CHAPTER 9
Acids and Bases
Why does lemon juice sting when it gets on an open cut? How does an antacid work? Why can HF be used to etch glass?
Naming Acids and Bases
By this point in this book you should have come across a few familiar acids and bases. Either you recognized the molecular formula of an acid or a base or you saw the name of a familiar acid or base. Acids and bases can be defined in a number of ways, as you will see shortly. It’s a good idea to see how acids and bases are named before you focus on the differences between them. You will see that the names do not follow the same rules used for other covalently bonded substances.
Here is a simple outline of how to name acids that have certain anions bonded within them.

Problems:
Name the following: HBr, H3PO4, and HClO2.
Solution: HBr is called hydrobromic acid because the ending to the compound is -ide, hydrogen bromide. H3PO4 is called phosphoric acid because of the phosphate ion present. HClO2 is called chlorous acid because of the chlorite ion present.
Operational Definitions of Acids and Bases
As mentioned previously, acids and bases can be defined in a number of ways. One way to define an acid or base is by what you “see” when an acid or base reacts with other substances. For example, your senses can help you identify an acid or base because acids taste sour and bases taste bitter. The sour taste of lemons can be attributed to the citric acid found in the lemon juice. In addition, bases have a slippery feel to them (please, do not touch or taste acids or bases).
There are also a few reactions that can help define acids and bases. Here are some examples:
• Acids and bases react to form a salt and water. This process is called neutralization. Proof lies in the fact that the water formed can be evaporated and the salt will remain behind. An example of this reaction is: HCl(aq) + NaOH(aq) → NaCl(aq) + H2O(l)
• Acids react with active metals, such as Zn or Mg, to form a salt and hydrogen gas. As the reaction proceeds, you can see the hydrogen gas being given off in the reaction. An example is: 2HCl(aq) + Mg(s) → MgCl2(aq) + H2(g)
• Acids can be formed from a reaction of nonmetal oxides and water; for example: CO2(g) + H2O(l) → H2CO3(aq)
• Bases can be formed from a reaction between metal oxides and water; for example: Na2O(s) + H2O(l) → 2NaOH(aq)
Finally, acids and bases can change the colors of certain indicators. Some examples are phenolphthalein and litmus. The chart below summarizes the colors of these two important indicators. A good mnemonic device to help you remember litmus indicators is BRA: “Blue turns Red in Acid.” Other indicators that can be used are methyl orange and bromothymol blue.

Conceptual Definition of Acids and Bases
Acids and bases can further be defined by three conceptual definitions as outlined below.
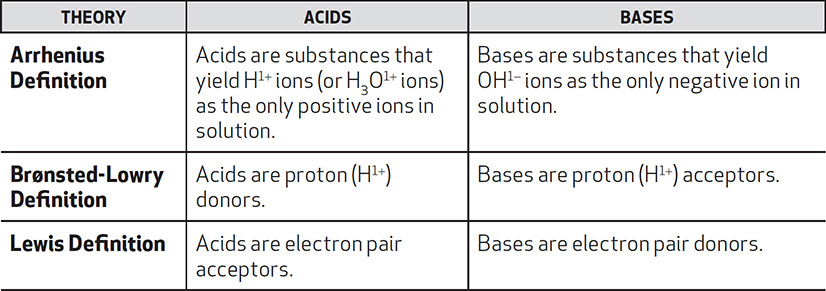
Arrhenius Acids and Bases
Arrhenius’s definition of acids and bases is the definition that most people know. Acids can be recognized because their chemical formulas have an “H” at the beginning, like HBr and HNO3. Bases are easy to recognize because their chemical formulas end with “OH,” like NaOH or KOH. There are a few strong acids and bases that you should be familiar with. These strong acids and bases ionize completely in solution and dissociate into as many hydronium ions and hydroxide ions as are available.

Strong acids and bases are also known to be strong electrolytes. That is, when they dissolve in solution they form ions that can carry an electrical current. This phenomenon is not limited to acids and bases; aqueous salts and molten salts have mobile ions that are also capable of carrying an electrical current.
THINK ABOUT THIS 
Brønsted-Lowry Acids and Bases and Conjugate Pairs
The Brønsted-Lowry definition of acids and bases does not replace the Arrhenius definition, but extends it. The Brønsted-Lowry definition of acids and bases requires you to take a closer look at the reactants and products of an acid-base reaction. In this case, acids and bases are not easily defined as having hydronium and hydroxide ions. Instead, you are asked to look and see which substance has lost a proton and which has gained the very same proton that was lost.
In the example: HCl + H2O → H3O1+ + Cl1−, which substance is the acid and which is the base? Look at the compound that contains chlorine. As a reactant the chlorine has one proton, but as a product the chlorine is an ion by itself. The chlorine compound has lost a proton and the HCl can be labeled as an acid. The water, or oxygen-containing compound, has two hydrogen atoms as a reactant but now has three hydrogen atoms/ions as a product. Therefore, the water is labeled as a base. (See Figure 9.1.)
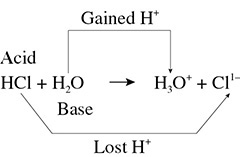
Figure 9.1 Proton Transfer HCl + H2O → H3O1+ + Cl1−
Let’s look at another situation involving the reaction H2O + NH3 → OH1− + NH41+. The water lost a proton and became a hydroxide ion. The ammonia gained a proton and became an ammonium ion. In this case, the water is an acid and the ammonia is a base. (See Figure 9.2.)
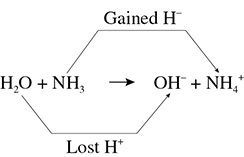
Figure 9.2 Proton Transfer H2O + NH3 → OH1− + NH41+
This brings up an interesting situation. Isn’t water a neutral substance? How can it react as an acid in one reaction and as a base in another? Water is a substance that can gain or lose a proton depending upon the environment it is in. Water is what is called an amphoteric substance because it can act as either an acid or a base.
THINK ABOUT THIS 
Problem:
Label the acid and base in the reaction: 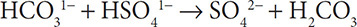
Solution: The compound containing the carbonate ion starts with one H1+ ion bonded to it and ends up with two H1+ ions. The HCO31− ion has accepted a proton and is the base. The compound with the sulfate ion started with one H1+ ion and ended up without any. The HSO41− ion has lost a proton and is labeled as an acid. Notice that in this example both reactants have “H” in their chemical formulas. Despite this, one of them behaved like a base.
What if an acid-base reaction was reversible? Could the products act as acids and bases in a reversible reaction? Look at the following: HF + NH3 ←→ F1− + NH41+. In the forward reaction, the HF is the acid and the NH3 is the base. The fluoride ion is called the conjugate base because it was formed from the loss of a proton and in the reverse reaction the fluoride ion is what accepts a proton. HF and F1− are what we call a conjugate pair, a pair of substances that differ by an H1+ ion.
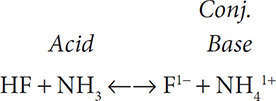
The opposite holds true for ammonia and the ammonium ion. Ammonia is classified as a base because it accepted a proton. The ammonium ion in the reverse reaction will be the proton donor. Because the ammonium ion was also formed from a base, the ammonium ion is called the conjugate acid. NH3 and NH41+ are another conjugate pair, a pair of substances that differ by an H1+ ion.
Problem:
What are the conjugate acids and bases for HS1−?
Solution: The conjugate base is formed from a substance that acts like an acid. If HS1− acts like an acid and loses a proton, then S2− remains as the conjugate base. The conjugate acid is formed from a substance that acts like a base. If HS1− acts like a base and gains a proton, then H2S remains as the conjugate acid.
Lewis Acids and Bases
Lewis acids and bases, because of their complexity, shall be examined briefly. Consider the structure of ammonia with its free pair of electrons. If the free pair of electrons were to make a bond with boron trifluoride, which substance is labeled as an acid and which one is the base? Because the boron accepted a pair of electrons it is considered to be the Lewis acid. Ammonia is the substance that donated the electron pair and is classified as the Lewis base. (See Figure 9.3.)
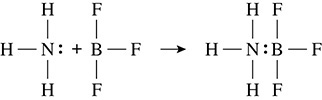
Figure 9.3 Lewis Acids and Bases
Titration and Neutralization
As discussed previously, neutralization is the process by which an acid is reacted with a base to form a salt and water. The general reaction for this process can be simplified by showing how the H1+ and OH1− ions combine to form water. Perhaps you have seen a commercial on TV where an antacid promises to neutralize more acid than another antacid. Perhaps you have seen a situation in the laboratory where an acid was spilled. The laboratory specialist should have neutralized the spill with baking soda. These are situations where the amounts of acid and base being neutralized are not completely measured out. There is a process where acids and bases can be measured out in exact quantities so that they neutralize each other exactly and do so without any excess acid or base. This process is called a titration.
In a titration, you are presented with an acid or base of an unknown molarity. You then use an acid or base of a known molarity to neutralize the unknown. The process is done slowly with the use of burets to deliver exact amounts of acid and base. Finally, you use an indicator to help determine the end point of the reaction. When the indicator changes color, the end point has been reached and the acid and base have neutralized each other. Titrations need to be so carefully controlled that often just one drop of acid or base added to the reaction flask can mean the difference between the solution’s being acidic or basic. This can be seen by the steep slope of the line in the graph in Figure 9.4.
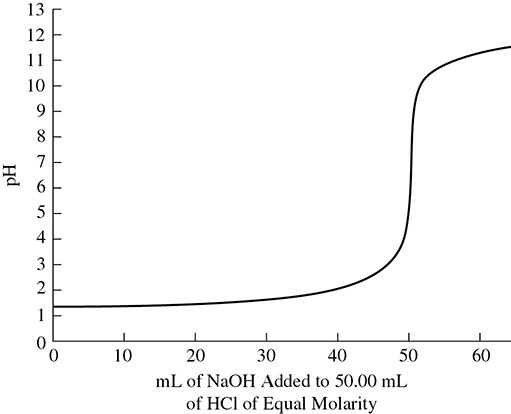
Figure 9.4 Titration Curve
The graph in Figure 9.4 shows that it took 50.00 mL of 0.100 M NaOH to neutralize 50.00 mL 0.100 M HCl. The equation used to make the necessary calculations in a titration is as follows: MaVa = MbVb, where Ma and Mb are the molarities of the acid and base and Va and Vb are the volumes of acid and base used in the titration process.
Problem:
A student is performing a titration in the laboratory. She delivers 15.00 mL of HCl of an unknown molarity to a flask via a burette. She then adds one drop of phenolphthalein indicator to the acid in the flask and the solution remains colorless. The student then begins the titration and delivers 0.100 M NaOH to the flask drop by drop. She finds that it takes 20.00 mL of the NaOH to make the indicator change from colorless to pink. What is the molarity of the HCl that was delivered into the flask?
Solution: The volume of the acid delivered was 15.00 mL (Va). The volume of the 0.100 M NaOH (Mb) used was 20.00 mL (Vb). We are asked to find the molarity of the acid (Ma). First write the equation used to solve titrations:
MaVa = MbVb then substitute:
Ma(15.00 mL) = (0.100 M)(20.00 mL) and solve:

Remember that this can be solved mentally as 20/15 is 1.33, which is 0.133 when multiplied by 0.100.
Hydrolysis
THINK ABOUT THIS 
Literally meaning “water splitting,” hydrolysis can be thought of as the opposite of neutralization. This is represented by the diagram shown in Figure 9.5.

Figure 9.5 Neutralization versus Hydrolysis
When a salt undergoes hydrolysis, it reacts with water to form an acid and a base. The acid and base formed can be considered to be the original reactants from which the salt was formed in a neutralization reaction. By determining the strengths of the original acid and base from which the salt was formed you can then determine if the salt is an acid, a base, or a neutral salt.
Consider NaCl, for example. To determine if this salt is acidic or basic, first locate the anion and cation in the salt. Na1+ is a cation and Cl1− is the anion. Then draw a line between the Na and Cl in the compound to indicate the split by the water: Na | Cl. The cation will bond with OH1− and the anion will bond with H1+. This leads to the fact that the base used to form NaCl is NaOH and the acid used to form NaCl is HCl. Because NaOH is a strong base and HCl is a strong acid, the salt they form, NaCl, is a neutral salt.
Problem:
Determine if the salt NaC2H3O2(s) is acidic, basic, or neutral.
Solution: Determine the cation and the anion present in the salt: Na | C2H3O2.
The cation was the cation from a base, in this case, NaOH. The anion was the anion from an acid, in this case, HC2H3O2. NaOH is a strong base and HC2H3O2 is a weak acid. Therefore, this salt is a basic salt that was derived from a strong base and a weak acid.
Ka and the Strength of Acids
Weak acids do not dissociate completely in solution and an equilibrium is established between the acid and the ions that it forms. This means that an equilibrium constant expression can be written and the concentration of the hydronium ions in solution can be found. For example, for acetic acid, which is a weak acid, you can write the equation: HC2H3O2(aq) ←→ H1+(aq) + C2H3O21−(aq) and write the equilibrium constant expression as:

Notice that the [H1+] is located in the numerator of the equation. If the value for Ka is greater than 1, then there is a greater concentration of H1+ present in the solution and the solution is more acidic. Therefore, a greater Ka value means that the acid is stronger. The reference tables in Appendix 4 show the Ka values of some common acids. Examination of these constants will show, for example, that hydrofluoric acid, used in the etching of glass, is a stronger acid than acetic acid, the acid that is responsible for the sour taste of vinegar.
Problem:
The Ka for HClO at 298 K is 3.0 × 10−8. What is the concentration of hydronium ions in a 0.2 M solution of HClO?
Solution: The reaction proceeds as: HClO(aq) ←→ H1+(aq) + ClO1−(aq). The equilibrium constant expression can be set up as:
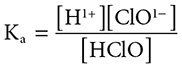
and substitution gives [remember that there is an equal concentration of H1+(aq) and ClO1−(aq)]:
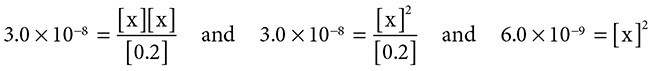
Solving gives [H1+] = 7.7 × 10−5 M.
pH and the pH Scale
The pH of a solution is a measure of the solution’s acidity. Before pH can be calculated, you must first know the concentration of H1+ in solution, thus the reason for calling it pH. The pH of a solution can be found by using the equation −log[H1+]. Consider the 0.2 M HClO from the previous section. The [H1+] was 7.7 × 10−5 M in that solution. Now you can calculate the pH of the solution. By substituting into the equation for pH you get: −log[7.7 × 10−5 M]. The log of 7.7 × 10−5 M is −4.1. Negating this as called for by the pH equation, you find that the pH is 4.1.
Besides finding the pH, the concentration of H1+ can help find the concentration of OH1− in solution as well. Pure water ionizes to form equal amounts of H1+ and OH1− as shown in the following equation: H2O(l) ←→ H1+(aq) + OH1−(aq). The equilibrium constant expression for this equation can be written as Kw = [H1+][OH1−]/1 (don’t include liquids in the equation). The equilibrium constant for the autoionization of water at 298 K is 1 × 10−14. This constant will help find the concentrations of H1+ and OH1− in acid and basic solutions. First, look at the pH of pure water.
In a solution of pure water the [H1+] is equal to [OH1−]. So if Kw = [H1+][OH1−] and [H1+] is equal to [OH1−], you can rewrite the equation as 1 × 10−14 = [x][x] = x2. Taking the square root of both sides of the equals sign, you get x = 1 × 10−7. If the [H1+] is 1 × 10−7 M, then the pH of pure water is −log [1 × 10−7 M] which equals 7. This is why a pH of 7 is neutral on the pH scale.
Problem:
A solution of a strong acid has an [H1+] equal to 1.0 × 10−2 M. What is the [OH1−] in this solution? What is the pH of this solution? Will the solution be acidic or basic?
Solution: Use the equation: Kw = [H1+][OH1−] and substitute to get 1 × 10−14 = [1.0 × 10−2 M][x] and x = 1.0 × 10−12 M. The [OH1−] is 1.0 × 10−12 M. To find the pH use the equation −log[H1+] and substitute to get −log[1.0 × 10−2 M], which is equal to a pH of 2. To determine if the solution is acidic or basic, compare the concentrations of H1+ and OH1−. Because the [OH1−] is 1.0 × 10−12 M and the [H1+] is 1.0 × 10−2 M you can see that there is a greater concentration of H1+ ions present. This tells you that a pH of 2 means that the solution is acidic.
With the help of the previous three problems, you can now set up the pH scale. The pH scale extends from numbers 1 through 14 with each increment meaning a difference in the [H1+] by a power of 10 (remember that you are using logarithms). You have already determined that a pH of 7 is neutral and that a pH of 2 is acidic. This means that the pH values above 7 will indicate a solution that is more basic. You can summarize these trends with the pH scale shown in Figure 9.6.

Figure 9.6 The pH Scale
Buffers
How is it that human blood can maintain a pH that is just above 7 considering all the factors that could change the pH of human blood? What is it that causes the resistance to the change in pH? Chemists, and the human body, prepare solutions called buffers (or buffer solutions if you wish) that help counter changes in pH. Buffers contain substances that are available to counter any H1+ and OH1− ions that may be added to a solution. This is achieved by preparing a solution that is equimolar of a weak acid or weak base and a salt of that weak acid or weak base. A common example is a buffer prepared from acetic acid (HC2H3O2) and the salt sodium acetate (NaC2H3O2). When the following equilibrium is established: HC2H3O2(aq) ←→ H1+(aq) + C2H3O21−(aq) and a base is introduced, the H1+ is consumed but the acetic acid will form more H1+ ions as the equilibrium shifts to the left. If an acid were introduced, then the acetate ion is available to consume the added H1+ and shift the equilibrium to the left. These shifts in equilibrium are what cause the pH to remain stable. There is one catch, however. For the buffer to be effective, the concentrations of the acid and its conjugate base must have a concentration far greater than that of any added acid or base.
REVIEW QUESTIONS
1. A stronger base
(A) is also a stronger acid
(B) is also a stronger electrolyte
(C) tastes sour
(D) yields fewer OH1− ions in solution
(E) is easier to neutralize
2. When HCl(aq) reacts with Zn(s) the products formed are
(A) water and a salt
(B) an acid and a base
(C) a salt and hydrogen gas
(D) a nonmetal oxide
(E) a metal oxide
3. A substance is added to a solution containing two drops of phenolphthalein. The solution then turns pink. Which substance would produce this color change?
(A) HCl
(B) H2CO3
(C) KOH
(D) CH3CH2OH
(E) CH3OH
4. Litmus is red when the H1+ concentration in the solution is
(A) 1 × 10−11 M
(B) 1 × 10−9 M
(C) 1 × 10−7 M
(D) 1 × 10−5 M
(E) 1 × 10−14 M
5. A substance is dissolved in water and the only positive ions in the solution are H1+ ions. This substance is
(A) KOH
(B) NaH
(C) H2SO4
(D) NH3
(E) CH4
6. Which is true about a solution that is acidic?
(A) [H1+] equals zero.
(B) [OH1−] equals [H1+].
(C) [H1+] is less than [OH1−].
(D) [H1+] is greater than [OH1−].
(E) Kw = 1 × 10−7.
7. According to the Brønsted-Lowry theory, a base can
(A) donate a proton
(B) yield H1+ ions
(C) donate an electron pair
(D) accept an electron pair
(E) accept a proton
8. What volume of 0.200 M NaOH(aq) is needed to neutralize 40.0 mL of a 0.100 M HCl(aq)?
(A) 100.0 mL
(B) 80.0 mL
(C) 40.0 mL
(D) 20.0 mL
(E) 10.0 mL
9. As an acidic solution is titrated with drops of base, the pH value of the solution will
(A) increase
(B) decrease
(C) remain the same
(D) approach zero
(E) none of the above
10. Which pH value demonstrates a solution with the greatest concentration of OH1− ions?
(A) 1
(B) 7
(C) 10
(D) 12
(E) 14
11. The reaction: HI(aq) + LiOH(aq) → H2O(l) + LiI(aq) is classified as
(A) a single replacement
(B) a neutralization reaction
(C) the process of hydrolysis
(D) a synthesis reaction
(E) an oxidation-reduction reaction
12. How many times stronger is an acid with a pH of 2 than an acid with a pH of 5?
(A) A pH of 2 is three times as strong.
(B) A pH of 2 is one thousand times as strong.
(C) A pH of 2 is three times as weak.
(D) A pH of 2 is one thousand times as weak.
(E) A pH of 5 is three thousand times as strong.
13. Which substance below is expected to be the strongest electrolyte?
(A) chlorous acid
(B) water
(C) acetic acid
(D) hydrofluoric acid
(E) hypochlorous acid
14. Which of the following statements is true?
(A) NaCl is a neutral salt.
(B) KC2H3O2 is an acidic salt.
(C) KOH is an acid.
(D) HCl and KOH react to form hydrogen gas and water.
(E) NaBr is basic salt.
15. Which pairing is not a set of conjugates?
(A) OH1– and H2O
(B) HC2H3O2 and C2H3O21–
(C) HCl and Cl1–
(D) NH3 and NH41+
(E) H2SO4 and SO42–
16. Which reaction below is incorrect based upon the reactants given?
(A) HF + LiOH → H2O + LiF
(B) 2HCl + Zn → H2O + ZnCl2
(C) SO2 + H2O → H2SO3
(D) K2O + H2O → 2KOH
(E) All of the above reactions are correct.
17. Which compound below is not correctly paired with its name?
(A) KOH is potassium hydroxide.
(B) H2SO3 is sulfurous acid.
(C) HI is hydroiodic acid.
(D) HClO2 is chloric acid
(E) H3PO4 is phosphoric acid.
18. Which of the following pairs would be a good choice for preparing a buffer solution?
(A) acetic acid and sodium acetate
(B) hydrochloric acid and sodium chloride
(C) perchloric acid and sodium chlorate
(D) sulfuric acid and sodium sulfate
(E) water and sodium metal
ANSWERS AND EXPLANATIONS
1. (B) Strong acids and bases will dissociate 100% into ions in solution that can conduct electricity.
2. (C) An acid and an active metal will yield a salt and hydrogen gas.
3. (C) Phenolphthalein will turn pink when it is basic. KOH is the only base in the choices given.
4. (D) Choice D corresponds to a pH of 5, which is acidic on the pH scale. All of the other choices are either neutral or basic.
5. (C) The question describes the definition of an acid. Of the choices given, the only acid present is sulfuric acid.
6. (D) An acidic solution will have more hydronium ions than hydroxide ions.
7. (E) Acids and bases can be defined multiple ways. A base can yield hydroxide ions, accept a proton, or donate a pair of electrons. The Bronsted-Lowry definition of a base calls for a substance to accept a proton.
8. (D) Using the equation MaVa = MbVb, we can calculate the volume of NaOH needed to titrate the acid. 20 mL of the base is needed to neutralize the acid.
9. (A) As a base is added to a solution, the pH value will increase toward the value of 14.
10. (E) The more hydroxide ions present in solution, the higher the pH value of the solution will be.
11. (B) The reaction of an acid and a base will yield a salt and water. This is a neutralization reaction.
12. (B) The solution with a pH of 2 is a stronger acid than that with a pH of 5. Because the pH scale works on powers of 10, a solution with a pH of 2 is 1000 times more acidic than a solution with a pH of 5.
13. (A) A stronger acid or base will be a stronger electrolyte. Of the substances listed, chlorous acid is going to form the greatest number of ions.
14. (A) NaCl is a neutral salt because it can form from a strong acid (HCl) and a strong base (NaOH).
15. (E) Conjugates will differ by a hydrogen ion. In choice E the two substances differ by two hydrogen ions.
16. (B) An acid and an active metal will yield a salt and hydrogen gas. Choice B is incorrect because of the water present.
17. (D) According to the rules for naming acids, HClO2 is called chlorous acid. The ending “-ous” is used because the ion involved ends in “-ite”.
18. (A) A buffer is best prepared with a weak acid with its conjugate base or with a weak base and its conjugate acid. Choice A includes a weak acid with its conjugate base.