19
The large body of work done on the dog genome has thrown up many interesting facets of the molecular mechanisms which lie behind the transformation from ancient wolf to modern dog. Unsurprisingly, the greatest successes have been in explaining the physical changes. They are, after all, considerably easier to study.
Modern geneticists live in a digital world where mutations in individual genes lie at the heart of their world view. There was a time, until about ten years ago, when we (and here I am counting myself among their number) dreamed that DNA would explain the whole of biology and medicine. Such youthful arrogance is characteristic of a new discipline and we were certainly not lacking in that department. As time went on, the cracks began to show. The ‘gene’ for something discovered in one laboratory had mysteriously disappeared when another research team tried to find it. As the geneticist, author and wit Steve Jones once remarked of the frustrations that plagued the gene hunters, the whole fiasco resembled the antics of T. S. Eliot’s feline hero Macavity:
Macavity’s a Mystery Cat: he’s called the Hidden Paw –
For he’s the master criminal who can defy the Law.
He’s the bafflement of Scotland Yard, the Flying Squad’s despair:
For when they reach the scene of crime – Macavity’s not there!1
In fact it was in an effort to ease the frustration caused by Macavity’s unpredictable peregrinations around the human genome that so much money and effort was poured into its canine equivalent, and for this we must be grateful to the cheeky feline. And, my goodness, the human genome is certainly covered in his rascally paw prints.
In a recent paper Jonathan Pritchard and his colleagues at Stanford University reviewed the findings of a large number of searches for genes controlling human height.2 There were a bewildering 697 locations in the human genome (and by implication at least the same number of genes) that between them accounted for only 16 per cent of the variance. Pritchard and his team searched again for the ‘missing’ genes, the equivalent of cosmology’s dark matter. They were dismayed to find that innocuous common variants, each with tiny effect, accounted for an astonishing 86 per cent of the heritability. They went on to make the sobering estimate that 62 per cent of all common SNPs affect height, very few of them lying in protein-coding regions.
Surely it would be easier to find important genes in the dog. And I am glad to report that so it has proved. This is largely because, unlike humans, dogs are drawn from a limited lupine gene pool in the first place, which has been further reduced by selection and, in pedigree dogs, closed breed formation, as we have seen. This makes gene-hunting a lot easier in dogs than it is in humans where, outside closed communities such as the Old Order Amish, mating is more or less random. In vivid contrast to the hopelessly muddled human situation, characteristics like body size in dogs are indeed controlled by only a few genes that in several cases have been identified. Even though the whole range of size, shape and general appearance is immensely wide in the domestic dog – more so than in any other species – only relatively few genes are involved in this morphological cornucopia. From the sleek and powerful Great Dane to the diminutive Pomeranian, from the deep-pile luxury of the handsome Komodoro to the Chinese Crested, practically amphibian in its nakedness, their varied morphologies are explained by just a few genes.
The first study to discover the genes contributing to overall size used a large collection of Portuguese Water Dogs, 330 in all, recruited through the Georgie project – a not untypical combination of public participation in a science project.3 Georgie was a Portuguese Water Dog that her owner Gordon Lark had acquired as a stray in 1986. In his own words, he quickly fell in love with her and with her breed, which had been a favourite among Portuguese coastal fishermen. As the name of the breed suggests, they love the water and are superb swimmers both above and below the surface. As a breed they are ‘fiercely loyal and with boundless energy’, though I must add here that I have never read a breed description that was anything less than flattering. Georgie died in 1996 of an autoimmune disease, and in a series of events reminiscent of the reaction to the tragic death of a child, the project that bears her name was born. Its stated aim was to use modern science to investigate and, one day perhaps, to find a cure for Georgie’s illness.
The project came to the notice of Elaine Ostrander, one of the handful of experienced canine geneticists, who chose the Portuguese Water Dog as the subject for her study of the genetic factors involved in skeletal morphology. Having myself been involved with projects requiring large numbers of (human) subjects, I can tell you that having the enthusiastic backing of motivated volunteers is absolutely central to their success. All the dogs were X-rayed and a series of measurements taken which described the morphology of each dog in mathematical terms, which could then be amalgamated into a single numerical description.
Importantly, the Portuguese Water Dogs used by Ostrander came from a limited number of founders made up of only 31 dogs some 24 generations in the past. Using 460 Portuguese Water Dogs and a series of 500 microsatellite markers, the forerunners of SNPs, Ostrander went through the genome segment by segment, looking for those markers that correlated most closely to the skeletal metrics. Knowing the details of each pedigree, she was able to assess the degree to which these metrics were inherited. It is certainly a complicated process but the outcome is relatively straightforward – a map of the genome showing the approximate locations of the genes involved in shaping the skeleton. Whereas as we saw earlier a rather similar analysis in humans by Jonathan Pritchard identified hundreds of places along the human genome that harboured genes controlling height, the Georgie project identified only six of any significance. These are gene locations rather than genes per se, so further work was needed to sift through the regions until the genes themselves were revealed. The forerunner to the dog genome project had already identified a promising gene called IGF1 very close to the most influential segment in the Portuguese Water Dog. IGF1, or, to give it its full name, insulin-like growth factor number one, is a protein involved in the activation of growth hormone and is widely implicated in bone and tissue growth. It is known to be faulty in the human growth disorder Laron dwarfism. However, despite high expectations there were no variations within IGF1 which could explain any effect on growth in the Portuguese Water Dog. More than likely the explanation lay in a genetic change close to the IGF1 gene which somehow modified its behaviour. However, what really excited Ostrander and her team was that the very same genetic segment containing IGF1 identified in the Portuguese Water Dog was present in all small dog breeds and rare or absent in all large breeds.4
The conclusion from this unexpected finding was that overall size in dogs was primarily controlled not by hundreds of different genes, as in humans, but by a single gene located very close to IGF1. The fact that it was found on the three different genetic backgrounds in various breeds suggests that either the mutation happened three times independently or is an old one which has spread by what is called a selective sweep into all miniature and toy breeds. There could not be a more dramatic demonstration of the huge morphological change introduced by a single gene. It meant that all toy breeds were originally created by crossing miniature versions of one breed with normal-sized dogs of another, followed by rounds of selection for the smaller offspring. Correspondingly, in Heidi Parker’s 2017 phylogram, toy breeds are grouped together irrespective of their affinity to their original functional grouping.5
Other research has taken a slightly different direction by concentrating on an intense study of known genes with suspected relevant actions. This so-called candidate gene approach can be very successful if the candidate is a good one, or it can be a complete failure if not. Two genes known to be involved in human skull development called MSX2 and TCOF1 were sequenced in ten different dog breeds, each having a different skull or face shape. A single mutation in the TCOF1 gene which introduced a single amino-acid change in the associated protein was highly correlated in the different breeds with faces that were short and broad.6
Not all candidate gene surveys hit the jackpot but they are nonetheless revealing.
Mutations in one of the many collagen genes, called COL10A1, are responsible for the rare inherited human long-bone disorder Schmid metaphyseal chondrodysplasia, whose characteristics are short stature combined with abnormally short arms and legs. When this gene was intensively studied in dog breeds such as Dachshunds and Corgis showing the same features as Schmid dysplasia, it was shown to be completely normal in every case.7
In contrast, what seemed like an unlikely candidate turned up trumps in a study of whippets. A mutation in the myostatin gene, which requires a double dose to have any effect, produced a double-muscled version known as the ‘Bully Whippet’. Whippets, like their close relatives the Greyhound, are slim bodied, deep chested and fast. Not surprisingly, breeders, with an eye to winning races, select the swiftest animals for mating to produce the next generation. They are acutely aware of the conformation of their dogs and several breeders noticed the occasional appearance of visibly enhanced muscles in a litter of pups. These pups grew up to become excessively heavy and strong and thus unsuitable for racing. They are usually put down. However, breeders also noticed that the parents of the Bully Whippets and some of the littermates were slightly more muscular than normal and also faster on the racetrack. This combination of mixed litters of Bully Whippets, partial ‘bullies’ and normal pups is typical of what is called a recessive genetic condition. The full ‘bullies’ had inherited two copies of the ‘bully’ gene, whereas the partial bullies received only one and the normal pups none. This pattern of inheritance is reproduced in all recessive genetic conditions, including the well-known human disorder cystic fibrosis.
Both the Bully Whippet and patients with cystic fibrosis and other recessively-inherited disorders are at a significant breeding disadvantage, the dogs because they are put down and the humans because they are likely to be incapacitated before they have children. This raises the very interesting question of why these conditions are as common as they are, since the genes responsible are being continuously eliminated by selection every time an affected individual, dog or human, fails to breed. In fact, not one but two copies of the gene are lost from the population as a whole when these ‘double dose’ individuals, or homozygotes to use the scientific term, die without leaving offspring.
The answer is an elegant and important one for understanding how genetic conditions become established. Although individuals, dogs or humans, with double doses of a mutant gene, that is the homozygotes, have fewer offspring, some of their siblings, or litter mates, will have one mutant and one normal gene. If these carriers, called heterozygotes, possess some advantage over individuals carrying two copies of the normal gene, they will prosper. In the case of Bully Whippets, carriers can run faster than regular dogs and will be chosen for breeding. The same rule applies to humans. Carriers of the cystic fibrosis gene must have had an advantage, not necessarily now but in the past, and the most likely is an increased resistance to cholera. In a thoroughly researched tropical disease called thalassaemia, which is very common in the Mediterranean and in south-east Asia, it is the advantage conferred on carriers in being able to resist infection by malaria that gives them the edge. The disease persists because, even long after malaria has been eliminated, as it has in the Mediterranean, the genes are still there.
The genetic explanation for the Bully Whippet is equally interesting and owes its solution, like many genetic mysteries, to the observation of a single human patient by a keen-eyed doctor. In this case the location for the breakthrough was the paediatric department of the University Medical Centre in Berlin.8 After a normal pregnancy, a healthy woman gave birth to a son. Soon after birth the boy developed sudden involuntary muscular contractions, called myoclonus, and was admitted to the neonatal ward for observation. He appeared to be extraordinarily muscular, with bulging thigh and arm muscles, but was otherwise completely normal. The myoclonus subsided after a couple of months and the boy continued to develop normally though he remained extraordinarily muscular. His case report and genetic investigation were written up when he was four and a half, by which age he was able to lift two 3-kilogram dumbbells with his arms straight out.
Research into the cause of his condition chose the candidate gene approach with the myostatin gene as its focus. Myostatin itself is a protein which, like IGF1, is a growth factor, but instead of being involved in bone growth its effects are on muscle development. The choice of myostatin as a candidate was a good one, and that was indeed the location of the mutated gene.9 However, the mutation itself, far from leading to an increased level of myostatin in the German boy, actually cancelled it altogether. It turned out that myostatin slows down muscle development, so when there is no myostatin its dampening effect is removed and muscle growth proceeds unchecked. This is a common type of mutation, called a ‘null’, giving the boy a homozygous null/null genotype. As it’s a recessive condition, both of his parents must have been carriers with null/wild type (wild type being the geneticist’s label for normal) genotypes, which, as we have seen, probably confers some advantage. Indeed, the boy’s mother was in her youth an Olympic-class swimmer and many of her relatives were unusually strong. This raises the interesting ethical point, entirely incidental to our consideration of the Bully Whippet, as to whether carriers of this condition have an unfair advantage in competition. The general consensus is that though the partial inactivation of myostatin might confer an advantage, it is but one of the multitude of genetic influences which elevate elite athletes above the rest and should not be discriminated against.
The myostatin mutation in the Bully Whippet, as with the young boy from Berlin, was one that inactivated the gene, but it did so in an interesting way. Most mammalian genes are separated into two classes of DNA sequence. The first, called the exons, contain the DNA sequence which directly encodes the amino acid sequence of the gene product, in this case the myostatin protein. Any mutation in the coding regions of these exons risks changing the amino acid sequence of the protein with potentially disastrous consequences. On sequencing the myostatin gene in twenty-two whippets, researchers discovered that all four ‘Bullies’ were missing a small DNA segment only two base pairs in length. This was enough to completely inactivate the gene because cells read genetic instructions in groups of three. The sequence determines not just the order of amino acids in the final protein but also their identity.
The two-base deletion causes what is called a frameshift mutation. The cell doesn’t know whether the DNA sequence it is reading is the correct one or not, it just carries on adding amino acids one by one in linear order according to the instructions it receives from the DNA sequence. The protein strand grows from one end and terminates at the other. In the Bully Whippet the myostatin strand, following instructions from the myostatin gene, grows normally for the first few hundred amino acids. Then it encounters the deletion, which causes a frameshift. Because the sequence is read in groups of three nucleotides, the amino acid sequence on the downstream side of the mutation is completely jumbled. Worse still, the mutation converts the normal three-base signal for the amino acid cysteine into one which promptly terminates the synthesis of the protein and destroys its function. All this because of a two-base deletion in a genome hundreds of millions of bases long. That is all it takes.
Strictly irrelevant in a book about dogs but nonetheless interesting is a historical observation dating back to 1807 of a similar double muscling in Belgian Blue cattle, which turns out to be due to a very similar mutation in the myostatin gene.10 Unlike whippet breeders, concerned as they are with speed, where the muscular Bully Whippets are too bulky to compete and are disposed of, breeders of beef cattle have been very keen to select for the bulky bovines. Although breeders knew nothing of the molecular genetics at the heart of the issue, herds of Belgian Blues and the closely related Piedmontese breeds have been built up by carefully controlled breeding so that all the animals now carry the myostatin mutation in double dose.
In situations like this where all the animals carry the mutation, it is said to be ‘fixed’. Fixation of the double muscling myostatin mutation is complete in those herds of Belgian Blues because breeders have intentionally selected it. In the more usual situation the population as a whole contains mainly wild-type individuals with a smattering of mutants whose future career depends on how fast they are eliminated by selection. Without a compensating carrier advantage, damaging gene mutations tend not to last for more than a few generations.
There is a well-known example of a mutation that has become fixed in one dog breed, the Dalmatian. All Dalmatians, not just some, suffer from very high serum levels of uric acid. In humans high serum uric acid leads to gout when the uric acid forms crystals in the kidney and in the joints, particularly the big toe. Uric acid is the final waste product from the breakdown of purines, one of the chemical components of DNA, but hyperuraemia, as a high blood level of uric acid is called, is only a problem in humans, great apes like gorillas and chimpanzees – and Dalmatians. All other species and all other dog breeds break down purines in a different and less troublesome fashion.
The human gene involved in gout had already been identified, so the first step to tracking down the Dalmatian mutation was to sequence the gene in the dogs. However, this led nowhere and the search for the gene had to begin all over again. After a great deal of work involving crosses between Dalmatians and Pointers, the gene was located to an area of the genome containing just four genes. Detailed sequencing of these genes revealed that the causal mutation was a single DNA base change in a gene called SLC2A9, which encodes a protein that helps glucose and uric acid cross cell membranes.11 Unlike the deletion we saw in the Bully Whippet, this change did not alter the reading frame but it did switch the amino acid sequence at that point, substituting a phenylalanine for a cysteine, which is enough to inactivate the gene. Although Dalmatians are the only breed in which all dogs suffer from hyperuraemia, a survey of other dogs which had been diagnosed with the disease showed that in two breeds, Bulldogs and Russian Black Terriers, some dogs carried precisely the same mutation. This must mean that the mutant gene had originated before Dalmatians became genetically isolated as a breed.
An interesting question is why the ‘gout’ mutation is fixed in the breed. Why are all Dalmatians homozygotes for the mutant gene, while it is very scarce in other breeds? The answer lies in a nearby gene which enhances the appearance of the characteristic spots on the dog’s coat, a genetic trait that appealed to breeders and led to selection not just for this benign coat-pattern but also for its far from harmless neighbour. Being so close to each other on the same chromosome, these two genes are inherited together every time.
In the Dalmatian, as in most dog breeds, appearance matters a lot. It’s unfortunate that the characteristic and highly desirable spotting pattern in the breed brings with it a serious disease just because of the proximity of the piebald coat-pattern and purine metabolism genes. Selection for one was inevitably linked to selection of the other, albeit unintentionally.
Appearance has always been important for dog breeders, as compliance to rigid breed standards is so influential in judging competitive shows. Important among these, perhaps the most important, are the characteristics of the coat – its colour, thickness, curliness and so on. It was natural therefore for the architects of the dog genome project to try and find the major genes responsible for these important features. Such is the variety of patterns and textures that one might be forgiven for thinking that dozens or even hundreds of genes might be involved. But one of the hopes of the dog genome project was that, in comparison to humans, apparently complex situations might be explained by just a few genes.
To test this important principle the team at the Broad Institute at Harvard that sequenced the dog genome threw their whole impressive technical armoury at the project. They chose to focus the search on two features already known to have a conventional form of inheritance – the ‘ridgeless’ form of the Rhodesian Ridgeback and white coat-colour in Boxers. Both traits are simple recessives, meaning that both parents have to be carriers. However, it is clear that this technical tour de force had wider ambitions than locating two quirky genes in two minor breeds (if Rhodesian Ridgeback and Boxer owners will forgive the slight). The Broad team was clearly trying to formulate a general approach to finding interesting dog genes, and these two were working examples to demonstrate the success of their methods. In the genetic equivalent of saturation bombing, they selected 27,000 equally spaced SNPs across the canine genome, then typed 250 dogs of various breeds and measured the average length of DNA haplotype blocks inherited through the generations. They found that haplotype blocks within breeds are generally long and contain only a handful of common haplotypes. This they interpreted as a reflection of the population history of the breed.
For example, although the Shiba Inu breed is an ancient one, its numbers were drastically reduced by the Second World War and it was nearly wiped out. A few dogs survived, and all today’s Shiba Inu are descended from these lucky few – and are all very closely related. Consequently, the average length of haplotype blocks in the Shiba Inu is long, the longest of any breed. In contrast, the Greyhound, also an ancient breed, has not suffered from a catastrophic population decline. The Greyhound has maintained a large breeding population and has the shortest average haplotype block length, abbreviated to LD, of any breed. Even so, the LD of all pedigree breeds is much longer than in dogs in general, and in feral dogs especially where mating is completely random. The longer LD in pedigree breeds is explained by the relatively small number of founder animals for any breed involved in its creation. LD values for dogs in general are much higher than the equivalent wild populations of wolves from which they descend. This level of LD is a reflection of the relatively small number of wolves involved in the creation of ‘domesticated’ dogs in the first place, followed by thousands of years of selection.
It did not take long for the Broad’s saturation mapping campaign to identify the regions of the genome wherein resided the two targeted genes. In the Rhodesian Ridgeback, the dorsal ridge of inverted hair which is the characteristic of the breed was soon located to a 750 kb region of chromosome 18 containing five known genes, three of which are growth factors involved in embryonic development.12 Having located the genes, the next step, rapidly accomplished, was to sequence them and find out what was going on in the Ridgeback. The answer turned out to be a different type of mutation than we have covered in the Bully Whippet and the Dalmatian. There the mutations had deleted short segments of DNA. In the Rhodesian Ridgeback the opposite had happened. A large chunk of DNA containing all three growth factor genes had been duplicated. As you will begin to appreciate, the genome can get up to all sorts of tricks. The effect of the duplication was to give the dogs a double helping of these growth factors which did them no good, other than creating the dorsal ridge which defines the breed. In the uncommon ‘ridgeless’ variant there was no duplication and just the normal arrangement of these growth factor genes, just like other breeds. So the ridge on the Ridgeback is a consequence of deliberate selection for the feature. Unfortunately, the ‘ridgeback’ mutation also predisposes dogs to neural tube defects similar to dermal sinuses in humans, a similar type of defect to spina bifida. Once again, as in the Dalmatian, selection for one characteristic feature also brings with it a less welcome companion.
When the Broad team turned its attention to the Boxer they were looking for the gene responsible for the absence of skin and coat pigmentation. Once again this is a recessively inherited trait, with carrier parents having an intermediate colouration with patches of white on a solid background. Earlier breeding experiments in the 1950s had suggested that the same genetic flaw was behind so-called ‘Irish spotting’ in the Basenji and Bernese Mountain dogs and piebald coats in Beagles, Fox Terriers and English Springer Spaniels.
The saturation mapping technique that had proven so successful with the Rhodesian Ridgeback soon narrowed the Boxer pigmentation gene to a one megabase (1 million base) segment that contained just one gene.13 This gene (MITF) is important in embryonic development and is mainly concerned with the production of melanocytes, the cells which produce the pigment melanin.
In the Boxer, it proved difficult to narrow down the mutation to a particular set of DNA bases because there was not enough genetic variation. This made it impossible to distinguish mutations which caused the pigmentation and others that were just normal variations. Lack of genetic variation is the flip side of hunting for causal mutations in closed pedigree breeds. In order to overcome this issue the team used a second species, the Bull Terrier, where the genetic variation was greater and which has a similar white version. Even so, they could not be completely confident of identifying the DNA change which caused the white pigmentation. This too is not an uncommon result, even with the impressive array of genetic tools recruited to the task by the Broad Institute, but they were eventually able to locate it to MITF encoding a cell transcription factor, a family of proteins involved with turning particular genes on and off.
The same gene (MITF) is also implicated in Waardenburg syndrome in humans. Here it is associated with deafness, cleft lip, a white streak in the hair and sometimes brilliantly blue eyes or even eyes of different colour – or heterochromia. The arresting visual appearance of heterochromia lends an alien quality to those who have it, notably David Bowie in his incarnation as Ziggy Stardust. And who can forget the arresting face of the young Afghan girl with piercing blue eyes who reminded us all of the human cost of that terrible war? Genetics makes the most unexpected connections.
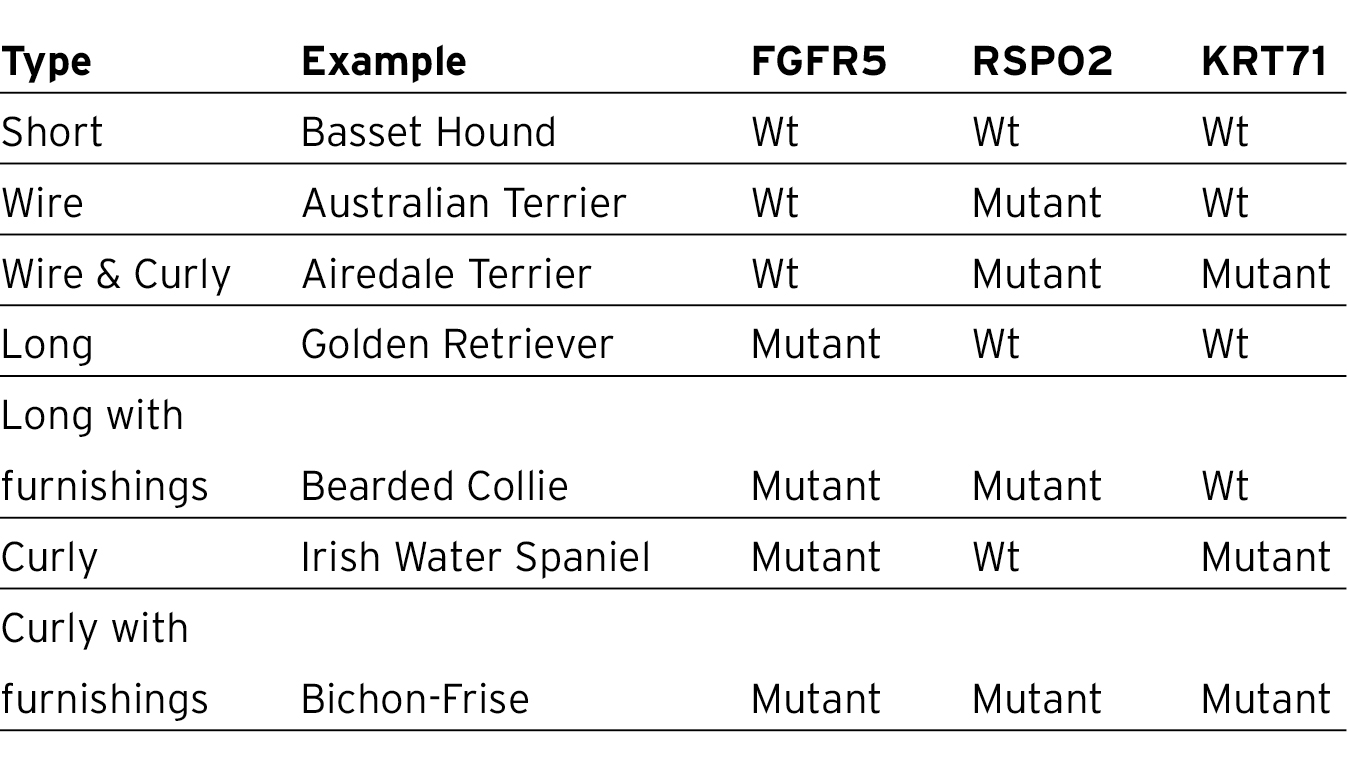
A couple of years later the veteran duo of Robert Wayne and Elaine Ostrander published a wide-ranging genetic study on coat variation which came to the remarkable conclusion that only three genes were between them responsible for the dizzying array seen in domestic dogs.14 Following the successful and by now familiar saturation mapping used by the Broad Institute team, Wayne and Ostrander concentrated on three coat characteristics. They chose first the presence or absence of ‘furnishings’, the name given to the eyebrows and moustache typical of wirehaired dogs such as the Highland Terrier. Second was hair length, and finally a third characteristic – whether the hair was straight or curly. They first typed the SNP panel in 96 Dachshunds with three different coat-colour varieties – wirehaired with furnishings, smooth, and finally long-haired. This located the furnishings gene to a segment of chromosome 13 that contained just one gene – RSPO2, an excellent candidate that had already been implicated in the development of hair follicle tumours which occur predominantly in breeds that have furnishings. As with some other canine genetic features there is a human equivalent – an East Asian hair type with some resemblance to wirehair in dogs. This is not caused by the same gene but by one called EDAR, which is also involved in the same developmental hair growth pathway.
The furnishings mutation turned out on detailed analysis to be yet another type that we have not yet encountered. There was nothing abnormal about the RSPO2 exons; the sequences were all entirely normal. However, there was an insertion just outside the gene itself which, it turned out, modified its expression, increasing it threefold and presumably leading to the hirsute character of the dogs that possessed it.
The team then turned their attention to fluffiness in Welsh Corgis, a breed in which dogs can be separated into long-haired, and therefore fluffy, and shorthaired non-fluffy individuals. Typing both varieties by saturation mapping pinpointed the mutation to one of the growth factor genes, FGFR5. This time the mutation was a familiar one, a straightforward substitution of amino acid, phenylalanine, for another, cysteine. You may have noticed that cysteine features a lot in the mutations we have illustrated. This is because cysteine forms connecting molecular bridges between protein chains and, more often than not, these connections are essential for making sure the protein chains come together in the correct orientation.
In order to investigate curliness the team chose that old favourite, the Portuguese Water Dog, which occurs in two forms, curly haired and wavy haired. The same mapping technique soon found that the gene responsible for these two hair types was a member of the keratin family and the mutation was a straightforward substitution of a single amino acid arginine for another, tryptophan, caused by a single base change.
What makes this energetic survey especially noteworthy is that by studying combinations of only three genes they had identified pretty well all variation in hair type seen in all breeds. For example, Basset Hounds have wild-type alleles at all three genes, resulting in a shorthaired non-curly coat without furnishings. Wirehaired Australian Terriers possess the wild-type variants (Wt) at FGFR5 and KRT71 but the mutant form of RSPO2 which confers a wired coat form. To avoid unnecessary repetition, I have summarised the outcome in the table below.

None of the three mutant genes was found in three grey wolves, an admittedly tiny sample, nor in any of the shorthaired dogs. This strongly suggests that the ancestral type was shorthaired, without furnishings and not curly. The implication of this is that all breeds with any of these derived features started off at some stage in the past with the ancestral type and that the mutant forms were introduced at a later stage during breed formation.
The saturation mapping that was so successful in localising these three genes also showed that the haplotypes surrounding each of them was exactly the same in all breeds. This means that, rather like the IGF1 gene for size, these three coat-type genes were each initially confined to one breed, even one litter, and then, through selective mating, spread to all the others.
Finally in this consideration of the effects of mutations I will turn to colour. In most mammals natural pigmentation is controlled by the melanocortin 1 receptor pathway. This pathway moderates the type and amount of melanin pigment made in the skin and hair. The huge range of colour that we see in the animal kingdom is due to just two forms of melanin: the red/yellow pigment pheomelanin, and the brown/black eumelanin. The subtle range of colouration is due to the way melanocytes moderate this very restricted palette. There are a few other genes involved, notably within what is called the ‘K locus’, which produces a protein capable of modifying the melanocortin receptor pathway and was found to be crucial in very black dogs. Melanistic dogs are unsurprisingly derived, presumably by selection, from the wild-type ancestor. However, the study that brought this to light came up with a major shock when it included melanistic wolves. Black wolves are only found in North America, except for a few in Italy.
In an effort to locate the melanistic gene, or genes, in North American wolves, the research team, which included the omnipresent Robert Wayne, made arrangements to study the Leopold pack, one of the wolf packs to form after the reintroduction of Canadian wolves into Yellowstone National Park in 1995. The subsequent activities and breeding structure of the pack have been intensively studied and a precise genealogy constructed. The Leopold pack contains both black and wild-type individuals, so it was comparatively straightforward (other than catching them, obviously) to see whether MCR1 or the K locus was inherited along with the colouration. This study showed a perfect split between genetic variants at the K locus and whether or not wolves were black. So far so good.

Resident Yellowstone wolf pack keeping American Elk in the river. Note the colour variation among the wolves. (Getty/Donald M. Jones/Minden Pictures)
The surprise came with the genetic analysis of the same region in melanistic domestic dogs. This showed beyond doubt that the mutation, a three-base pair deletion, and adjacent sequences were exactly the same in both wolves and dogs. This had to mean that, contrary to the usual flow of genes from the wolf to the dog, the K locus had gone the other way both in North America and, it transpired, also in Italy. The black wolves were black because they had inherited the melanistic mutation from domestic dogs. This gene, which has spread into the wild wolf population since domestic dogs reached North America from Asia along with the first humans some 12,000 to 15,000 years ago, is itself under the influence of selection. However, the selection among wild wolves is not artificial but natural, as a survey in the Canadian Arctic showed. In the far north and east where the barren tundra predominates, black wolves are rare, presumably because they are at a disadvantage during the winter when they stand out against the snow, making it harder for them to track prey. However, further west and south the proportion of black wolves increases as tundra gives way to forest, where the dark colouration is a helpful camouflage. It has been known for a long time, and Darwin himself noted it, that Native Americans encouraged the occasional mating of their own dogs with wild wolves in order to introduce a fresh dose of the wild into their dogs. They would tether females in heat to trees and leave the rest to nature. There would need to be some modifications to transfer dog genes to wolves, but somehow that is what happened.
So far we have only considered genetic traits in the dog that affect its physical aspects – size, coat-colour, musculature and so on. These have a rich variety of causes involving a range of different mutation types in a large number of genes. The genetic complexity of these changes, great as they are, have been solved thanks to our recently acquired detailed knowledge of the dog genome. Through this, researchers have identified candidate genes which, given a bit of luck, have often turned up trumps and have been shown to be the location of causal mutations. However, the creation of a set of equally spaced genetic markers covering the entire genome has led to the ability to locate genes when inspired guesswork fails. These saturation mapping techniques make no assumptions about the nature of the genes involved and just plough through the genome until their locations are revealed.
Once those are mapped, like an X marked on a treasure chart, detailed sequencing around the location soon reveals the gene itself and usually the exact mutation behind the trait. The modern structure of pedigree breeds, which have become increasingly closed genetic systems, helps a great deal in these gene mapping enterprises, although, as we have seen, the accompanying genetic isolation promotes inbreeding and the appearance of often damaging recessive traits. All the recent successes depend not only on the extravagant technology but also on a basic requirement of the genetics. To map a gene you need a feature which varies within the breed and which is relatively straightforward to score. For example, the experiments with coat-colour mapped the melanism gene because it was easy to tell which dogs were black and which were not. The trait is said to ‘segregate’ within the breed. If a trait does not segregate and all dogs are the same, then the genes responsible cannot be mapped however many markers are used. Candidate genes can still be scrutinised for obviously disabling mutations like frameshift deletions, but, as we have seen, this is very much a hit-or-miss affair.
The great successes over the last decade or so since the dog genome was published in 2005 have been in identifying genes responsible for physical genetic traits like coat-colour or inherited disorders. Of at least equal and perhaps greater interest is the genetics which lies behind differences not in appearance but in behaviour. These might be between breeds or between different individuals within the same breed. For example, a bloodhound makes a good hunting dog but is hopeless at herding sheep. Equally an Old English Sheepdog will control a flock of sheep with ease but would be next to useless following a scent through thick undergrowth. These differences between breeds have been known for millennia and indeed have been under intense selection for almost as long as dogs have been our companions. They have a genetic basis, but it has proved extraordinarily difficult to identify the genes involved, for a number of reasons.
First of all, whether or not the trait segregates is an essential requirement for mapping. Certainly, some individual bloodhounds will be better than others at following a scent, and some sheepdogs will be better than others at herding. But unlike coat-colour, where all sorts of variation is tolerated, bloodhounds that have no sense of smell and sheepdogs who just don’t get it when it comes to herding are soon dispatched or at the very least not chosen for breeding. This severely limits the range of variation within a breed for geneticists to work with.
Next comes the issue of assessment. It is easy to score a dog as black or white, but how do you measure herding ability, for example, in a reproducible way? Various approaches have been tried, from expert assessment (very slow and expensive) to owner-directed surveys of performance (difficult to achieve consistency). These two very practical considerations have held back advances in understanding the genetics behind these most interesting behavioural differences between breeds. Nonetheless the good old-fashioned candidate gene approach has met with some limited success.
The candidate gene approach succeeded in unravelling the cause of the sleep disorder narcolepsy in Dobermanns.15 Dogs with this condition tend to fall asleep at a moment’s notice, presumably not an asset in a breed created primarily for guard duty and the personal protection of the breed’s creator, German tax collector Louis Dobermann. Nevertheless a colony of Dobermanns exists that segregates for the condition, and this allowed researchers to test a number of candidate genes to see if any of them follow the same route through the pedigrees. One of them did and it was identified as the hypocretin receptor gene, where an insertion had disrupted its normal functioning in the brain.16
This is a rare example of successful gene hunting in a behavioural trait, but the genetic basis for most dog behaviours, though doubtless genetic in origin, is still shrouded in mystery. Unfortunately this includes most behaviours of interest or even concern to owners. For example, a few breeds, particularly the Bull Terrier, suffer from a disorder which is very reminiscent of obsessive compulsive disorder (OCD) in humans. It is easy to score, as affected dogs chase their own tails. Thus far the gene has not been identified, although the successful treatment of the condition with serotonin re-uptake inhibitors such as clomipramine, which is also used to treat OCD in humans, suggests there may be a common genetic pathway in both species.
Aggression is another behaviour of considerable interest to owners and breeders alike, but studying it comes with its own issues. Many in the dog fanciers’ community argue that there is no such thing as a bad dog, only a bad owner, and that no breed should be penalised because of inadequate or careless owners. Nevertheless some breeds have been outlawed, notably the American Pitbull. Undoubtedly this breed, until recently, and probably even now, bred for illegal dog-fighting, has been selected for its performance in the fight-pit. But that does not mean that Pitbulls are intrinsically vicious, as might be indicated by a consistent genetic mutation. One reason I love genetics is the multitude of ethical questions it raises. The issue of the genetic components of aggression in the Pitbull is immediately applicable to our own species. How far does individual responsibility for violent actions extend if it turns out that the cause is a mutation in the genome? That question puzzles and will go on puzzling ethicists and lawmakers for years to come.
I want to end the chapter with a remarkable example recently revealed which may even hold the secret of dog domestication, for so long the holy grail of researchers. It is also a gripping scientific detective story and shows how a single unusual event, observed by lively minds, can lead to amazing revelations. It began in 1961 in Auckland, New Zealand, at Green Lane Hospital, an offshoot of the city’s main infirmary. Three cardiologists led by Dr J. C. P. Williams had noticed four young patients suffering from supra-valvular aortic stenosis. The principal feature of this condition is a narrowing of the aorta, the main blood vessel leading from the heart. Not surprisingly, it can have serious or even fatal consequences. The doctors noticed that as well as the cardiovascular problems these four children shared other features including various degrees of mental retardation and unusual faces. These were referred to as ‘elfin’, owing to slightly pointed ears and crowded teeth. They all had a wide philtrum, the gap between the upper lip and the bottom of the nose, the overall effect making them easily recognisable. Most remarkable of all, these children had extraordinarily engaging characters. They were always smiling, always cheerful and very easy to approach. Where there are disorders that display a variety of symptoms, these are referred to as syndromes and are often given the name of the principal investigator, so this was named after Dr Williams.17
Other cases conforming to this syndrome were soon noticed in cardiology clinics around the world. It seemed likely that the aortic stenosis in Williams syndrome might have something to do with the structure of the aortic wall. Like all arteries the aorta is like a thick, elastic tube which expands and contracts to even out the flow from the left ventricle as the heart pumps blood around the body. The component responsible for the elasticity of the aortic wall is the protein elastin. I worked on elastin for my PhD many years ago and it is a remarkable protein which, as the name implies, really is elastic. It is found not just in blood vessel walls but also in the skin, where thin fibres of elastin keep it taut – at least when you’re young. As time goes by the connections that join the elastin molecules together break down, and this is accelerated by the action of sunlight – to, on the one hand, the despair of sun-worshippers and, on the other, the delight of manufacturers offering products that claim to halt or even reverse the ageing process.
Soon after the elastin gene was located on human chromosome 7, scientists checked to see if there were any obvious elastin gene mutations in other conditions, like Williams syndrome, where it might be implicated. This soon led to the discovery that the gene resided in one of those parts of the genome which tended to get muddled by losing whole chunks of DNA. The genome is not as stable as we might imagine. In Williams syndrome patients, deletions did indeed take out the elastin gene and some of the genes on either side of it. Large gene deletions like this usually come out of the blue, so there are often sporadic cases with no family history. The sporadic cases also showed similar large-scale deletions involving the elastin gene. This meant that Williams syndrome patients, both familial and sporadic, had only one working elastin gene when they should have had two. This wouldn’t usually matter where an enzyme defect is involved, which is why carriers of most recessive disorders are symptomless, getting by perfectly well on just one copy. Where the protein forms part of a structure, like the arterial wall, producing only half as much as normal has consequences. In all cases where one of the elastin genes is deleted, the result is aortic stenosis.
Following this discovery in Williams syndrome, some other patients with aortic stenosis were also found to be missing one of the elastin genes. But they didn’t have the other features of Williams syndrome, including the remarkably open personality. Why not? The most likely explanation is that there was another gene lying close by which is missing or mutated in the Williams syndrome patients but not in patients with aortic stenosis alone. What, I hear you ask, has this to do with dogs?
Once again, we are indebted to Elaine Ostrander from the US National Institutes of Health and Bridgett vonHoldt from Princeton and a mixed team of research scientists in different disciplines drawn from a number of other US universities for making the connection. As we know, Dr Ostrander has a long-standing interest in the domestication of dogs. She and the team wondered whether the exaggerated sociability of Williams syndrome patients may also be a feature of domesticated dogs that was absent in their wild ancestor the wolf.
The first hint came from a saturation mapping project of 701 dogs from 85 breeds and 92 Arctic wolves.18 The question asked was straightforward. Were there any places in the genome that were substantially different in dogs and in wolves? Two regions stood out. The region of greatest difference between the two lay within a gene called SLC24A4, which is mainly concerned with hair and tooth construction. Not far behind in the rankings was another gene called WBSCR17, which lies very close to the elastin gene on dog chromosome 6. There is a remarkable consistency in gene location and order in mammalian genomes, which hinted that this gene might also be responsible for the other features of Williams syndrome in humans. By then, it was known that the Williams syndrome deletion was about 1.5 megabases long and took out a segment containing roughly twenty-eight genes, one of these being WBSCR17.
There followed a series of behavioural tests of the same sort used to diagnose Williams syndrome in humans on eighteen dogs and twelve captive wolves who were accustomed to being with humans. One was puzzle-solving, another measured hypersociability, and the third assessed social interest in strangers. Without going into details of all these tests, let me summarise the results. In the puzzle-solving test, dogs spent most of their time looking at the humans for cues while the wolves just got on with it and achieved higher scores as a result. Next the researchers measured sociability. In the first test the researcher sat quietly, ignoring the subject but looking at the floor on which was drawn a circle one metre in circumference. In a variation of this, the researcher called the animal by name (remember that the wolves were already socialised to humans) and encouraged them to approach. In this test dogs spent far more time within the circle than the wolves, both when the experimenter was known to them and, slightly less often, when they were strangers.
Though the number of animals in the experiment was small, it was enough to convince the scientists that, on the one hand, dogs but not wolves showed the hypersociabilty associated with the Williams syndrome but lacked the ability to act without human guidance. When it came to pinning down the mutations in the dog that might be responsible, there were mixed results. There was plenty of genetic variation in the region, with mutations present in members of the transcription factor II genes. No one is yet quite certain how these genes work or what effect the mutations might have, but I suspect the answer won’t be long in coming. What is clear is that this suite of genes is under strong positive selection favouring those animals showing Williams syndrome-like hypersociability above those that do not.
There is a lot more to be done, but this fascinating piece of research, stemming from the observations of three curious New Zealand doctors, has provided the first solid clue that ‘domestication’ has a significant genetic basis and was not just a consequence of behavioural adaptation.
The research culminating in the fascinating potential link between an obscure human disorder and a genetic basis for domestication involved some of the most sophisticated technology ever applied to the dog genome and has yet to reveal all its secrets. Had artificial selection applied by man picked out genetic traits that made dogs on the one hand friendly towards us and yet on the other unable to think for themselves?