6
The Red Mist
In September 2007, an attending physician at the Austin Medical Center (AMC) in Austin, Minnesota, examined a patient with numbness, tingling, fatigue, and weakness in the legs and feet—symptoms that suggested damage to the peripheral nervous system, the network of nerve cells that transmit information between the brain and spinal cord (the central nervous system) and other parts of the body. Through an interpreter, the doctor learned that the patient, an otherwise healthy and physically fit Hispanic immigrant, worked at Quality Pork Processers (QPP), a local meatpacking plant.
The Spanish interpreter was surprised. She had interviewed other patients at the hospital with tingling, pain, and difficulty walking—and she was pretty sure some of them worked at the same factory.1 Sure enough, a review of medical records by the nursing staff turned up ten cases involving muscle weakness, pain, or tingling going back to December 2006. How had they missed this? Taken individually, the patients’ complaints were varied and fairly vague, but taken together—with QPP as a common link—the cluster could be evidence of a disease outbreak.
But an outbreak of nerve damage? That was odd. The attending physician called the local field office of the Minnesota Department of Health (MDH), which in turn called MDH headquarters in St. Paul. Muscle pain and weakness are common in slaughterhouse workers who are on their feet all day, doing the same physical task over and over. The MDH occupational health office thought the patients might be suffering from repetitive motion injuries caused by muscle strain.
Two weeks later, a medical epidemiologist at the Mayo Clinic—which owns the Austin Medical Center—called the state health department with more details. He spoke with Richard Danila, one of the MDH medical detectives who solved the mystery described in Chapter 5. The problem was not muscular, but neurologic. The patients reported tingling and numbness, not just pain and weakness, and their reflexes were abnormal. Could it be an inflammatory nerve disorder—neuropathy—caused by infection? Later the same day, Danila’s office was contacted by the chief occupational nurse at QPP, who had referred three workers complaining of tingling, pain, and “heavy legs”2 to the Mayo Clinic for special testing. She was worried that an epidemic might be spreading through the plant.
Medical detective Aaron DeVries was assigned to the case. He was new to MDH, having joined in July after completing an infectious diseases fellowship and assisting in post-hurricane Katrina recovery efforts as a Medical Reserve Corps volunteer. A minister’s son who “grew up all over the country,” DeVries is even-tempered, observant, and thorough. Over the course of one long day, he visited the Mayo Clinic in Rochester (about eighty miles from St. Paul) and then the Austin Medical Center and the meatpacking plant, both in Austin (forty miles further on). He learned at the Mayo Clinic that the QPP workers were suffering from polyneuropathy, a neurologic disorder involving damage to multiple peripheral nerves. The symptoms included head and neck pain, muscle weakness, numbness, and pins-and-needles sensations or burning pain in the hands and feet. A few of the patients had lost sensation in their lower bodies and required walkers or wheelchairs.
Polyneuropathies can have many different causes, including an underlying disease like diabetes or physical traumas like sports injuries or car crashes. DeVries could rule out diabetes and traumas as unlikely explanations for a cluster of cases. A more probable cause for a slaughterhouse outbreak was either chemical poisoning or a contagious disease spread from animals to humans. Infectious diseases are, in fact, the most common cause of polyneuropathies.3
The pathogen most often passed from hogs to humans is Trichinella, a parasitic roundworm ingested in undercooked pork. However, Trichinella infection causes stomach cramps and diarrhea and is seldom seen in farm-bred pigs, thanks to modern farming practices and USDA rules and inspections.4 In any case, the QPP outbreak did not appear to involve consumption of contaminated food—a major concern during a food-factory outbreak. The people who worked at QPP, and not the people who ate QPP meat products, were getting sick.
Since the symptoms were neurologic, DeVries also considered the possibility of a degenerative brain disease caused by a prion—a special kind of protein that causes other proteins in the brain to fold abnormally. Prion diseases have been reported in several mammalian species, though not (so far) in pigs. Examples include mad cow disease in cattle, scrapie in sheep, and chronic wasting disease in deer. Prion diseases that affect humans include Creutzfeldt-Jakob disease (CJD), which causes progressive dementia; a variant form of CJD caused by eating beef from a cow with mad cow disease; and kuru, a fatal neurologic disease identified in the 1950s among New Guinea tribes who consumed the brains of deceased relatives as part of funeral services.5
However, prion diseases attack brain tissue rather than peripheral nerves, and they are progressive and incurable. Fortunately, that was not the case here. The first neuropathy patient seen at the Mayo Clinic—later identified as the outbreak’s “index patient”—was a young Mexican immigrant who had improved after treatment with steroids, a standard anti-inflammatory treatment, during a two-week hospitalization.2, 6–8 His illness had begun back in December with flu-like symptoms and muscle pain that he attributed to standing on his feet all day. But when he lost all sensation in the lower half of his body, his family had rushed him to the emergency room. Since then he had recovered twice, but his symptoms relapsed each time he returned to work at QPP.
After completing his consultation with physicians at the Mayo Clinic, DeVries drove over to Austin, where he spoke with the AMC staff and reviewed patient records, looking for additional clues. By this time he was convinced that the disorder was neither food-related nor due to repetitive stress. He had also ruled out an infection transmitted from person to person, because none of the patients’ family members were sick.
His next stop was the QPP factory, which is located on the bank of the Cedar River, next to the headquarters of Hormel Foods, the corporation that produces Spam, Dinty Moore stew, and other canned convenience products. Austin, a city of about twenty-five thousand people, is known as “Spam Town USA” because Hormel is its biggest employer. The QPP factory used to be part of Hormel, but was spun off in the 1980s—a time of increased automation and consolidation in the meatpacking industry—after a thirteen month strike that ended with Hormel workers accepting a significant cut in wages.9 Today, QPP continues to buy hogs from Hormel and provide meat products to Hormel for packaging and sale. Many of its 1,300 employees are Spanish-speaking immigrants from Latin America.
As he entered the factory, DeVries could see the huge holding pen where the hogs, excited and screeching, are unloaded off massive livestock trailers.10 He met with Kelly Wadding, QPP’s owner and chief executive officer, and then with the factory’s head nurse, who showed him around the occupational health clinic. She said that some of the workers, whose jobs are physically taxing, were having difficulty climbing the stairs to her third-floor office.
Wadding and the QPP director of human resources gave DeVries a quick tour of the factory so he could see how a modern abattoir operates. In addition to the holding pen, the factory included three large sections: the entry room where the hogs are stunned by electroshock and killed, the “warm room” where the carcasses are butchered, and the “cold room” where the cuts of meat are prepared and packaged.
The warm room is a huge, barn-like area where everything is planned out in great detail. The first step is to hang the hogs by their rear legs, after the blood has been drained and the skin removed. The last step is to put the meat into the freezer for processing in the cold room. Between those steps the hog is disassembled, with each piece removed for packaging as a food product. The pork-processing assembly line is like the assembly line in a car factory, but in reverse (a “dis-assembly line”), starting with a live animal and ending with packaged body parts. As in a car factory, each worker stands at a designated workstation and performs the same task over and over, as each partially processed product comes down the line.
DeVries returned from the tour with a major epidemiologic clue. Kelly Wadding and the human resources manager had made a quick sketch of the warm room, indicating the workstations of the people who had fallen ill. Most were in one small area of the very large plant. DeVries and his boss, Ruth Lynfield, the Minnesota State Epidemiologist, decided to arrange a full-scale inspection of the QPP factory. Whatever the cause of the outbreak—a microbe, a toxin, or something else—they would find it in the warm room.
A New Yorker long re-settled in the Midwest, Lynfield is an articulate, energetic, out going person who knows how to make things happen. She advised DeVries to call James Sejvar, a CDC medical detective who had studied neurology at the Mayo Clinic and graduated from the CDC Epidemic Intelligence Service (EIS). A person trained in both neurology and epidemiology could be a valuable resource. Lynfield and DeVries were pleased to reach him on the first try, because he is in demand and travels a great deal.
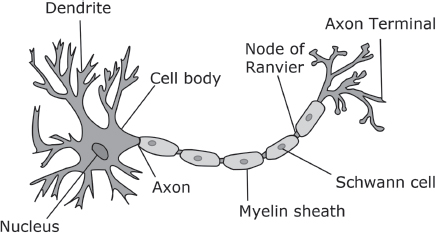
Sejvar was familiar with the research work at the Mayo Clinic neurology laboratory. During his medical residency he had met the legendary neurologists Peter J. Dyck and P. James Dyck—father and son—who are experts in peripheral neuropathies. He had also met Daniel Lachance, a research scientist with a special interest in neurologic disorders, in which immune cells or antibodies attack a patient’s own nerves as if they were germs or foreign bodies. The close proximity of three such experts was an amazing piece of good luck for the public health investigators. As it happened, Lachance and James Dyck had already examined several ill workers from the pork processing plant before an outbreak was suspected. They thought the index patient’s relapsing symptoms suggested an autoimmune condition like chronic inflammatory demyelinating polyneuropathy (CIDP), which involves destruction of the myelin sheath, the protective protein coat that insulates the axon, the long threadlike part of a nerve cell that conducts nerve impulses. CIDP can be triggered by infection, most often by Campylobacter bacteria, a common cause of gastrointestinal disease. Here was another reason to suspect that the workers’ illness might involve an infection, perhaps an infection acquired from swine.

A Nerve Cell. Image courtesy of Quasar Jarosz - CC-BY-SA-3.0.
With this idea in mind, Lynfield asked three animal health experts to attend the factory inspection, as well as an infection control specialist and a toxicologist. She invited a scientist from the Minnesota Department of Agriculture, a veterinarian from the Mayo Clinic with expertise in pathogens that infect both humans and animals, and a veterinarian named Stacy Holzbauer, who was an EIS trainee assigned to MDH. Although most EIS officers are doctors, in recent years EIS has prioritized recruitment of veterinarians, recognizing that most new infectious diseases—including AIDS, severe acute respiratory syndrome (SARS), Ebola hemorrhagic fever, hantavirus pulmonary syndrome, and pandemic influenza—have emerged from animal reservoirs.11
Before entering the cavernous warm room, which was large, steamy, and smelly, the inspection team, led by Lynfield and DeVries, donned plastic gloves, booties, masks, and plastic gowns. Wadding supplied a factory map and provided the toxicologist with material safety sheets on chemicals used in the factory. The inspection team walked along the assembly line, observing the tasks performed at each workstation. Cutting up the hog carcasses was a messy and repetitive business. Each worker made a particular cut, over and over, in each successive hog. Some used large knives or other cutting tools, while others used sophisticated machinery. The workers wore different types of protective clothing, depending on their jobs.
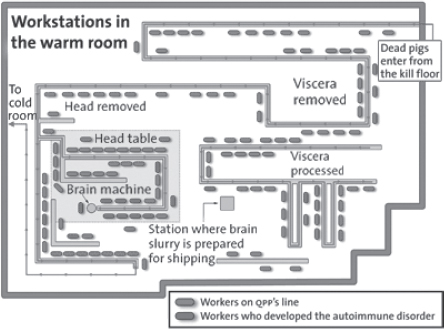
At the very end of the line the inspection team came to the “head table,” the place where the hogs’ heads were disassembled and their brains removed. About thirty-five workers stood around this table, removing the tongues, cheeks, temples, snout, ears, and jaw of each hog using knives, chisels, and automatic cutting tools. (The factory people liked to say that they utilized “everything but the squeal,” so that nothing went to waste.) The next to last job was called “backing heads,” chopping and removing muscle from the backs of the skulls with a circular knife, and the last one was “blowing brains,” removing the brains from the skulls. These jobs were relatively well-paid and sought-after by the QPP workers, who bid for them on the basis of seniority and ability.12 A head came down the line about every three seconds, and it required speed, dexterity, and sustained focus to remove as much muscle and brain as possible.
The head muscles and brains of hogs are commercially valuable as specialty food products. Head muscle can be made into sweet or spicy sausage or into headcheese, a meat jelly that can be served with crackers or bread, like goose liver pate. Scrambled eggs with brains is a popular dish in some southern states, where hog brains are sold in supermarket cans as “pork brains in gravy” or bought at meat markets as fishing bait. However, the greatest demand for hog brains is in China and Korea, where they are deep-fried, stir-fired, or cooked in hot pots. From an economic point of view, U.S. hog brain sales to Asia are part of the modern globalization of trade.
The brain-harvesting job was called “blowing brains” because the brain-removal worker “blew” the brains out of the skull with a blast of pressurized air. Standing at the very end of the head table, the brain-removal worker placed each skull, right-side up, lower jaw and skin removed, on the metal hose of a pressurized air device, with the hose fitting through the foramen magnum, the “great hole” where the spinal cord enters the skull. Placement of the skull on the nozzle automatically tripped a wire that caused pressurized air to rush into the skull, liquefying the brain matter into a soggy pink mass that drained into a ten-pound bucket. Another worker periodically carried the pooled brains to the cold room for further processing.
It was remarkable to see the brain-removal procedure in action. The head table worker stuck the skulls on the nozzle as fast as he could, one after the other, generating some splatter, trying hard to keep up. Walking ahead with Kelly Wadding, Ruth Lynfield could see a strange reddish mist blowing around the room, propelled by air currents generated by an overhead fan. (Even though it was November, it was warm in the warm room.) Pink particles settled on the people at the head table, especially on the brain-removal worker and the person backing heads. A Plexiglas shield near the pressurized air device provided little protection, nor did the workers’ goggles, coats, and cut-resistant gloves, which left their arms exposed from the shoulder to the wrist. (Few wore apron sleeves, which can be hot and uncomfortable.) Moreover, none of the head table workers wore respirators or face masks, so there was nothing to prevent them from breathing in the aerosolized brain matter, minute by minute, day after day.
Staring spellbound at the head table and its workers—who looked both strong and vulnerable—Lynfield said to Wadding, “Kelly, what do you think is going on?”13 Wadding responded by deciding, then and there, to stop using air-blasting machinery to harvest brains. On the spot, he ordered the device dismantled and brought to his office. He also agreed to provide the head table workers with additional protective equipment.
Though they did not realize it at the time, the brief exchange between Lynfield and Wadding ended the outbreak of polyneuropathy in Austin, Minnesota. Eliminating the factory’s brain mist turned out to be a totally effective prevention measure. Later on, the medical detectives would compare it to “taking the handle off the Broad Street pump”—the iconic action taken by the Englishman John Snow, the “father of epidemiology,” to end an 1854 cholera epidemic transmitted through drinking water. In Austin, as in long-ago London, the simple action that ended the outbreak was performed on the basis of a hypothesis. John Snow thought that cholera was caused by a waterborne microbe, and Lynfield thought the polyneuropathy was caused by the brain mist. Neither had proof.
Over the next few days, the medical detectives designed a formal study to confirm or disprove the brain mist theory. At the same time, they considered other possibilities, just in case they were chasing a red herring. One possibility was that the illness was caused by poisoning from a toxic chemical used in cleaning or processing; another was that an airborne toxin or pathogen was spreading through the factory’s air vents. However, colleagues from the CDC’s National Institute for Occupational Health (NIOSH) ruled out the air-vent theory after reviewing the factory’s blueprints and maintenance records. The NIOSH inspectors also confirmed the toxicologist’s conclusion that the factory did not use chemicals whose ingestion or inhalation could cause neurologic symptoms.
Lynfield asked Holzbauer, the veterinary epidemiologist, to lead the epidemiologic study, because she was the only member of the MDH team who was familiar with pork processing plants. Holzbauer grew up in South Dakota as the eldest of eight children on a “grain, cattle, and kid farm.” Her plan was to become a large-animal veterinarian, marry a cowboy, live on a ranch on the Great Plains, and raise cowboys. However, after a few years as a large-animal vet, she enrolled in a master of public health program for veterinarians, inspired in part by Laurie Garrett’s public health classic, The Coming Plague. She was surprised to discover that public health and large-animal medicine share a basic world view, because epidemiologists (like large animal vets) essentially view their patients as a herd—members of a single species that live together in large groups—rather than as individuals. (Small-animal medicine, caring for individual pets, is more like human medicine.) After working on a CDC project to promote appropriate use of antibiotics for veterinarians, Holzbauer—convinced she had found her niche—applied to the EIS program. Today, she is happily married (though not to a cowboy) and the mother of twin baby girls.
Holzbauer knew all about hogs. Her family raised them when she was in grade school, and she had studied the parts of a hog’s lifecycle—birth, growth, finishing, slaughter—in college and vet school. Holzbauer thought that the QPP factory was fairly typical, though she had never before seen compressed air used to harvest hog brains. In fact, when she first saw the compressed-air brain-removal device, she thought it was a good idea—less chance of cutting off fingers with axes or bandsaws!
Holzbauer developed a questionnaire to help the investigators identify disease risk factors by comparing the experiences of the QPP patients and the workers who had remained healthy. Thirteen of fifteen patients identified by AMC, the Mayo Clinic, and the QPP nursing staff enrolled in the study, as well as two groups of healthy workers who served as controls: forty-nine who worked at the head table and fifty-six who worked anywhere in the warm room.12 All thirteen neuropathy patients had worked in the warm room, and seven had worked at the head table. Eight were women, and 80 percent were Hispanic. Their symptoms included numbness; prickling or tingling in the legs, feet, hands, or arms; decreased strength; and absent or decreased reflexes. Some reported severe fatigue, searing skin pain, leg cramps, or a feeling that the soles of their feet were on fire. The diagnosis of peripheral neuropathy was confirmed by advanced electromagnetic testing at the Mayo Clinic that indicated damage to the nerve axons or myelin sheath.
The patients and the healthy controls were interviewed about their health histories, jobs and work histories, and potential sources of exposure outside the factory. It was requested that each interviewee stop by the factory’s medical station to provide a throat swab and blood sample. The study went smoothly, with the plant managers arranging for workers to be taken off the line, three at a time, for forty-five minute interviews. They also provided blueprints so the team could measure the distance between the patients’ workstations and the site of brain removal. Lynfield was pleased at the degree of cooperation. She says it is rare that things are done appropriately, with support rather than resistance. She and Wadding held joint press conferences and met with the workers to explain the purpose of the study.
It was especially important to gain the trust and cooperation of the factory workers, including QPP’s Hispanic workers. Holzbauer’s team provided informational materials in Spanish and arranged for native Spanish speakers to conduct interviews. The interviewees were not required to give their names and were assured that the investigators’ mission was to improve the plant’s health conditions and not to enforce any laws or regulations. DeVries remembers being at the factory late at night in the middle of winter to talk with night shift workers. Being there was important, because it helped demonstrate that the team was interested in the employees’ welfare.
The results were clear: the thirteen neuropathy patients were 6.9 times more likely than the warm-room controls to have ever worked at the head table and 7.5 times more likely than the head-room controls to have either blown brains or backed heads.12 Blowing brains was the job held by the index patient, whose illness had relapsed twice, and backing brains was the job of a patient who told a New York Times reporter that she “always had brains on my arms.”2 These two individuals were the most severely ill. Like the index patient, the woman who backed brains had fallen sick months before the outbreak was detected. Her illness began with tingling in her hands and knots in her legs and progressed over months until she could no longer stand long enough to do her job.2, 7, 14 She had not realized her illness was due to an outbreak until she saw a report on television.7
No correlation was found between illness and prior vaccinations, medications, insecticides, pesticides, contact with other animals, travel history, or activities conducted outside the factory. However, while proximity to the brain-removal station was the strongest predictor of disease, not every neuropathy patient had worked at or near the head table. Danila recalls that one of the patients worked in a different section of the warm room, some distance away from the brain-removal device. The epidemiologists considered her an “outlier” who did not fit their hypothesis. However, it turned out that her best friend worked near the brain harvest station and that she spent her coffee breaks, twenty minutes a day, standing next to her friend as she worked. (She did not sit, because there were no chairs at the head table.)
While the study was in progress, Sejvar and colleagues at the CDC and the USDA surveyed U.S. factories to identify other plants that used compressed-air devices to process hog brains. Of twenty-six federally-inspected swine abattoirs in thirteen states, they found six that processed swine brains, including three that used pressurized air: one in Delphi, Indiana, one in Fremont, Nebraska (owned by Hormel), and the QPP factory in Austin.12 Sejvar also notified the World Health Organization, the World Organization for Animal Health, and the European Centre for Disease Prevention and Control,15 but those organizations found no examples of overseas factories that used compressed air to harvest hog brains.
Like the Minnesota factory, the pork processing plants in Indiana and Nebraska had designed their own home-made pressurized-air devices. But the devices in Indiana and Nebraska did not use an automatic trigger. In Indiana, the device had a foot pedal, which provided the operator with more control. In Nebraska, the device was like a gun, completely portable. It had an attachment for a hose like the ones on air pumps for filling tires. Squeezing the attachment let loose the blast of air.
The managers at the Indiana and Nebraska plants did not recall any cases of muscle weakness, numbness, or pain, and no documented cases of neurologic disease were recorded in their occupational health records. However, the medical detectives decided to look further, aware that plant managers and workers might be reluctant to speak with government officials. The Indiana Department of Health and the CDC eventually turned up five cases in the Delphi area, after distributing health-alert notices to local neurologists, healthcare facilities, and county health officials. All five had previously worked at the Indiana factory.12, 16 A single case of peripheral neuropathy was eventually identified in Nebraska, involving a brain-removal worker who had terminated employment for medical reasons.12
In January 2008, a CDC/NIOSH team, including Holzbauer and Sejvar, visited the plant in Indiana to conduct a second risk-factor study, similar to the one completed in Minnesota.16 The study was led by an EIS officer assigned to the Indiana Department of Health. Holzbauer helped conduct interviews and collect blood samples, and Sejvar conducted on-site assessments of interviewees who reported weakness, tingling, or pain. He identified four additional cases of polyneuropathy among people currently working in the factory, bringing the total to nine. More than half of the illnesses occurred in women, most of whom had been hospitalized, and all of whom were Hispanic.
In Indiana, a worker with a warm-room job called “hanging heads” removed each hog’s head from its carcass and carried it to a separate room—called the “head-boning room”—for further processing. The workstations of the ill workers were clustered in the head-boning room, near the brain-removal device. The results of the Indiana study were also clear-cut and statistically significant. All of the patients who participated in the study had harvested brains at least once, and most reported that brain tissue had entered their eyes, nose, or mouth while they worked. Moreover, the amount of exposure time was correlated to risk. Each additional three-month period spent harvesting brains increased the likelihood of illness by 1.5 percent.16
The identification of polyneuropathy patients at two other “brain mist” plants was further evidence that hog brain material was associated with the cases of nerve damage. However, one piece of information did not fit: the compressed air devices were not new. They had been in use in Minnesota and Nebraska since 1998 and in Indiana since 1993. Intensive case-finding had not turned up any cases earlier than 2006 in Minnesota or 2004 in Indiana. The single case in Nebraska was identified in 2006.
What had changed? The medical detectives checked the delivery records of the factories in Minnesota, Indiana, and Nebraska but found no common source of hogs during the years preceding the outbreak. The hogs came from farms all over Canada and the United States, mostly from Minnesota, Iowa, Wisconsin, and South Dakota. Moreover, there were no reports of illness at other plants that received hogs from the same farms.
Another possibility was that workers had been falling ill for some time but no outbreak had been detected because of the high turnover at the plant. There was some anecdotal evidence for this, including a patient in Indiana who said she had replaced a worker who got sick while harvesting brains,17 and an immigrant worker seen at the Mayo Clinic in 2005 who had gone back to Mexico before her tests were complete.7, 18, 19 It was certainly possible that other workers had left the factory rather than seek help or take medical leave.
Another idea, suggested by the patients themselves,7, 18 was that the head table workers had breathed in greater amounts of brain mist since 2006 because QPP was slaughtering hogs at a faster rate. The line speed had increased from 1,200 to 1,300 hogs per hour in April 2003, and then to 1,350 hogs per hour in 2006, the year the index patient fell ill. (For comparison, the line speed in 1996, when the device was new, was about 900 hogs per hour.7) According to the workers, the need to remove a brain every few seconds made it difficult to place each skull fully on the nozzle, causing misfiring, skull breakage, and greater spray of aerosolized particles. More material escaped into the air, so that the workers stood in a constantly replenished haze of the reddish-grey brain mist observed by Lynfield and her colleagues.
The Nebraska and Indiana factories also reported production increases that fit the outbreak’s timeline. The line-speed in Nebraska increased from 1,200 to 1,250 hogs in 2006, the year that a brain-removal worker became too ill to work. At the Indiana plant the situation was a little different because not all hog heads were harvested for brain. Instead, the percentage varied from year to year, based on market demand. However, the Indiana plant had increased its brain harvest rates by 250 percent between 2004 and 2007, and the neuropathy cases were reported between April 2005 and December 2007.16
QPP apparently sped up the assembly line because of increased demand for cheap meat products like Spam as the recession took hold. Instead of hiring additional workers, QPP encouraged overtime work that increased the exposure of individuals who worked at the head table. In Nebraska, the increased demand for brains (as opposed to hog meat in general) may have reflected the decreased availability of cow brains after 2004. This was due to a USDA rule that banned the sale of bovine brains from older cattle because of concerns about mad cow disease.16
So far, so good. But the question of how the hog brain material had caused the workers’ illness was still unanswered: Was an infectious agent present in the brain mist? Lynfield, DeVries, Holzbauer, and Danila returned to the factory to retrieve samples of not-yet-sold hog brains stored in QPP’s cold room. Hog pathogens are well studied, because of their impact on the agricultural industry. A few, like hepatitis E, are known to infect humans; several cause neurologic symptoms (e.g., encephalomyocarditis virus and pseudorabies virus) or embryonic and fetal death (e.g., porcine parvovirus); and others are the porcine relatives of human viruses that cause such diseases as influenza. Despite an exhaustive search, however, no bacterium, virus, or parasite was found in either the hog brain samples or the human throat swab samples collected during the risk-factor study. To leave no stone unturned, the detectives also arranged for testing of stool samples from four Minnesota and two Indiana workers as part of the CDC Unexplained Diarrhea project, knowing that CIDP can be triggered by Campylobacter bacteria, a common cause of gastrointestinal symptoms. However, no consistent evidence of a diarrheal infection was found.12
But what if the brain mist contained a rare or unknown pathogen for which they had no test? Infection of slaughterhouse workers with unusual animal-borne pathogens is not unheard of. In 1999, for example, a previously unknown virus called Nipah—first identified on pig farms in Malaysia—caused an outbreak of respiratory and neurologic disease among abattoir workers in Singapore who slaughtered pigs from the affected Malaysian farms.20, 21 Moreover, Q fever—an animal-borne disease described in Chapter 4—has caused outbreaks in sheep or goat abattoirs in France,22 Australia,23 Scotland,24 the Netherlands,25 and California.26 However, additional testing, including a search for rare or unusual microbial ribosomal RNA sequences, yielded only negative results.
There was one more possibility: An infection that triggered an immune disorder could have been over before the throat swabs were taken. In that case, the causative microbe might be gone, but microbe-specific antibodies might remain in a patient’s blood. This explanation, too, was ruled out, after extensive laboratory testing.
Having found no evidence of infection—either as a direct cause of the neurologic symptoms or as a precipitating factor—the medical detectives began to entertain a more unusual hypothesis that initially struck them as bizarre. Could the brain mist itself—rather than a toxin or pathogen carried along with it—elicit a human autoimmune reaction, similar to a very severe allergic reaction?
The evidence for an autoimmune response was solid, despite the lack of an infectious trigger. The Mayo Clinic doctors had suspected an autoimmune disease from the beginning, because of the pattern of destruction of the peripheral nerves. When they measured the velocity of nerve impulses along the patients’ axons, they detected damage at the nerve roots (where the nerves emerge from the spinal cord) and the extremities (where the nerves connect with the muscles or sensory organs), with the mid-nerve sections remaining unharmed. This finding is consistent with immune-mediated nerve damage, because antibodies have greater access to the ends of the nerves, which are well supplied with blood vessels. Damage to the nerve ends was confirmed by several other tests, including magnetic resonance imaging (MRI), electron microscopy, and nerve biopsies. For example, the MRI found inflammation (irritation and swelling) of the nerve roots. An autoimmune condition was also suggested by the patients’ improving when no longer exposed to the brain mist or when given steroids or other immunotherapies.
Additional data implicating an autoimmune disease was provided by Ian Lipkin, the molecular biologist who contributed to the investigation of the New York City outbreak described in Chapter 1. When Lipkin tested the patients’ blood serum samples for cytokines and chemokines—proteins released by immune system cells to activate and sustain an immune response—he found high levels of interferon-gamma (IFN-y), a cytokine associated with autoimmune disorders.12 The Mayo Clinic neurologists confirmed this result and identified many other markers of autoimmune diseases, including high levels of protein in the patients’ cerebrospinal fluid.27
The idea that the brain mist itself had caused an autoimmune response began to seem less bizarre and more likely. As Sherlock Holmes told Watson, when you have eliminated the impossible, whatever remains, however improbable, must be the truth.28 A literature search turned up a few, mostly long-ago, examples of neurological tissue from one species causing nerve damage in another. For instance, Louis Pasteur’s first rabies vaccine, made in 1895 from virus extracted from canine brain and spinal tissue, had caused neurologic symptoms in some patients.29 Moreover, in 1935 scientists developing an animal model of acute neurologic disease induced encephalomyelitis (inflammation of the brain and spinal cord) in monkeys by injecting them with liquefied rabbit brain tissue.30 In the 1980s, encephalomyelitis was reported in patients who received a rabies vaccine contaminated with myelin basic protein, which is a major building block of the myelin sheath.31 More recently, in the 1990s, an experimental treatment involving injection of bovine tissue contaminated with brain proteins induced Guillain-Barré syndrome (GBS)—an acute form of CIDP—in some patients.32
Our modern understanding of molecular biology suggests that Pasteur’s observations and some other neurologic autoimmune events might be due to “molecular mimicry,” a phenomenon in which antibodies recognize part of a foreign body—an “epitope” or “antigenic determinant”—that is similar to part of a normal human protein.33 Bovine brain tissue, for example, could have caused GBS in humans by eliciting antibodies that bind not only to cow-brain epitopes but also to human epitopes with a similar molecular structure. Because the human epitopes “mimic” the bovine ones, the human immune system cannot distinguish between them and attacks them both. Similarly, inhalation of aerosolized hog proteins by the QPP workers could have generated “self-antibodies” that attacked both the hog material and the patients’ own nerves, causing the sustained inflammation that characterizes autoimmune disease. This explanation is plausible because in fact hogs and human share many proteins in our nervous systems.
Lachance and his colleagues tested this idea by examining the pattern of neuronal self-antibodies generated by the patients from Minnesota and Indiana. The scientists used a well-established laboratory assay developed by a Mayo Clinic colleague, Vanda Lennon,18 to detect antibodies generated in cancer patients against both cancer cells and normal nerve cells. The assay uses a fluorescent stain to detect human antibodies that bind to mouse neural tissue immobilized on a slide. The result was surprising: antibodies from all of the neuropathy patients—as well as one third of head table workers who had no symptoms—bound to mouse nerve structures, including the myelin sheath, in a pattern the researchers had never seen before, despite having tested thousands of patients with cancer, CIDP, or GBS.27, 34, 35 They regarded this pattern as the unique biological signature—or biomarker—of a new autoimmune disease.
The new disease has variously been called progressive inflammatory neuropathy (PIN)36, 37 or occupational inflammatory polyradiculoneuropathy.38 From a clinical and public health point of view, its identification and characterization marked the end of the outbreak investigation. The source of the outbreak was known, and the patients had been identified and treated with immunotherapies. Above all, the John-Snow-like intervention had worked! No more factory workers were afflicted with the alarming combination of pain, tingling, and loss of sensation. Moreover, Sejvar and his USDA colleagues had alerted the swine industry and abattoirs across the nation and around the world about the dangers associated with the air pressure method of hog-brain removal.
Nevertheless, many scientific questions remained unanswered. A group of Mayo researchers, including Jeffrey Meeusen, Vanda Lennon, Lachance, and Peter Dyck, decided to investigate the mechanism of disease by creating an animal model of “breathing brain.”39, 40 They smeared liquefied hog brain tissue on the noses of anesthetized mice and took periodic blood samples to check for the generation of self-antibodies, which increased in a dose-dependent manner. When the mice inhaled hog-brain material twice a day, five times a week—mimicking the occupational exposure of the QPP head table workers—their immune systems generated the same biomarker pattern of self-antibodies as the QPP workers. Although the scientists could not tell whether the mice experienced tingling sensations or numbness, MRI testing revealed the same swelling of the nerve roots observed in the human patients.
The next step was to determine which nerve structures were harmed by the autoimmune response. Using standard immunologic tests, the neurologists detected self-antibodies to a variety of neural proteins in the blood of the exposed mice, including myelin basic protein and the channel proteins that create membrane pores that allow ions to flow in and out, generating the nerve impulses that travel along a nerve’s axon. Which were most significant? Two pieces of evidence—one achieved through careful measurement and one through serendipity—pointed to voltage-gated potassium channels (VGPC) as the antibodies’ principal target. First, quantitative testing indicated that the highest levels of antibodies were directed against VGPC protein complexes. In addition, a lab technician noticed that the mice began to shake when administered an anesthetic called isoflurane that works by interfering with potassium channels. The neurologists remembered that a shaking behavior related to isoflurane had been reported before, in the literature on Drosophila fruit flies, a major experimental organism for molecular geneticists. In fact, the first gene encoding a potassium channel protein was cloned in 1987 from a Drosophila fruit fly mutant called “shaker” because it shook its legs vigorously when exposed to isoflurane.41 Thus, the self-antibodies may be binding to a VGPC epitope that is common to swine, humans, and possibly also fruit flies.
The neurologists concluded that the factory workers’ disease was a channelopathy—a neurologic disease caused by disruption of nerve impulses involving membrane channels that allow passage of calcium, sodium, or potassium ions. Other channelopathies include cystic fibrosis, myasthenia gravis, and some seizures and migraine headaches. These diseases may be caused by inborn mutations or by an autoimmune reaction triggered by a foreign body. In the case of the Austin outbreak, the antibody attack on the potassium channels had led to weakness, pain, and (in some cases) lower-limb paralysis among a group of hard-working people whose jobs required substantial strength and stamina. Although motor function improved in all of the treated slaughterhouse workers,27, 42 a year and a half later many continued to experience headaches or pain in their necks, lower backs, or feet, apparently due to stretched nerve roots and irritated nerve terminals.43 Several required medical accommodations, such as sitting for fifteen minutes every two hours, in order to return to work.
The QPP patients were legally entitled to workers’ compensation benefits, if they agreed not to sue. (Minnesota is one of nearly two dozen states that allow undocumented workers to be covered under workers’ compensation laws.) Twelve eventually received settlements of about half a year’s pay ($12,500), and the index patient, who had suffered permanent nerve damage, received $38,600.7 QPP apparently made some attempts to accommodate the ill workers by providing them with lighter duties. Nevertheless, some workers who filed Workers Compensation claims were eventually fired for working under forged or stolen identities,7, 44 and the index patient’s settlement required him to leave the factory for good.7 Few left voluntarily, because the QPP jobs, though low-paying and numbingly repetitive, provided a steady livelihood, with health benefits and overtime pay.
Looking back, the medical detectives recall the surprising complexity of the investigation and the intensity of working on a severe and debilitating neurologic disease that had not been identified before. The MDH team worked with experts from academia, industry, and government who examined the issues from many different angles, including animal health, environmental health, neurology, toxicology, immunology, food safety, and agriculture. Holzbauer and DeVries—the junior members of the team—gained invaluable experience in solving a medical mystery through collaborative investigation, and Lynfield and Danila gained new insights into occupational health by seeing factory workers process hogs at a rate of nineteen thousand a day. In regard to the scientific findings, Sejvar notes that the Mayo Clinic mouse model has already advanced our understanding of autoimmunity by demonstrating that self-antibodies are not just a biomarker but actually play a direct role in causing disease. He is continuing to work with colleagues at the University of Minnesota to learn more about the binding of anti-hog-brain antibodies to human neural tissue.
Above all, however, the medical detectives recall the amazing John Snow moment that stopped the outbreak in its tracks—a moment that all epidemiologists imagine, but few experience.