATIONS
1. (B) The modern periodic table is arranged by atomic number and not by mass, as it was originally.
2. (A) A larger atomic radius within a period is found on the left side of the table. Group 1, period 3 is sodium and it will have a larger radius because of its low electronegativity and low ionization energy.
3. (E) The nonmetals are located in the upper right of the periodic table.
4. (E) Elements in the same family will have the same valence electron configuration and the same oxidation state. The alkali metals lithium and potassium are in the same family.
5. (D) The reason elements in the same family react similarly is because they have the same number of valence electrons.
6. (D) The halogens, Group 17, have gases (F and Cl), a liquid (Br), and a solid (I).
7. (A) Noble gases are stable because of their complete outermost principal energy levels. This makes them inactive and not likely to give up an electron.
8. (C) Because of their valence electrons being in the d orbitals, the transition elements are going to have multiple oxidation states and many colored salts/salt solutions.
9. (C) As one moves from left to right and approaches fluorine, there is a decrease in metallic character because the nonmetals are located on the right side of the table.
10. (B) Being used in thermometers for years, mercury is a liquid at room temperature.
11. (D) Because of their low reactivity, noble gases do not form compounds.
12. (A) Nonmetals are poor conductors of heat and electricity, and they tend to be brittle.
13. (C) Electronegativity can vary greatly in a group or family. However, the other properties in this problem tend to stay constant from one element to the next.
14. (C) The question describes the properties of the transition metals.
15. (D) Flame tests are an excellent way to try to identify a metal. Copper will have a bluish-green color when placed in a flame.
16. (T, F) Potassium atoms are larger than their ions because metals will lose electrons and have a smaller ionic radius than atomic radius.
17. (F, T) Oxygen and nitrogen are not in the same family. They are in the same period. However, the second statement holds true regarding valence electrons, ions, and chemical bonds.