8
Evolving questions
Although the basic concepts are firmly established, there are many issues with our understanding of evolution that are still live – and often controversial. Should the idea that evolution is directionless be revised? What role does epigenetics play in evolution? Can organisms adapt first, then mutate later? And is evolution predictable?
Is it time to put progress back in the picture?
The concept of progress has been purged from evolutionary theory, but could it be time to let it back in?
The celebrated palaeontologist Stephen Jay Gould once wondered what would happen if we could rewind the tape of life. If it were possible to turn the clock back half a billion years and then let evolution happen all over again, what would we see? Gould famously argued that the history of life would not repeat itself. The world would be unfamiliar, and would probably lack humans.
His point was to demonstrate that evolution is not a process of inexorable progress but of contingency. Mutations happen unpredictably. Sexual reproduction combines genes at random. Droughts, ice ages and meteorites strike without warning and kill off fully fit individuals and species.
We tell ourselves stories of evolutionary progress but these are just wishful thinking. Life produces abundant variations; most fail. The few that survive we call the most advanced, but that is a profound error which conflates ‘latest’ with ‘best’. As Gould wrote in his classic book Wonderful Life (1989): ‘Life is a copiously branching bush, continually pruned by the grim reaper of extinction, not a ladder of predictable progress.’
Gould also had little time for humanity’s hubris. Far from being the pinnacle of evolution, we are just another product of contingency. ‘Perhaps,’ he wrote frostily, ‘we are only an afterthought, a kind of cosmic accident, just one bauble on the Christmas tree of evolution.’
Gould’s view is the orthodoxy of evolutionary theory. Yet it remains hard to reconcile with the intuitive sense that life has indeed progressed over time. All life was once single-celled, yet now a single organism can contain tens of trillions of cells. The number of cell types has increased, too, from one kind in single-celled organisms to 120 in mammals. Brains have grown larger. And humans have accelerated this trend in the past 50,000 years with our own uneven but powerful ascent.
For many years, a small but energetic group of researchers has been trying to rehabilitate the concept of evolutionary progress and explain it in theoretical terms. They hope to show that Gould’s view of evolution is too bleak and that certain kinds of biological progress are not merely accidental or illusory, but necessitated by physical law. If these researchers succeed, it could lead to a crucial modification of current theory.
Gould and those who followed in his footsteps accepted that life has increased in size, complexity and diversity. However, they argue that this is not because evolution is inherently progressive.
Instead, it is an illusion. By definition the first life was very simple. As variation increased, some organisms inevitably became more complex. Humans pay the most attention to the complex ones, leading to a belief in an upward march. As Sean B. Carroll, a professor of molecular biology at the University of Wisconsin–Madison, puts it: when there is nowhere to go but up, some species will go up.
Development-oriented theorists accept these passive increases in complexity. But they argue that there are also ‘driven’ processes that bias evolution toward increasing complexity. John Smart, a member of the evolution, complexity, and cognition research group at the Free University of Brussels in Belgium and a leading thinker in this field, argues that evolution and development can be reconciled. That is, it will be possible to define progress in objective terms and explain why it must happen. The case laid out by Smart and other theorists is based on at least four arguments.
The first concerns a new way of thinking about progress – a concept that is notoriously difficult to define, largely because what counts as progress depends on who is doing the defining. More complexity seems valuable to us, for example, but many organisms – especially parasites – are successful thanks to a reduction in complexity.
Energy flows
Rooting a new definition in basic physics would be one way around this problem.
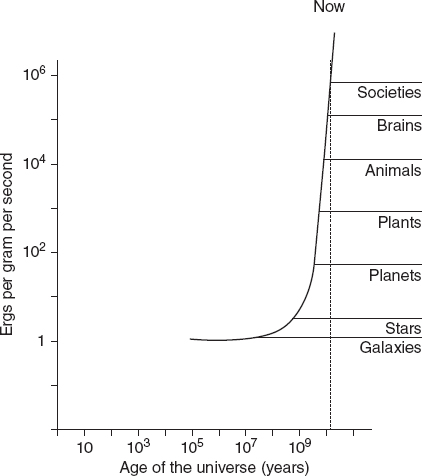
Eric Chaisson, an astrophysicist at the Harvard-Smithsonian Center for Astrophysics, Cambridge, Massachusetts, has put forward the idea of energy rate density, a measure of how much energy flows through each gram of a system per second. A star, for all its spectacular output, has a much lower energy rate density (2 ergs per gram per second) than a houseplant (3000 to 6000 ergs per gram per second). This sounds counter-intuitive until you remember that stars are just balls of gas.
Humans do better still, with a basic energy rate density of 20,000 ergs per gram per second. Societies, too, can be measured in this way. Chaisson estimates that hunter-gatherer societies have an average energy rate density of 40,000 ergs per gram per second, while technological societies use 2 million ergs per gram per second.
Chaisson argues that energy rate density is a universal measure of the complexity of all ordered systems, from planets and stars to animals and societies. Furthermore, when he plots the energy rate density of such ordered systems against the time they first appear in the history of the universe, the line goes unequivocally upwards, indicating a general increase in complexity over time (see Figure 8.1).
FIGURE 8.1 Growing energy density: as the universe ages, ever more complex systems evolve.
Thermodynamics
The second argument concerns thermodynamics. At first sight, the second law of thermodynamics is a gloomy affair. It seems to indicate that increases in disorder are inevitable and irreversible and that the universe is running out of the energy needed to create and sustain complex entities such as living things.
A literal reading of this law implies that the ascent of life is extremely unlikely. More nuanced readings, however, have been used to argue that local increases in complexity are not merely permitted by the law, but required by it, and order can and does emerge spontaneously from chaos.
Physicist J. Miguel Rubí of the University of Barcelona in Spain says that, strictly speaking, the second law of thermodynamics applies only to systems in equilibrium, a state in which nothing changes. This condition is rarely present in the universe. Earth, for example, is heated by the sun, creating energy gradients on its surface. Where energy gradients exist, pockets of complexity can arise even as the system as a whole decays into disorder. These pockets provide a foothold for further increases in complexity. Energy gradients thus provide a loophole in the second law that permits life to arise and ascend.
Convergent evolution
Argument number three is convergent evolution. Taking a different view to Gould’s argument, the tape of life has been rerun many times – at least partially. In many cases, very different species living in similar environments have independently evolved in similar ways.
In his book What Technology Wants (Viking Press, 2010), Kevin Kelly, the founding editor of Wired magazine, gives numerous examples of convergent evolution to support his argument that the outcomes of evolution – of which he considers technology one – are not accidental. Flapping wings evolved independently in birds, bats and pterodactyls. Dolphins, bats and several species of cave-dwelling bird separately hit on echolocation. Fish in the Arctic and the Antarctic independently evolved antifreeze compounds. Perhaps the best-known example is the camera eye, which has evolved independently at least six times. The implication, Kelly writes, is that many outcomes of evolution are not accidental but inevitable. These outcomes include not just organs but brains, minds, societies, and technologies.
Intelligence may be another convergent property. Nicola Clayton, a professor of comparative cognition at the University of Cambridge, and Nathan Emery, a cognitive biologist at Queen Mary, University of London, argue that while primates and crows are far apart on the evolutionary tree and have very different brain structures, they have independently evolved many similar kinds of cognition, including tool use, deception and complex social groupings. The implication, again, is that intelligence always emerges in favourable conditions.
Catastrophes
Last of all, a theory of development must account for catastrophism. The occurrence of unpredictable, planet-altering events is a challenge for any developmental perspective on evolution. Had the dinosaurs not been killed by an impact, critics of the theory say, mammals would not have had the opportunity to expand into new niches and there would have been no evolutionary sequence leading from primates to tool-wielding, language-using apes. In short: no impact, no us.
Simon Conway Morris, a palaeontologist at the University of Cambridge, counters this by arguing that while catastrophes delay or accelerate the developmental process they do not significantly change it. The key is convergent evolution.
Suppose the deadly meteorite had sailed harmlessly by, Conway Morris suggests. The dinosaurs would have survived for the next 30 million years until Earth’s next glaciation. The cold would have killed off those dinosaurs living north and south of the tropics, opening up niches for the warm-blooded mammals and birds that co-existed with them. Eventually tool-users not unlike us would have evolved and sooner or later any dinosaurs remaining in the tropics would have been hunted to extinction. ‘The mass extinction of the dinosaurs would then have been under way, perhaps 30 million years behind schedule in comparison with the real world,’ Conway Morris writes.
So whatever catastrophe hits, the tape of life would probably run more or less the same way. It might delay the developmental process by reversing an advance, but the advance would eventually happen again. Or it might accelerate the process by opening up an environmental niche. In either case, the outcome would not change substantially, only the timing would.
If these four arguments hold up, it would mean a significant expansion of evolutionary theory is needed, showing that life not only evolves, it develops.
The implications would be profound. Development, unlike evolution, has a direction: an acorn becomes a tree, an embryo becomes a newborn. It never goes the other way. And while the outcome is not fully determined, it is powerfully constrained.
Direction and constraint, however, do not imply design and purpose. A developmental view of evolution needs no help from teleology. Such a theory of evolution offers no support for intelligent design. Indeed, it would strike another major blow to it by offering a cogent naturalistic explanation for the emergence of complexity.
Perhaps more profoundly, admitting progress into evolution would give a different perspective on our own existence. In offering a naturalistic explanation for the emergence of intelligence and its offspring, language and technology, it would cast them as predictable outcomes of the cosmos rather than as accidents of contingency. Far from being ‘just one bauble’, we would have an explicable, even inevitable, place in the order of things.
Adapt first, mutate later: is evolution out of order?
We used to think evolution had to start with random mutations; now walking fish and bipedal rats are turning our ideas on their head. We have long known that our muscles, sinews and bones adapt to cope with whatever we make them do. A growing number of biologists think this kind of plasticity may also play a key role in evolution. Instead of mutating first and adapting later, they argue, animals often adapt first and mutate later. This process could even play a role in major evolutionary transitions such as fish taking to land and apes starting to walk upright.
The idea that plasticity plays a role in evolution goes back more than a century. Some early biologists thought that characteristics acquired during an animal’s lifetime could be inherited by their offspring: giraffes got their long necks by stretching to eat leaves, and so on. The French naturalist Jean-Baptiste Lamarck is the best-known advocate of this idea, but Darwin believed something similar. He even proposed an elaborate mechanism to explain how information about changes in the body could reach eggs and sperm, and therefore be passed on to offspring. In this way, Darwin suggested, plasticity produces the heritable variations on which natural selection can work its magic.
With the rise of modern genetics, such notions were dismissed. It became clear that there is no way for information about what animals do during their lifetime to be passed on to their offspring (although a few exceptions have emerged since). And it was thought this meant plasticity had no role in evolution.
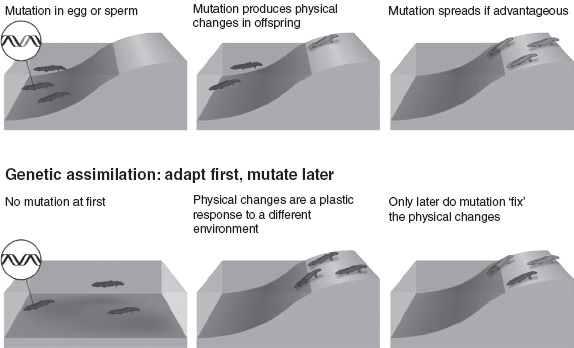
Instead, the focus shifted to mutations. By the 1940s, the standard thinking was that animals mutate first and adapt later. A mutation in a sperm cell, say, might produce a physical change in the bodies of some offspring. If the change is beneficial, the mutation will spread through the population. In other words, random genetic mutations generate the variation on which natural selection acts. This remains the dominant view of evolution today.
Standard model: mutate first, adapt later
FIGURE 8.2 Evolving without evolving.
The dramatic effects of plasticity were not entirely ignored. In the 1940s, for instance, the Dutch zoologist Everhard Johannes Slijper studied a goat that had been born without forelegs and learned to hop around, kangaroo-like, on its rear legs. When Slijper examined the goat after its death, he discovered that the shape of its muscles and skeleton looked more like those of a biped than a quadruped.
Few biologists considered such findings relevant to the evolutionary process. The fact that changes acquired during an animal’s lifetime are transient seemed to rule out that possibility.
Transient response
But what if the environmental conditions that induce the plastic response are themselves permanent? In the wild, this could happen as a result of alterations in prey animals, or in the climate, for instance. Then all the members of a population would develop in the same, consistent way down the generations. It would look as if the population had evolved in response to an altered environment, but technically it’s not evolution because there is no heritable change. The thing is, the only way to tell would be to ‘test’ individuals by raising them in different circumstances.
In this way at least, plasticity can allow animals to ‘evolve’ without evolving. The crucial question, of course, is whether it can lead to actual evolution, in the sense of heritable changes. The answer, surprisingly, seems to be yes. In the 1950s, British biologist Conrad Hal Waddington showed that it is feasible in an experiment involving fruit flies. Waddington found that when pupa are briefly heated, some offspring develop without crossveins in their wings. He then selected and bred those flies. By the 14th generation, some lacked crossveins even when their pupa were not heated. A physical feature that began as a plastic response to an environmental trigger had become a hereditary feature.
How is this possible? Plastic changes occur because an environmental trigger affects a developmental pathway in some way. More of a certain hormone may be produced, or produced at a different time, or genes are switched on that normally remain inactive, and so on. The thing is, random mutations can also have similar effects. So in an environment in which a particular plastic response is crucial for survival, only mutations that reinforce this response, or at least do not impede it, can spread through a population. Eventually, the altered developmental pathway will become so firmly stabilized by a genetic scaffolding that it will occur even without the environmental trigger, making it a permanent hereditary feature.
Genetic assimilation
Waddington called this process genetic assimilation. It may sound like Lamarckism, but it is not. The acquired characteristics don’t shape the genetic changes directly as Darwin proposed, they merely allow animals to thrive in environments that favour certain mutations when they occur by chance.
Waddington’s findings have been regarded as a curiosity rather than a crucial insight. But in the past decade or two, attitudes have begun to change. One reason for this is a growing appreciation of the flexibility of genes. Rather than being rigidly preprogrammed, we now know that the environment influences many aspects of animals’ bodies and behaviour.
Such discoveries have led some biologists to claim that developmental plasticity plays a major role in evolution. A few, such as Kevin Laland at the University of St Andrews, UK, even argue that the conventional ‘mutate first, adapt later’ picture of evolution needs a rethink (see Chapter 11). Most biologists have yet to be convinced.
The sceptics point out that genetic assimilation does not overturn any fundamental principles of evolution – in the long run, evolution is all about the spread of mutations, whether or not plasticity is involved. Yes, say the proponents of plasticity, but the key point is that plasticity can determine which mutations spread, so its role should be given the prominence it deserves.
All this still leaves open the question of whether genetic assimilation can ‘fix’ traits that first appear as a result of plasticity. A decade ago, Richard Palmer at the University of Alberta in Edmonton, Canada, found a way to search for evidence in the fossil record. Most animals have some asymmetric traits. In our case, it’s the position of the heart and other organs, which is encoded in our genes. But in other species, asymmetries are plastic. For instance, the enlarged claw of male fiddler crabs is as likely to be on the left as on the right.
What Palmer showed by examining the fossil record of asymmetry in 68 plant and animal species is that on 28 occasions, asymmetries that are now hereditary and appear only on one side started out as non-hereditary asymmetries that appeared on either side. ‘I think it’s one of the clearest demonstrations that genetic assimilation has happened and that it is more common than expected,’ says Palmer.
There is a caveat here, though. The ancestral non-hereditary asymmetries may have been a result of random genetic noise, says Palmer. So while his work does show genetic assimilation in action, it was not necessarily fixing traits due to developmental plasticity.
What is the role of epigenetics in evolution?
The term ‘epigenetics’ refers to an array of molecular mechanisms that affect the activity of genes. Epigenetic ‘switches’ turn gene activity up or down. They have long-lasting effects that can persist through cell division, and sometimes through sexual reproduction too. Here, geneticist Adrian Bird examines the evidence that epigenetic traits can be passed down through the generations.
We started to get the first glimpse of some of the mechanisms involved in epigenetic phenomena in the 1970s and 1980s. The first to be discovered was DNA methylation, which involves a small chemical subunit called a methyl group being added to DNA. Other epigenetic mechanisms include chemical changes to the proteins that package DNA. One of the most interesting aspects of epigenetics is the route it offers for the environment to influence our bodies and behaviour, rather than our genes – and that these traits might be transmitted to our offspring.
What is the evidence for epigenetic inheritance? For plants, it is strong. For example, a particular flower shape found in some toadflax plants is faithfully passed on between generations, yet it does not appear to involve any difference in DNA sequence. This ‘peloric’ flower form, which has been known for more than 200 years, turns out to be caused by silencing of a gene through DNA methylation. However, there is no evidence to suggest this is ‘adaptive’. In other words, the plant is not learning something in one generation that it commits to ‘memory’ epigenetically and then transmits to the next generation.
Evidence for transgenerational effects in animals is more scarce. The best example involves the effect of diet on an unusually coloured type of mouse called agouti. Normally, a litter of these mice has a range of coat colours from yellow to dark brown, thanks to the agouti gene. But if the pregnant mother is fed a diet high in certain vitamins and amino acids that are rich in methyl groups, she gives birth to more brown pups.
Another striking example is rat pups that are neglected by their mothers in the nest grow up to be skittish and timid adults. There is evidence that this is achieved through DNA methylation at a gene that regulates the response to stress. Methylation turns down the ‘volume control’ on the gene, resulting in permanent anxiety. According to the evolutionarily adaptive theory, this prepares the rats for a tough environment by making them more risk averse. This, however, is a within-generation effect, not one that is transmitted down the generations.
One (controversial) 2013 mouse study suggested that even the fear of a particular smell can be passed down epigenetically. Mice whose father or grandfather learned to associate the smell of cherry blossom with an electric shock became more jumpy in the presence of the same odour, and responded to lower concentrations of it than normal mice. And a 2016 study on frogs provides the strongest evidence yet that a father’s lifestyle may affect the next generation. The research showed that sperm epigenetic tags change gene expression in embryos.
What about humans? Might the epigenetic consequences of starvation, neglect or disease be inherited across human generations? One study showed that people with a grandparent who went through a famine as a teenager died earlier, on average, if they were the same sex as the starved grandparent. The implication is that the experience of starvation changed the epigenome and that this effect was faithfully transmitted over two generations to compromise the health of the grandchildren. Studies of this kind are statistical and retrospective, so it is hard to investigate what is going on at a molecular level. Besides, it is difficult to rule out that these effects were transmitted through culture rather than epigenetics. Large-scale mapping of human epigenomes in relation to experience and disease is under way and may help resolve this conundrum. Even then it will be difficult to rule out the possibility that culture and behaviour within families is responsible for the transmission, rather than epigenetics.
Evolution through epigenetics?
If individuals can acquire characteristics through interaction with their environment and then pass these on to their offspring, does this force us to rethink the gene-centred view of evolution and the idea of the gene as the basic unit of inheritance? A growing number of biologists think so (this is discussed in detail in Chapter 11). But not everyone agrees.
‘The possibility that characteristics acquired during an individual’s lifetime are epigenetically memorized and transmitted to the next generation has led to a revival in some quarters of the hitherto discredited theory of evolution known as Lamarckism,’ says geneticist Adrian Bird of the University of Edinburgh. ‘The consensus view, supported by a mountain of evidence, is still that evolution proceeds by natural selection among genetic variants that arise by accident. Much of the data is unreliable, but this has not stopped the spread of the idea that the environment “talks” to the epigenome and can ensure transmission of desirable (or, more often) undesirable traits without mutations. Nevertheless, epigenetic mechanisms could play some minor evolutionary role.’
In theory, what does Richard Dawkins, author of The Selfish Gene think? ‘The “transgenerational” effects now being described are mildly interesting, but they cast no doubt whatsoever on the theory of the selfish gene,’ he says. He suggests, though, that the word ‘gene’ should be replaced with ‘replicator’. This selfish replicator, acting as the unit of selection, does not have to be a gene, but it does have to be replicated accurately, the occasional mutation aside. ‘Whether epigenetic marks will eventually be deemed to qualify as “selfish replicators” will depend upon whether they are genuinely high-fidelity replicators with the capacity to go on for ever. This is important because otherwise there will be no interesting differences between those that are successful in natural selection and those that are not.’ If all the effects fade out within the first few generations, they cannot be said to be positively selected, Dawkins points out.
Is evolution predictable?
Evolutionary biologists have long debated whether rewinding the tape of life and replaying it would give similar results, or whether outcomes depend largely on chance events that push the course of evolution onto radically different tracks.
The two alternatives yield very different views of the history of life on Earth, with some prominent biologists, such as Simon Conway Morris, arguing that human-like, intelligent beings are inevitable products of evolution.
Others, such as palaeontologist Stephen Jay Gould, who popularized the tape of life metaphor, argue that if it were possible to turn back the clock, the history of life would not repeat itself. The world would be unfamiliar, and most likely lack humans.
Many studies have tested the reproducibility of evolution at the genetic level. In one, an international team took advantage of a natural experiment. Three different groups of terrestrial mammals have at some point in their evolution re-colonized the ocean, giving rise to what we now know as whales, walruses and manatees. Comparing the genetic changes in the three lineages, the researchers reasoned, should reveal whether evolution followed similar or very different paths in each case.
They sequenced the genomes of manatee and killer whales and bottlenose dolphins. The comparisons showed that many genes changed independently in each lineage, suggesting that randomness did indeed play an important role in their evolution.
But for 15 genes, natural selection led to exactly the same genetic changes occurring in all three lineages. This suggests that for some of the challenges of life in the sea, evolution repeatedly arrived at the same solution – that is, replaying the tape does indeed give much the same result again and again. This is a high-resolution replay of the tape, looking at what would happen to individual lineages, rather than what overall diversity would eventually result, which is what Gould looked at.
However, this result may say less about the predictable creativity of evolution than about a paucity of viable options. When the team performed a similar analysis of the genomes of dog, elephant and cow – related mammals that remained on land – they also found a comparable amount of convergence in their mutations, even though those animals share few similarities of lifestyle.
Lack of options
This may imply that the vast majority mutations are lethal, so that evolution stumbles on the same few viable ones over and over again. Perhaps there is only so much that can be changed and still be functional.
Another study of the predictability of evolution looked at populations of the bacterium Pseudomonas fluorescens that rapidly diversify by mutation and selection into distinct types or ‘morphs’ when grown in test tubes. It found that when these mini-worlds are seeded with genetically identical microbes and the population size is large (around a billion cells per millilitre), each ‘replay’ results in highly similar patterns of evolutionary change. After just one week, P. fluorescens evolves into two new morphs called ‘wrinkly’ and ‘fuzzy’ spreaders. But this doesn’t happen if the mutation supply rate is limited, reducing it by more than two orders of magnitude. Evolution only repeats itself if certain phenotypic innovations have a high probability of arising and are strongly favoured by selection.
If bacterial colonies that start out as identical clones evolve down different routes to reach a similar end point, what about colonies that start off as distinct? This has been studied using different P. fluorescens starter cultures with deleted genes encoding critical components of wrinkly spreaders and then allowing these ‘defective’ colonies to evolve. In all instances wrinkly spreaders eventually emerge by co-opting alternative genetic systems and structural components to bring about the necessary change. So, in the face of similar selective conditions, different lineages can find similar solutions to the same problems.
Replay life’s tape, then, and while Homo sapiens may not evolve there is a high probability that introspective bipedal organisms with binocular vision will.