The Mammary Gland
Barbara Davis1 and Suzanne E. Fenton2*, 1Innogenics, Inc., Harvard, MA, United States, 2National Institute of Environmental Health Sciences, Research Triangle Park, NC, United States
Abstract
The development of breast cancer, the most common malignancy of women, is clearly influenced by environmental factors particularly during key developing periods or “windows of susceptibility.” With a better understanding of this important interplay, there is heightened awareness and increased implementation of more rigorous methods for hazard identification using in vitro and in vivo models. This chapter provides background for mammary gland evaluations including details of biology and development, species and sex comparisons, hazard identification and study design approaches, morphologic evaluation and diagnosis, and mechanisms of mammary toxicity and carcinogenicity.
Keywords
Mammary gland; breast cancer; methodology; histopathology; rat; whole mount; wet mount; mammary carcinogen; toxicants; endocrine disruptors; chemical carcinogen; adenocarcinoma; genetically engineered mice; developmental abnormality; nipple; canine; nonhuman primate; puberty; pregnancy; lactation; involution; vaginal opening; estrous cyclicity; terminal end bud; lobule; inflammation
Introduction
Breast cancer is the most common invasive cancer and the leading cause of cancer-related mortality in women. As such, there remains heightened concern about the potential causes and risk factors, including those from environmental exposures, drugs or dietary intake that may contribute to the development or progression of this deadly disease. Lifetime exposure to estrogens (early age of menarche, older age at menopause, nulliparity, unopposed estrogen) and genetics (family history of breast/ovarian cancer) are major factors that increase risk for breast cancer, and early age of first pregnancy (<22 years old) is associated with decreased risk. Other biological indices, such as obesity and breast density, are highly correlated with risk of breast cancer and may be affected by environmental factors.
This interplay of reproductive and hormonal influences generally holds across species. For example, mammary cancer is the most common malignant cancer in intact bitches (dogs) greater than 6 years old. Neutering prior to first estrus essentially negates the risk, while risk increases with neutering after consecutive cycles. In old world macaques, the role of reproductive status is not clearly defined, but mammary gland biology and breast cancer development are generally comparable to that in women. In a population of captive macaques the lifetime risk for breast cancer was estimated to be about 6% and considered comparable to women (1 in every 8, or 12%). The types of cancer, intraductular carcinomas and invasive and metastatic cancers, are also morphologically like those in women. The role of estrogens and progestins in mammary proliferation are also comparable in macaques and women. In experimental studies in castrated cynomolgus and rhesus macaques, estrogen treatments alone stimulated epithelial proliferation, progesterone treatments alone had negligible effects, while both estrogen and progesterone treatment together stimulated proliferation exceeding that of estrogen alone.
In rodents, hormonal and reproductive status and genetics have significant effects on the development of mammary cancer. Mammary cancer can be induced with estrogen treatment in both rats and mice and incidence of mammary cancer increases with increasing age in many strains of rats. In rats, as in women, pregnancy is protective, although pregnancy does not have the same protective effect in mice. Mice also deviate from rats, monkeys, and women in that the most common types of mammary cancer in mice are lobuloalveolar, noninvasive, and not metastatic. In contrast, mammary cancers in women, monkeys, dogs, and rats are ductular, derived from the most primitive epithelial cells of the terminal ductal units, and are invasive and malignant.
Animal studies also show that there are windows of increased susceptibility to external influences during gland development, which may enhance the predisposition to cancer later in life. Consequently methods in safety assessment and hazard identification using animal models should be designed to assess developmental abnormalities as well as preneoplastic and neoplastic changes in adults. The purpose of this chapter is to provide an overview of mammary gland biology of different species with relevance to humans, and review models, methods, mechanisms, and definitions of mammary gland toxicity and carcinogenicity.
Mammary Gland Structure and Function
Mammary glands are modified tubuloalveolar glands of the skin (see Chapter 24: The Integumentary System), and are a complex organ composed of epithelial lined ducts and alveoli within an adipose-based fat pad surrounded by fibroblasts, immune/inflammatory cells, lymphatics, and blood vessels. Mammary development resembles that of epidermal appendage sweat glands, but under control of pituitary and gonadal hormones in addition to hormones produced by mammary fat pad components. These hormonal influences transition the gland through numerous phases of growth, differentiation, involution and atrophy, and have varied effects on males and females.
Stages of Mammary Gland Development
Mammary gland development has distinct anatomical and functional changes during embryogenesis and prepuberty that include bud formation and early ductal tree formation, and during postpuberty and pregnancy that include ductal elongation, lobuloalveolar differentiation, and pregnancy and lactation.
Bud and ductal tree formation: Gland formation begins during embryogenesis with the development of linear bilateral ectodermal thickenings, referred to as the mammary line or ridge, which overlie a specialized mesoderm. The ectodermal cells of the ridge migrate and aggregate, forming “mammary epithelial buds” at the location of the future mammary gland. These solid ectodermal cords grow into the underlying mesenchyme, followed by limited epithelial branching and canalization to form mammary sprouts. Support structures, including adipose tissue, which later forms the fat pad containing blood vessels, lymphatics, and connective tissue, develop from mesenchyme coincidental with mammary bud development. Myoepithelial cells that eventually surround epithelial structures (ectoderm) and nerves (neuroectoderm) differentiate separately, but simultaneously, with the mammary buds. There are some species differences in the number and complexity of sprouts prior to birth as sprouts form the papillary ducts of each gland. Each sprout also communicates externally with epithelium that will form the teat/nipple. Some species have collecting ducts that drain multiple primary ducts into a teat (mice, cows), whereas the human has numerous ducts that drain directly into the nipple.
Ductal elongation: Initial growth of the gland occurs through linear ductal elongation promoted by a distinct bulbous structure at the distal end of the duct called the “terminal end bud” (TEB) (Figure 19.1). TEBs are composed of multiple layers of epithelial cells and myoepithelial cells lining the basement membrane that interact closely with the surrounding mesenchymal stroma of the fat pad to determine length and patterning of the gland. The leading portion of the TEB is covered by a distinct layer of “cap cells” overlying more numerous layers of “body cells,” each containing stem cells with the capacity to form ductal or luminal alveolar cell types. Canalization occurs at the trailing end of the duct to form a single layer of luminal cuboidal epithelial cells and basally located myoepithelial cells supported by a distinct basement membrane. A continuous basement membrane is principally composed of type IV collagen, laminin, nidogen, and heparin sulfate proteoglycan. Both luminal epithelial and myoepithelial cells produce laminin subunit chains, distinguished by type: epithelial cells deposit α3 and α5 chains, myoepithelial cells deposit α1 chains. The stroma surrounding the ducts consists of fibroblasts and adipocytes, macrophages and eosinophils. These interstitial cells support ductal elongation and branching. Functionally and microscopically, mammary gland morphology and duct elongation is characterized both as a proliferative and an apoptotic process.
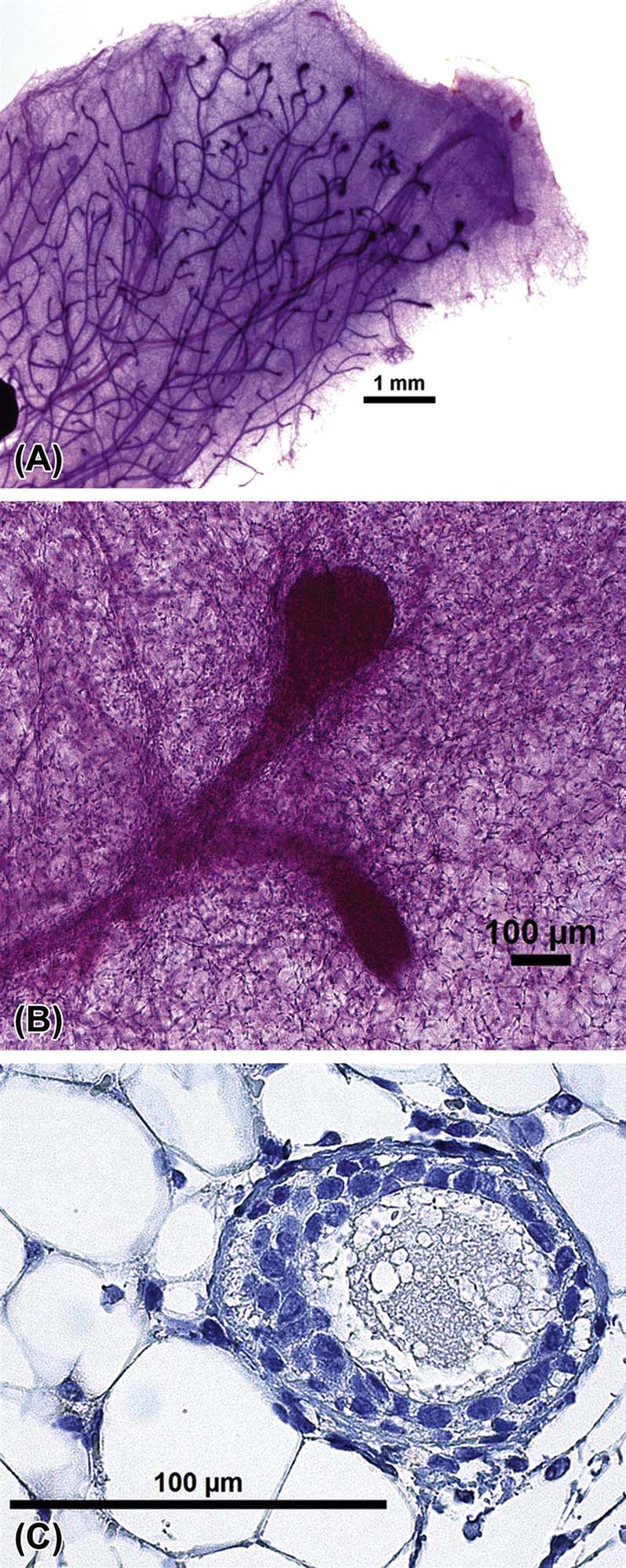
Lobuloalveolar differentiation: In all species, there is extensive growth of the mammary gland and branching of TEBs into lobular structures during adolescence. Although there are species differences in promoting growth, in all species exponential mammary epithelial growth and development is ignited just prior to other outward signs of puberty and is coincident with a relative burst of ovarian hormones. In most species, including humans, rapid mammary development occurs prior to cycling, suggesting that more than just ovarian hormones control this process. In the adult, mammary glands are arranged into lobules of compound branched alveolar sacs, separated by dense interlobular connective tissue and fat (Figure 19.2). Lobules are arranged into distinct lobes with its own excretory duct, also called the lactiferous duct, and its own opening on the teat or nipple via the papillary duct. Cuboidal basal and superficial columnar epithelial cells line the lactiferous duct. The secretory units are alveoli, which respond to hormonal signals of lactation. An alveolus is lined by secretory cuboidal or columnar epithelium and an outer layer of myoepithelial cells that lie between the epithelium and the basement membrane.
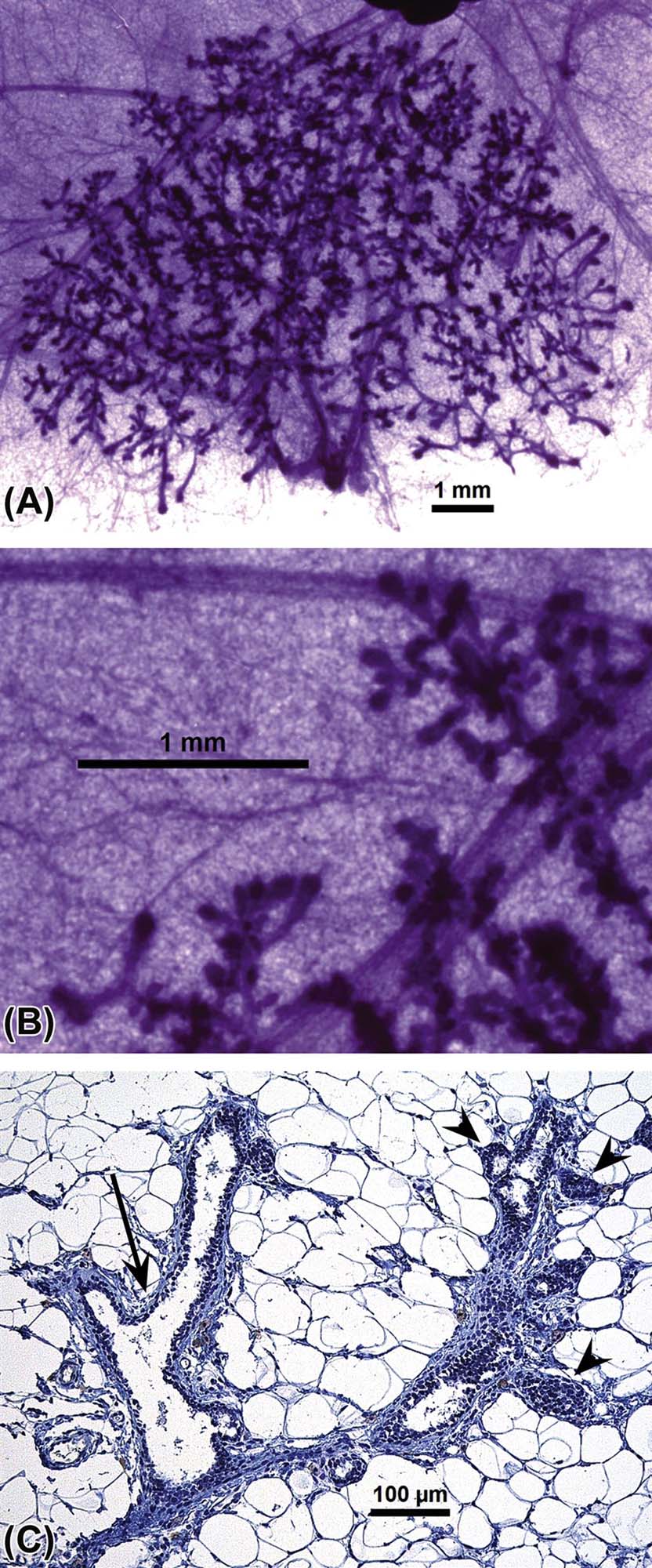
Pregnancy and lactation: Marked development and differentiation continues through pregnancy and lactation (Figure 19.3). During pregnancy and lactation, extensive growth and alveolar maturation occurs to form milk-producing glands. With growth of the gland, there is a concurrent reduction in the amount of intra- and interlobular connective tissue. The secretory alveoli are lined by cuboidal epithelium, surrounded by a layer of myoepithelial cells, basement membrane, and an intimate network of capillaries and lymphatics. The continued growth of the mammary gland during the second half of pregnancy is due to increases in the height of epithelial cells and an expansion of the lumen of the alveoli. These cuboidal cells produce and secrete the milk components, which are expelled from lobuloalveolar units by contraction of myoepithelial cells. During peak lactation, adipocytes are rarely observed in tissue sections. After lactation or end of suckling the mammary glands undergo an apoptotic process called involution by which remaining milk is phagocytized and epithelial cells degenerate. The few remaining alveoli are lined by low cuboidal, nonsecretory epithelial cells, and prominent myoepithelial cells. Stromal cells, adipocytes, and the amount of interstitial and connective tissue are increased as involution proceeds. Corpora amylacea, which are small concretions of protein, may be found in alveoli, ducts, or interstitial areas.

Species Variations in Mammary Gland Development
Species differences to consider include the relative timing of development, the extent and complexities of the ductal and lobuloalveolar development, and male mammary characteristics. Understanding these species/sex variations are critical to choosing an appropriate research model and in interpreting the effects of xenobiotics.
Rodents: In the mouse, mammary gland development begins about embryonic day 10.5 with the appearance of 5 placode sets on the mammary ridge. Further development occurs along each of the two mammary lines from embryonic day 11.5 to day 13.5. The mesenchyme adjacent to the mammary epithelium becomes dense and begins to regulate elongation of the mammary buds. Epithelial cords are formed and continue to elongate as terminal ends form and grow into the fat pad through fetal days 15–16.5. Ductal branches and a lumen form just prior to birth.
The rat differs from the mouse in that mammary placode development begins about embryonic day 12.5 with 6 sets of mammary buds present by embryonic day 15.5 that develop into a more complex branched pattern around the time of parturition. Both male and female demonstrate the branched pattern and lumens that are present at the time of birth. Prior to vaginal opening, ductal budding is apparent, and the TEBs begin to cleave into clusters of 3–5 smaller alveolar buds each with a centrally located lumen surrounded by a layer of cuboidal epithelial cells. The glands of the prepubertal female rat are characterized by scattered ducts lined by a single or double layer of cuboidal epithelium, which, after branching several times, becomes multilayered to form the TEBs. TEBs are comprised of 3–6 layers of medium-sized epithelial cells with scant cytoplasm and oval nuclei.
Growth and branching of the mammary structure in rodents continues after birth reaching peak growth rate from 21 to 55 days of age. With each estrous cycle the alveolar buds form complex lobules of smaller diameter with a distinct lumen lined by a single layer of low cuboidal epithelium. The glands of mature, virgin female rats are tubuloalveolar, characterized by abundant branching, narrower ducts, and more numerous alveolar buds and lobules. As rodents age or experience periods of prolactin and progesterone-prominent pseudopregnancy, glands will have more extensive budding and lobular formation. There are also slight changes in budding/lobule formation depending on the estrous cycle stage, therefore stage of cycle should be determined when evaluating mammary tissue for effects of xenobiotics. When some strains (e.g., Charles River Sprague-Dawley or Fisher 344) become middle aged (from 8 to 14 months of age) and reproductive senescence ensues, prolactin levels increase leading to the development of several morphologic changes including inappropriate secretory activity, ectasia of ducts, formation of cysts or galactocoeles, epithelial hyperplasia, and periductal fibrosis.
Although the mammary gland becomes a vestigial organ in the male, it may be more susceptible to endocrine modulation during development and retains the capacity to develop cancer in the adult. In male mice the mammary rudiment degenerates on or near gestational day 14 in response to androgen-dependent condensation of the surrounding mammary mesenchyme. A small stalk of epithelium may remain. There is no nipple formed in either male mice or rats. However, in male rats mammary glands are difficult to distinguish from female glands prior to puberty, but there is considerable sexual dimorphism in adulthood. Compared to the tubuloalveolar glands in the adult female rat, the adult male has lobuloalveolar glands lined by a single layer of cuboidal vacuolated epithelial cells, and pseudostratified or stratified epithelium. The male rat mammary gland remains responsive to endogenous and exogenous hormone agonists and antagonists throughout its life.
Dogs: When dogs are used in safety assessment studies, they are usually young, sexually immature, which limits the assessment of toxicity. Prior to puberty, dogs have rudimentary glands composed of large interlobular ducts with few laterally projecting TEBs within a dense connective tissue stroma. Like other species, marked proliferation of TEBs, linear growth and tertiary branching into lobes occurs with the onset of puberty and requires the influence of estrogens and progestins.
In cycling bitches the gland of proestrus is characterized by interlobular ducts, a few small lobules and extensive connective tissue stroma. During estrus, there is marked intralobular ductal epithelial proliferation and formation of many small ductules lined by a multilayered epithelium within loose connective tissue. By default in the bitch, corpora luteal function is maintained by prolactin during the 2 months (about 63 days) postovulation regardless of whether the bitch is pregnant or not (see Chapter 18: Female Reproductive System). Mammary gland development during the early half of this period (early diestrus if not pregnant, or early pregnancy) is characterized by greater development of the ducts, the stroma becomes mucinous and fibroblasts within the interlobular stroma demonstrate increased mitotic activity. During the last half of this period, late diestrus or late pregnancy, secretory alveoli begin to develop. Alveoli are filled with bright eosinophilic proteinaceous secretion and lined by cuboidal to flattened cells and elongated or stellate myoepithelial cells and supported by minimal intralobular stroma. Stroma remains prominent around the ducts. If pregnant the mammary gland is stimulated by the hormones oxytocin and prolactin, and becomes secretory. If not pregnant the mammary gland involutes. Involution is characterized as reduction and shrinkage of the alveoli with vacuolated and apoptotic epithelium. Because of such variations, evaluations of cycling females should always be conducted with consideration for cycle stage.
Nonhuman Primates: As in humans a rudimentary ductal tree is formed early in life. TEBs form the leading edge, surrounded by myxoid stroma. Rapid growth and the appearance of “invasive” epithelium are normal in nonhuman primates, particularly macaques. Most of mammary gland development occurs during puberty. In rhesus macaques, puberty occurs between 2- and 3-years old, and in cynomolgus monkeys it occurs slightly later. The epithelial cells are surrounded by myoepithelium and stromal support consisting of fibroblasts and adipocytes. Most of the developing gland is adipose and fibrous connective tissue. The lactiferous ducts are lined by stratified squamous epithelium as they approach the surface, and in the nonlactating animal they are typically filled with keratin. As the mammary gland continues development there is rapid elongation and branching of major ducts to form a dense gland of arborized lobuloalveolar units. Thus even early in puberty with the onset of menstruation, there may be well-differentiated, densely branched lobuloalveolar units lined with secretory epithelial cells.
Toxicology studies of nonhuman primates often employ few and younger animals that may be just entering puberty. Mammary gland development can be quite mixed—varying from dense branching ducts to lobuloalveolar morphologies—which may be misinterpreted as toxicant effects. There are also subtle cycle-related changes characterized by ductal proliferation during the luteal phase of the cycle and alveolar proliferation during the follicular phase. During pregnancy and lactation, extensive alveolar maturation and differentiation to a milk-producing phenotype occurs. Involution is typically extensive with regression of alveoli to a nulliparous state. Experimental studies have shown that during aging and menopause, or with surgical castration, atrophic ducts maintain estrogen and progesterone receptors and respond to exogenous steroids for some time. It should also be noted that marmosets do not undergo menopause so older females will maintain more developed glands while rhesus macaques reach menopause at about 22–24 years old, accompanied by atrophic changes in the mammary gland.
Humans: Mammary milk lines develop as early as 4–5 weeks of gestation and form one pair of mammary placodes. The primary ectoderm bud is present by 12 weeks of gestation and small ductal outgrowths as a primitive gland are present at birth. Also, rather than forming a single ductal tree, each human anlagen forms several trees initiating at the nipple. Before birth the specified mammary epithelium grows from the nipple into the fat pad to form a small, branched ductal network. The advancing margins of undifferentiated TEBs are surrounded by a loose myxomatous connective tissue matrix. TEBs express both estrogen receptors by gestational week 30 and progesterone receptors at birth, evidence of the role that steroid hormones play in normal breast development with implications extending to the potential deleterious influences of endocrine disrupting compounds. During childhood and adolescence, breast growth keeps pace with overall body growth until greatly accelerating at puberty.
Age of puberty in girls is significantly decreasing across continents, with genetics, nutrition, or increasing exposure to natural and synthetic estrogenic compounds all implicated as potential causes. Pubertal development is classified in five Tanner stages, which describe breast and pubic hair growth prior to the onset of menarche. According to median Tanner stages over time, accelerated age of puberty in girls is characterized as menarche occurring possibly a few months earlier, while breast development can occur up to 1–2 years earlier than it did decades ago. Thus breast development is not only significantly advancing in the human population, but there is also dissociation between the pubertal hormonal control of breast development and menarche. These observations underscore the enhanced sensitivity of the mammary gland to genetic and environmental influences compared to other reproductive tissues (see Chapter 18: Female Reproductive System). The advancement of breast development increases the overall time that TEBs remain as susceptible targets, which increases the risk for development of cancer. Indeed, earlier age of menarche and later age of menopause are known risk factors for breast cancers. Thus assessing the maturation of the mammary gland in rodent toxicology studies provides an important means to identify potential human health hazards.
Morphologic Evaluation
Thorough morphological evaluation of mammary glands incorporates routine assessment in histological hematoxylin-eosin (H&E) sections, quantitative morphometric analysis and morphological evaluation of whole mounted mammary glands (see Further Reading for protocols). Factors to consider are the age of the animal and expected developmental maturity of the gland, the species, sex, strain, stage of cycle, pregnancy, lactation, or involution.
In rodent studies, all gross lesions are collected and historically the fifth mammary gland was dissected with or without skin from the same side in both sexes. However, Figure 19.4 demonstrates the major advantage of using the fourth and fifth gland of the rodent, cut in longitudinal sections, allowing a greater chance of detecting a lesion if it exists in the mammary tissue. The contralateral fourth and fifth glands should be isolated for mammary gland whole mounts. Male mice typically lack mammary epithelium, thus would not be evaluated unless nipple/areolae retention is noted early in life. Sampling in dogs includes the nipple and surrounding gland. More extensive collection is needed for nonhuman primates as mammary epithelial growth extends far into the fat. Glands are placed flat in a histocassette or are flattened onto fiberboard or index card and processed through formalin fixation to a 5-micron section on a charged glass slide stained with H&E. Temporal biopsies in nonhuman primates (see Further Reading), add the power of analyzing changes over time without, importantly, the need for sacrifice.
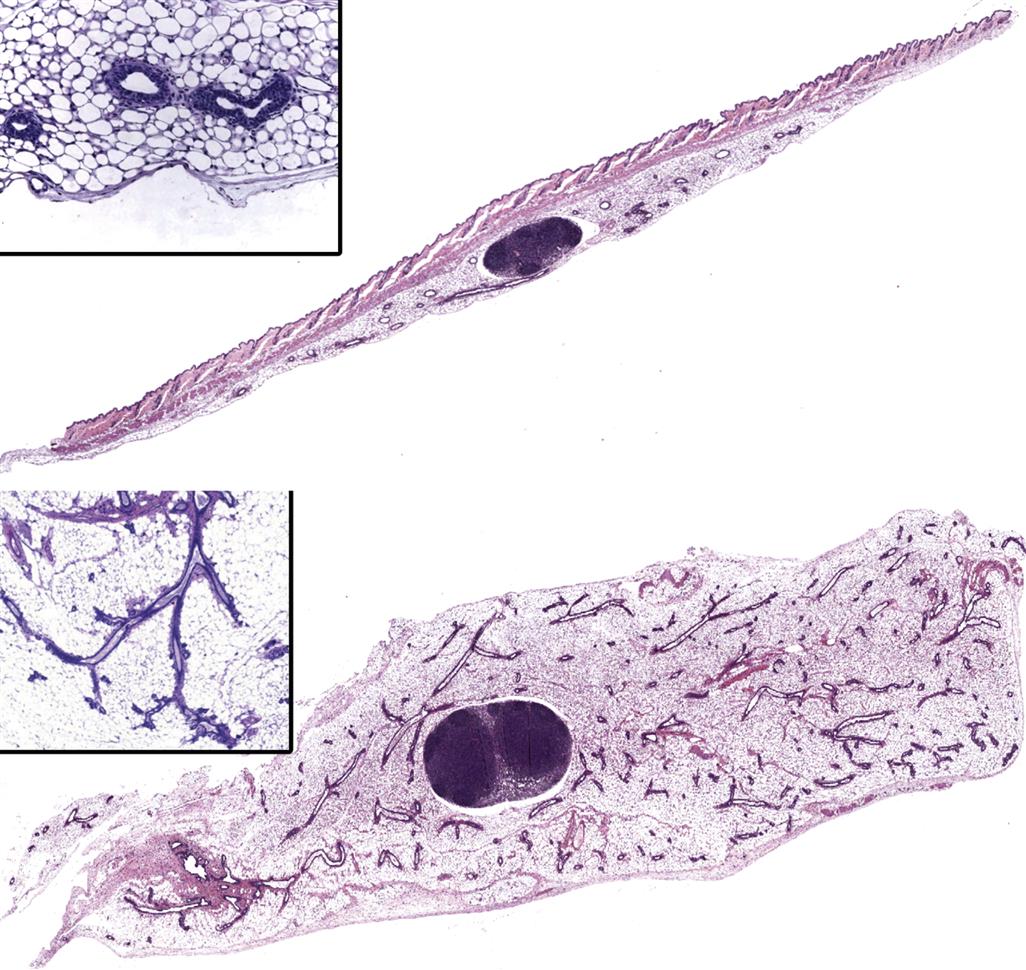
Increasingly, sectioned mammary tissue receives qualitative assessments, coupled with semiquantitative morphometric analysis. Parameters evaluated include length of the ductal tree along the longitudinal axis, total area occupied by epithelial ducts, branching density, total number and size of TEBs all relative to the total area of mammary epithelium, and total area of the fat pad. These parameters can be measured in the routine sections using image analysis software systems or with eye-piece reticules, but are subjective unless the entire gland is sectioned. Immunohistochemistry end points including cell proliferation and apoptosis measurements are similarly quantified with respect to location of cells (epithelial, myoepithelial, mesenchymal, ductal, bud, alveoli, and fat pad) and total number of cells evaluated. Increases in epithelial density and cell proliferation in early development have been correlated with the development of cancers later in life in both rodents and nonhuman primates. Thus morphometric analysis of glands, coupled with immunohistochemical staining with PCNA or Ki67 cell proliferation markers, provide excellent markers of mammary gland changes.
Whole Mount Preparations: While histologists and pathologists are well versed in light microscopic techniques and evaluation, whole mount preparation (the entire fourth and fifth gland mounted onto a slide) and evaluation is not yet a routine procedure in all laboratories. However, evaluation of mammary gland morphology in whole mounts allows assessment of the branching complexities and glandular densities. Whole mounts are also more amenable to quantitative assessments of TEBs, alveolar buds, duct, and branching development, and growth into the fat pad because the entire gland is represented. Processing mammary gland for whole mounts requires some training, but is relatively inexpensive and does not require pathology experience. Procedures are detailed in Davis and Fenton (2013). The whole mount should be prepared from the contralateral gland processed for standard H&E slide examination. Masses or questionable lesions observed in whole mounts can be excised and reprocessed through paraffin for H&E microscopic examination (Figure 19.5; see Further Reading for protocol). Mammary gland development may be scored according to the criterion detailed in Davis and Fenton (2013) and briefly described here.
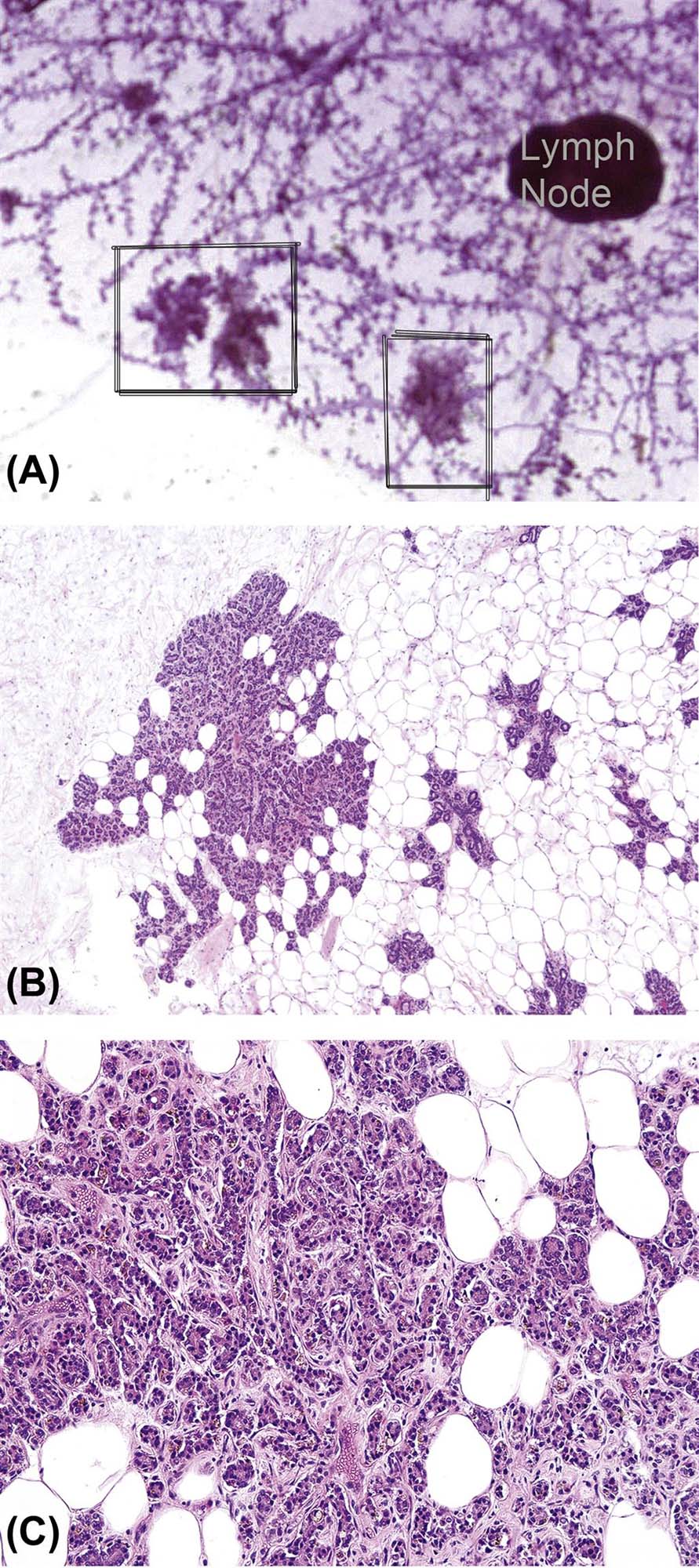
Developmental Assessment: To evaluate the whole mounted mammary gland, the normal structures of the mammary gland, including the difference between TEBs and terminal ducts, identification of a bud, lobule, duct, lymph node, and lateral branches, and age-related shifts in morphology should be familiar. Morphology may differ slightly between strains within species, therefore, it is imperative that the slides from the control group are evaluated first, to become familiar with the normal mammary gland morphology for the strain, sex, and age of animal in that particular study. This should include reviewing controls of males and females separately, at the ages evaluated. When assessing the mammary morphological development, determine (1) the number of TEBs relative to the number of duct ends and the morphology of the TEBs, (2) the degree of lateral duct branching, (3) the degree of ductal bud and lobule formation, (4) growth of the gland (measure the length and width of the glands), and (5) the glands may be given a morphological developmental “score” as described in Davis and Fenton (2013). In some cases a more quantitative evaluation of the glands may be required. Some suggested methods are provided in Further Reading.
Evaluation of Toxicity
In Vitro Techniques
A variety of in vitro techniques are useful as screening assays for mammary toxicants as well as for mechanistic studies. The idea that chemicals can be screened for estrogenic activity in vitro was validated by Soto and Sonnescheim and colleagues describing the E-Screen assay. This assay takes advantage of endogenous estrogen receptor–mediated events in breast epithelial cancer MCF7 cells. An “A-screen” has been similarly developed for assessing androgen receptor (AR) activity using prostate carcinoma or MCF7 cells transfected with AR. Yeast-based steroid hormone receptor gene transcription assays and in silico ligand-dependent reporter assays are also used in drug screening, drug development, and toxicology studies. Compounds showing activity in these screening assays should be considered as potential mammary gland toxicants.
Cell culture experiments have provided important mechanistic knowledge of mammary gland biology and cancer. Neoplastic MCF7 and nonneoplastic MCF10 have been widely used, although there are many well characterized mammary (breast) epithelial cells available including cells derived from human breast. Another important and increasingly popular in vitro system is the 3-dimensional (3D) mammary gland culture. 3D cultures were originally described by the Bissell lab using MCF10A cells first suspended in medium containing 2% Matrigel and then plated on a solid layer of Matrigel. When mammary epithelial cells (such as MCF10A cells) are cultured on laminin-rich extracellular matrix, they produce a basement membrane and arrange themselves in spherical acini with a centrally located lumen. Modulation of genes or stromal elements can affect the formation of the acini. Such 3D cultures provide an advantage in toxicology studies by providing capabilities to assess both morphological and molecular changes and may demonstrate relevant cell-type interactions.
Animal Studies
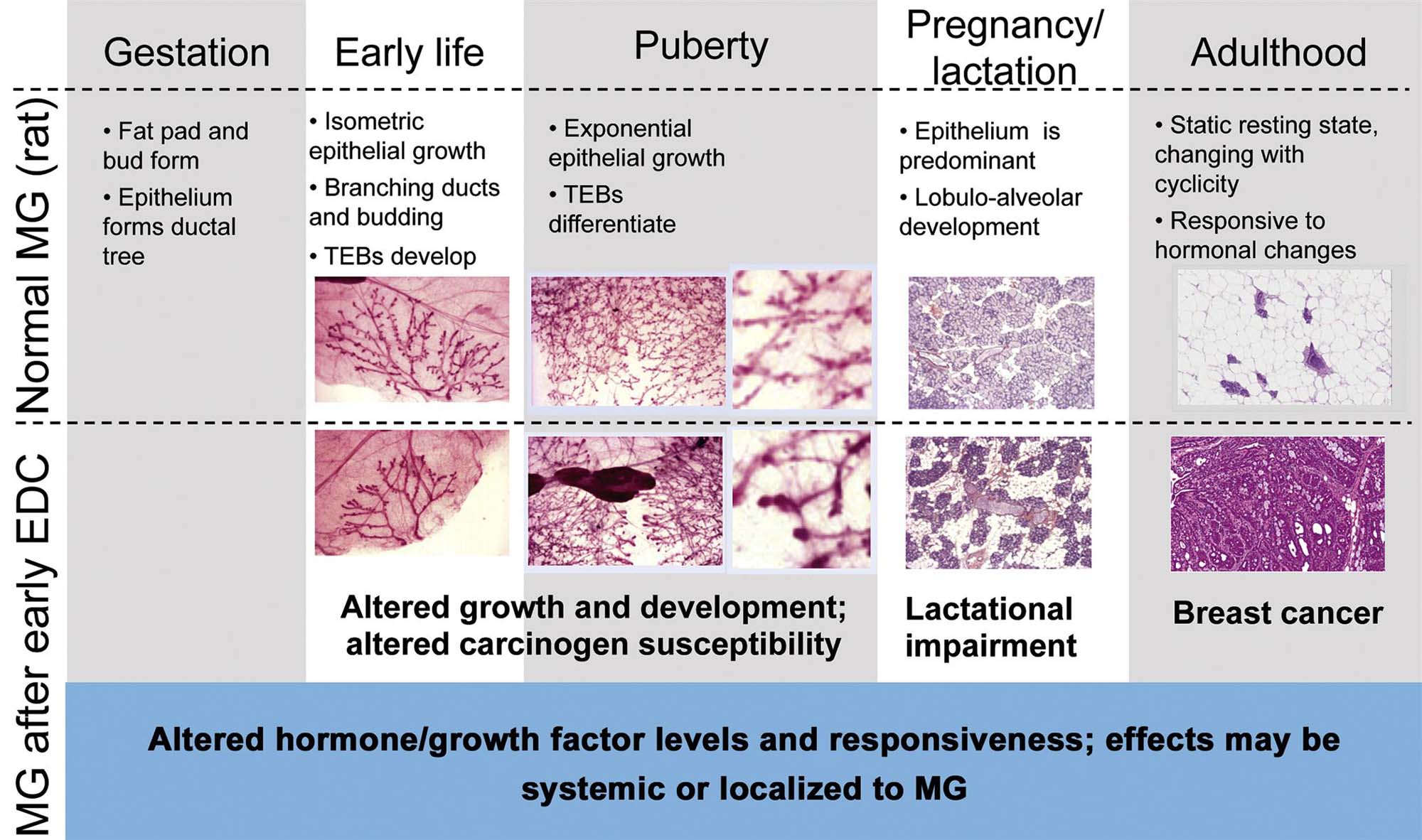
Although there are developmental and biological differences between mammary glands of various species of animals and humans, modeling effects of chemicals in animals has proved necessary and critical to advance our understanding of mammary gland biology, response to injury and cancer. The use of animal models (especially rodents) has demonstrated that sensitive life stages (especially fetal development, puberty, and pregnancy) impart a unique sensitivity to some chemical exposures leading to later life disease risk and that toxicants can act by mechanisms other than as frank carcinogens (i.e., as endocrine disruptors) to confer an increased risk of cancer or other late-life effect, such as insufficient lactation (Figure 19.6).
Bioassays in Spontaneous Rodent Animal Models: Bioassays conducted by the NTP have historically relied on B6C3/F1 mice and F344 inbred rats exposed up to maximally tolerated doses of single chemicals from sexual maturity (6–8 weeks old) up to 2 years. However, because of the high background of spontaneous tumors in some strains (Fisher 344), the NTP has recently changed rat strains to the Harlan Sprague-Dawley or Wistar Han. Industry-sponsored studies more often use CD-1 mice and CD-1 Sprague-Dawley, Harlan Sprague-Dawley, or Wistar rats. These different strains of animals have different sensitivities and tumor susceptibilities, which should be considered in study design and study interpretation. For example, mammary gland fibroadenomas are the most common spontaneous tumor in female Sprague-Dawley rats with incidences reported as high at 70% in chronic studies. Female Fischer rats have reported incidences of about 40%. Fibroadenomas are not considered a premalignant lesion in humans nor are rat mammary fibroadenomas considered predictive of carcinoma in women. Spontaneous mammary adenocarcinomas, which are considered relevant to women, are more common in Sprague-Dawley rats, with reported incidences of 11% in Harlan Sprague-Dawley rats. Mice, as compared to rats, have a lower background incidence of mammary tumors. B6C3F1 mice are relatively resistant to spontaneous mammary gland tumor development and the parental strains, C3H/He and C57BL/6, are relatively resistant to spontaneous and carcinogen-induced mammary gland tumorigenesis.
Following an NTP-sponsored expert panel workshop, and with the accumulation of data linking early exposures with later life mammary gland lesions, recent NTP carcinogenicity bioassays have incorporated early life or multigenerational exposures. Morphologic evaluation of the mammary gland includes mammary gland whole mounts and advanced sectioning techniques such as those demonstrated in Figure 19.4. European studies have also modified their protocols in recent years to include mammary gland evaluation of males and females. In addition to morphological evaluation of the mammary gland, careful evaluation of serum hormones, and other hormone-related end points in multigeneration studies such as timing of gonadal control of puberty (vaginal opening, heat, first estrus, etc.), cyclicity patterns, retention of nipples/areolae in males, and possibly anogenital distance measurements are important to aid in identification of potential mammary gland toxicants or carcinogens. Finally, a greater emphasis is now being placed on using “environmentally relevant doses” instead of maximum tolerated doses in toxicity studies as our understanding of how endocrine disrupting compounds mediate their effects on cells.
Several chemicals are known to interfere with the full development of a lactational mammary gland (e.g., dioxin, atrazine, perfluorooctanoic acid (PFOA)). Such an effect should be investigated in reproductive studies reporting decreased postnatal survival, decreased litter weights, or altered growth curves in nursing pups. Nursing pups should have stomachs full of milk. When lactation deficiencies are suspected, functional assessments (often referred to as a “lactational challenge”) of dam-pup interactions should be made. Such evaluations incorporate timed nursing experiments in which dams are separated from pups from 2–8 hours. Immediately prior to reunion, the litter is weighed, the dam is reintroduced for a fixed amount of time (15–30 minutes), nursing behavior is assessed and postnursing litter weights are measured. The pre- and postnursing litter weights serve as a surrogate for milk volume. Histological examination of the dams’ mammary glands is essential to help differentiate underlying morphological alterations versus function alterations (or both). Milk protein measurements from collected milk samples (collected at more than a single time, if possible) may provide valuable biomarkers of effect without having to sacrifice the animal. Lipid profiles, protein content, and other nutritional information may be collected using expressed milk samples.
Chemical carcinogenesis in spontaneous rodent models (also see Chapter 6: Carcinogenesis: Manifestation and Mechanisms): The coadministration of cancer inducing agents with a test article of interest is a commonly used and well-accepted method in toxicology studies to identify potential mammary carcinogens. For example, spontaneous mammary gland cancers in rats, generally adenocarcinomas, can be enhanced with treatment of genotoxic carcinogens like 7,12-dimethylbenz[a]anthracene (DMBA), N-nitrosomethylurea (NMU) and N-ethyl-N-nitrosourea (ENU), or irradiation. DMBA is most effective when administered after puberty. Part of the age-susceptibility pattern to DMBA is attributed to the development of the enzymes necessary to metabolize DMBA to its carcinogenic form after puberty. In contrast, NMU is most effective when administered before puberty due to a deficiency of a DNA repair enzyme in the immature rat gland and the induction of Hras mutations in the mammary epithelial cells. Irradiation is most effective when done in the postpubertal period and enhanced by short-term estrogen treatment during this time. Another consideration for susceptibility to these carcinogens is the relative abundance of TEBs in the gland at the time of administration. The TEBs contain stem cells and have high mitotic indices, thus are susceptible to damage from these chemicals.
Genetically Engineered Mice (GEM): Mice, which tend to have lower incidence of spontaneous mammary cancers that rarely are malignant, have nonetheless been exploited as models for breast cancer. Historically, it was discovered that infection with lactationally transmitted mouse mammary tumor virus (MMTV) caused hyperplastic lesions and mammary tumors through activation of Wnt, fibroblast growth factor, and notch signaling pathways that are also critical in early mammary gland morphogenesis. Hyperplastic lesions and spontaneous tumors that develop in naturally MMTV-infected mice are characteristic of well-differentiated hyperplastic alveolar nodules and alveolar tumors. Some have promoted these lesions to represent preneoplastic lesions comparable to such lesions in women’s breast tissue, but others maintain that the biology and morphology of these hyperplasias and benign tumors are features that should be considered mouse specific. Nonetheless, MMTV has also been exploited in the development of many GEM models of mammary cancer by targeting and driving gene expression using MMTV as a promoter. Another commonly used mammary-specific promoter is the whey acidic protein, or WAP promoter.
A tremendous effort has gone into characterizing GEM to bridge morphologic and genotypic variations of mouse mammary cancer to breast cancers in women. More than 40 models have been classified and categorized according to (1) lesions that resemble spontaneous mouse mammary tumors, (2) lesions that are unique and specific for the transgene, and (3) lesions that resemble human breast lesions. However, for most models and transgenes tested, there is limited evidence that these show malignant characteristics and they are not transplantable or immortal. Nonetheless, these models remain informative and recent characterizations further support their importance in understanding mammary gland biology and cancer. There is another effort that is generating diversified outbred mice, genetically unique lines of mice, which will help to identify susceptible subpopulations and allow linking of genetic underpinnings to disease. Phenotypic understanding of their mammary tumor susceptibility is in its infancy, but these may prove to be valuable in testing certain pharmaceuticals/xenobiotics for effect on the mammary gland in the future.
In summary the use of animal models (especially rodents) has demonstrated three critical factors that would have taken decades to discern in humans. First, that sensitive life stages (especially fetal development, puberty, and pregnancy) impart a unique sensitivity to some chemical exposures leading to later life disease risk. Second, that toxicants can act by mechanisms other than as frank carcinogens (i.e., as endocrine disruptors) to confer an increased risk of cancer or other late-life effect, such as insufficient lactation. The use of transgenic, knockout, and other gene modified rodents (primarily mice) have identified highly important details of mechanisms in disease development and progression. Finally, the nonhuman primate is an excellent model to understand pleiotropic effects of toxicants or drugs on mammary gland development as well as carcinogenic potential. It is often overlooked because of costs or availability.
Response to Injury
Physiologic Response to Injury
The most recent emphasis in toxicology has been placed on understanding the direct or modifying effects of endocrine disrupting compounds. These are often reported as causing either accelerated maturation or delayed differentiation of the gland in females (Figure 19.6), or in altering glands in the male from a lobular phenotype to a more female-like tubuloalveolar morphology. Retained nipples in male rodents are also characteristic of antiandrogen effects. Accelerated mammary growth may be characterized as increased TEB formation at early time-points and decreased numbers at later time periods. Delayed maturation may be characterized by the presence of TEBs until much later in development, extending the window of time that these carcinogen-sensitive structures are present in the gland. Inhibition of development may also be reflected as a decrease density of the gland, as well as an overall decrease in TEBs formed. Ductal hyperplasias and dysplasias can be induced with various estrogen-like compounds, but, as mentioned previously, many of these effects depend on timing of exposure and the extent of mammary gland development within the animal of interest. For example, exposure to estrogenic compounds through the perinatal period in rats, when TEBs are developing, are associated with hyperplasia of the buds, while exposure during the peripubertal period, when ducts are developing, is associated with ductal hyperplasia. Neonatal exposure to estrogen, progesterone, or both in mice causes irreversible effects in adults, including secretory stimulation, dilated ducts, and abnormal lobuloalveolar development. Phytoestrogens, such as genistein and resveratrol, and the mycoestrogen zearalenone act similarly to estrogen agonists in their effects on the gland. Changes include delayed development, ductal hyperplasia, alveolar hypoplasia, reduced apoptosis in TEBs, increased or decreased numbers of terminal ducts or lobules, and accelerated alveolar differentiation, again depending on time of exposure.
Altered mammary gland development following perinatal exposure has also been observed for other endocrine disrupting compounds, including atrazine, bisphenol A (BPA), dibutylphthalate, dioxin, methoxychlor, nonylphenol, polybrominated diphenyl ethers, PFOA, and others. Some of these compounds such as methoxychlor act as estrogen agonists, but most of these compounds have pleomorphic effects on hormone receptors or hormone signaling in many tissues, and thus correlating a specific physiological and morphological response to classes or specific compounds is certainly complex.
Systemic hormonal changes and correlative mammary morphologies related to spontaneous aging and testing of pharmaceutical-based hormone receptor agonists and antagonists have been nicely characterized in rats (see Lucas et al. (2007) in Further Reading). Common changes in the aging adult male rat include a tubular alveolar pattern with formation of central lumens. This is occasionally referred to as mammary gland “feminization” and is attributed to increased prolactin and growth hormone levels. Because both these hormones increase in aging rats, particularly strains with high incidence of pituitary tumors, such changes in male rat morphology can be an indirect effect of a treatment as well as represent an adverse effect of endocrine disruption. In the adult female rat mammary gland, lobuloalveolar hyperplasias with or without ductal ectasia and secretory activity are associated with increased levels of circulating prolactin, growth hormone, or estrogen levels often associated with endocrine disruptors. Lobuloalveolar morphology, sometimes referred to as “virilization” in the female gland, occurs with androgen stimulation or higher levels of circulating testosterone. The varied effects of pharmaceutical agents are consistent and predictable. Thus when such morphologies are observed in the mammary gland of rats in study, careful consideration should be given to determining hormonal effects as well as potential for direct effects on mammary gland development.
Molecular and Biochemical Response to Injury
At the biochemical and molecular levels, complex and varied responses occur after injury. Molecular signaling through hormone and growth factor receptors is altered by changes in hormone receptor expression, receptor levels, receptor affinity to ligands, or receptor localization. These are further altered by production of local growth factors and hormones as well as genetic mutations that result from injury.
Gene expression profiles from chemically induced mammary gland cancers in Sprague-Dawley rats show distinct differences from spontaneous mammary tumors. Compared to spontaneous carcinomas, carcinomas induced by either 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP), DMBA, 2-amino-3,8-dimethylimidazo[4,5-f]quinoxaline (MeIQx), NMU, or 4-aminobiphenyl (4ABP) show higher expression of genes associated with mammary epithelial cell growth and proliferation, such as cyclin D1, PDGFα, and relatively lower expression of differentiation marker genes, such as β-casein, WAP, and transferrin. Additionally, several components of the prolactin/prolactin receptor/Stat5a/cyclin D1 signaling pathways are found in the chemically induced rat mammary gland carcinomas. Mammary cancer in DMBA-treated FVB mice show elevated expression of the aryl hydrocarbon receptor (AhR), c-myc, cyclin D1, and hyperphosphorylated retinoblastoma (Rb) protein compared to normal mammary gland tissue. Mammary cancer associated with benzene and ethylene oxide exposure to mice had increased mutations in Tp53 protein and Hras mutations in a chemically related pattern distinguishable from spontaneous mutations. However, for most of the compounds associated with mammary gland injury and dysmorphogenesis, the molecular pathways remain to be defined.
Morphologic Response to Injury
The response of the mammary gland to injury recapitulates a wide spectrum of nonneoplastic and neoplastic changes. Standardized nomenclature provides consistency of diagnoses across studies and captures patterns of lesions that represent xenobiotic effects with biological significance. This is commonly done for rodents used in toxicity studies, but should be done for all species, including nonhuman primates for which there appears to be a propensity for medical pathologists to diagnose malignancy and veterinary pathologists to diagnose benign.
Rodents: The Mammary Gland Organ Systems Working Group of the International Harmonization of Nomenclature and Diagnostic (INHAND) Criteria for Lesions in Rats and Mice has standardized the nomenclature summarized in this chapter for rodents. An important note is that the various strains of mice and rats will have their own classifications of background lesions for which an effect of chemical needs to be evaluated. Historical background incidences from various studies are often available from the supplier or study site.
Nonneoplastic changes manifest as degenerative, necrotic, inflammatory, and vascular lesions or, in relation to alterations in growth, manifest as atrophy, hypertrophy, or hyperplasia. Degenerative changes affecting the epithelial and myoepithelial cells of the ducts and alveoli are most commonly associated with aging or occasionally observed as a test-article effect. The changes are characterized by epithelial vacuolization, loss of cell layers, and ductal dilation with accumulation of proteinaceous material. Cellular necrosis within the mammary gland is rarely observed but fat necrosis and inflammation occurs as incidental findings. Regeneration of epithelial cells is usually observed in areas of degeneration as well, and degeneration, necrosis, and regeneration typically present together in repeated mammary gland injury.
Inflammation in rodent mammary glands is usually limited to small infiltrates of leukocytes and should be differentiated from the lymphocytic and eosinophilic infiltrates that accompany ductular morphogenesis. Acute inflammation is characterized by epithelial degeneration, vascular congestion, edema, and an admixture of neutrophils, lymphocytes, and few plasma cells. In chronic inflammation, infiltrates of macrophages, and fibrosis will accompany epithelial regeneration, hyperplasia, or metaplasia. Older rats occasionally develop granulomatous inflammation associated with ruptured galactocoeles or dilated/ectatic ducts. Periductular fibrosis is a common age-related change in rats and has been associated with epidermal growth factor treatment in mice. Recent studies have also demonstrated the increasing incidence of toxicants affecting the stromal and adipose-rich areas of the mammary gland, specifically enhanced macrophage infiltration, stromal hyperplasia, and altered fat cell size or number have been noted.
Alterations in growth are commonly associated with age as well as observed as a test article–related effect in younger animals. For example, dilation and ectasia or galactocoeles of ducts or alveolar hyperplasia with or without epithelial hypertrophy or hyperplasia occur as an age-related change in females, but should also be considered as a test article–related effect in younger female rodents. Compared to the female rat in which alveolar hyperplasia may be considered age or test article-related, the normal morphology in male rats is characterized by lobuloalveolar differentiation. Estrogen receptor–α agonists cause lobuloalveolar hyperplasia and secretory activity in female rats, but “feminization” of the mammary gland in male rats. Feminization in the male rat is characterized by a predominance or transformation from tubuloalveolar to ductular morphology. AR agonists have no effect in the male but will cause virilization and increased secretions in the female rat. In contrast, AR antagonists cause atrophy of the male rat mammary gland with no effect on the female rat mammary gland. Progesterone receptor antagonists cause lobuloalveolar hyperplasia and secretions in female rat mammary glands but have no effect on male rat mammary gland, as the male mammary gland does not express these receptors (see Filgo et al. (2016), in Further Reading). Importantly, dopamine receptor antagonists, which stimulate prolactin secretion, will cause hyperplasia in female and feminization in male rat mammary glands—an effect often seen in aging rats with elevated prolactin levels.
However, in the male the normal development and morphology of the glands may be confused with what is diagnosed as the female is not to be confused with what is normal ductular morphology in the adult male. Compared to the tubuloalveolar glands in the adult female rat, the adult male has lobuloalveolar glands lined by a single layer of cuboidal vacuolated epithelial cells, and pseudostratified or stratified epithelium. The male rat mammary gland remains responsive to endogenous and exogenous hormone agonists and antagonists throughout its life. However, ductular ectasia with alveolar epithelial hypertrophy and hyperplasia has been observed. The lesion may be considered secondary to effects on the hypothalamic–pituitary–ovarian (HPO) axis.
Neoplastic changes in rodent mammary glands occur as spontaneous and test article–related benign and malignant tumors. Ductal epithelial atypia a (Figure 19.7) and ductal carcinomas in situ (DCIS) are increasingly being recognized in studies, particularly in studies of rats exposed to chemical carcinogens or endocrine disruptors during development. These lesions are characterized using the same criteria as used for human breast. DCIS show disruption of the epithelial bilayer and atypical disorganized masses of cells bridge across or fill the ductal lumens. The basement membrane remains intact.
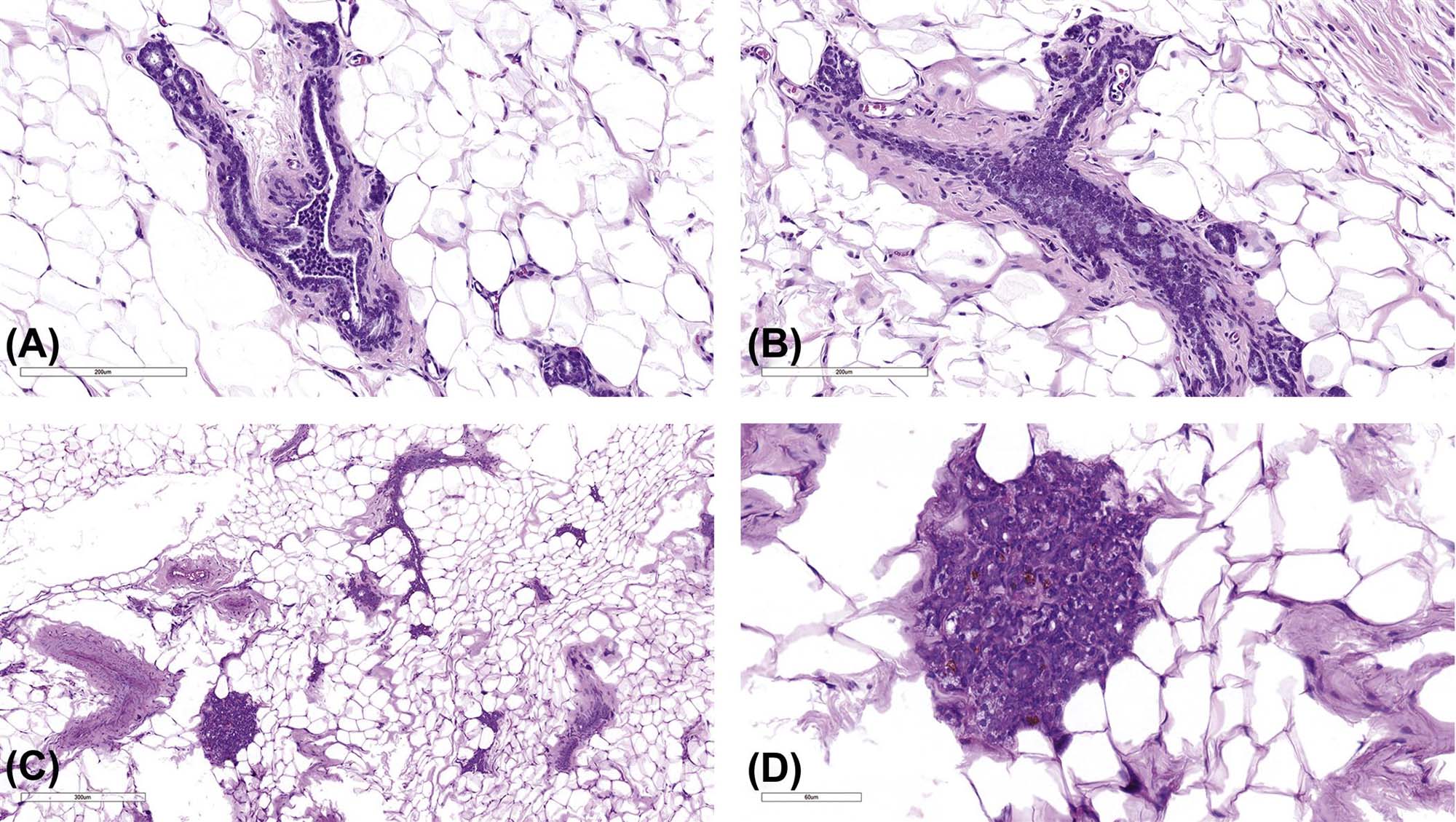
Benign neoplasms of epithelial origin include adenomas, fibroadenomas, and benign mixed tumors and malignant tumors include adenocarcinomas, adenocarcinomas arising in fibroadenomas, and malignant mixed tumors (Figure 19.8). In the chemical carcinogen–treated rat model, benign tumors arise from alveolar buds and lobules while carcinomas arise from epithelial cells in less differentiated TEBs and terminal ducts.

Adenomas are usually visible as nodules. Histologically they are characterized as well-demarcated, encapsulated, and expansive masses that compress surrounding normal tissue. They are composed of cysts, alveoli, or papillary fronds of single or multiple layers of epithelial cells aligned on a fine fibrovascular stroma. The epithelial cells are cuboidal, well differentiated, and may show secretory activity. Focal areas of squamous metaplasia may occur. Although there are morphological subtypes, it is not standard to subclassify tumors in toxicity studies.
Fibroadenomas (see Figure 19.8) represent a proliferation of glandular epithelium surrounded by with varying proportions of well-differentiated epithelial tubules or glands and dense fibrous stroma. Mitotic activity is low. Rarely, carcinomas can arise within fibroadenomas and should be diagnosed appropriately as “adenocarcinoma arising in fibroadenoma.”
Benign mixed tumors are rare in rodents but are the most common spontaneous tumor in dog. These mammary tumors are neoplastic proliferations of both epithelial and myoepithelial cells with differentiation of the latter into islands of cartilage, bone, adipose, or sometimes hematopoietic marrow and diagnostically classified differently from fibroadenomas because benign mixed tumors do not have a predominant fibrous component. The distinction between benign and malignant mixed tumors is based on the extent of invasion of the surrounding tissues by the epithelial cells or resemblance of the mesenchymal component to osteosarcoma or chondrosarcoma.
Adenocarcinomas are malignant proliferations of pleomorphic columnar or cuboidal epithelial cells arranged in papillary, cystic, tubular, solid, or cribriform or comedo patterns (Figure 19.8). There may be complete loss of lobular–alveolar structures and acini may become cystic or blood filled. Adenocarcinomas can induce a marked schirrous response. These cancers can be locally invasive and are metastatic. If more than about 25% of the tumor has squamous differentiation, then it should be diagnosed as an adenosquamous carcinoma, more commonly seen in mice than rats.
Nonhuman primates: Nonneoplastic lesions manifest as cystic changes in lobules and ducts, and apocrine metaplasia with alveolar dilation and secretory changes resembling apocrine sweat glands. These are generally incidental, related to aging and reproductive status.
Hyperplastic lesions are focal and multifocal lobular proliferations seen in older rhesus, pigtail, and cynomolgus macaques. The lesions are characterized as enlarged or distinct nodules of well-differentiated alveoli but proliferate independently of hormonal stimulation. They can be found within generalized lobular hyperplasia of lactation or estrogen and progestogen treatment. Ductal hyperplasias are characterized by focal increased epithelial cells into 2–3 layers, with maintenance of polarity and size. These are common spontaneous lesions in intact middle-aged rhesus macaques.
DCIS is distinguished from hyperplasias by increased layers of disorganized, pleomorphic epithelial cells that bridge and occlude the lumens of tubules. The basement membrane remains intact. Loss of basement membrane is diagnostic of intraductular carcinomas, which can be invasive and metastatic. DCIS occur commonly in mammary gland of cynomolgus and rhesus macaques, but development of larger carcinomas are not common.
Mechanisms of Toxicity
Mammary gland toxicity and carcinogenicity have been observed in animal models exposed to a wide variety of agents including estrogens, androgens, antiandrogens, thyroid-active chemicals, aryl hydrocarbon receptor agonists, genotoxic compounds, and mutagens. Several rodent mammary gland carcinogens are epoxides or epoxide metabolites. These include chemicals such as 1,3-butadiene and related chloroprene and isoprene that are metabolized to epoxides. Although the mechanisms related to epoxide-induced mammary cancer are unknown, one hypothesis is that the mammary gland is efficient in metabolizing the chemicals to their epoxides. Epoxides are mutagens and associated with K-ras mutations in rodents. Isoprene and 1,3-butadiene also cause chromosomal aberrations, which may be related to mammary carcinogenicity. Brominated species are also associated with rodent mammary cancers. Chemicals, such as 2,2-bis(bromomethyl)-1,3-propanediol are proposed to act either through oxidative damage or formation of DNA adducts and then DNA damage. Many of these chemicals also induce cytochrome P450 metabolizing enzymes, which, like DMBA, can activate chemical reactivity or be metabolized to electrophilic oxygenated species that bind DNA.
Endocrine disrupting chemicals have a variety of effects. Atrazine, brominated diphenyl ethers, and dioxin, all have been shown to delay mammary gland development following neonatal exposures through interactions with hormone responses. Estrogenic compounds like DES or BPA increase protein expression and functional activity of histone methyltransferases, which have been linked to breast cancer risk and epigenetic regulation of tumorigenesis. It is also common for endocrine disrupting compounds to act via indirect mechanisms to induce persistent effects in mammary tissue. Furthermore, recent data suggests that epigenetic mechanisms—those changes to DNA that cause a heritable modification without changing the DNA code—may have important roles in not only mammary, but many cancers over the lifetime.
Summary
The high incidence of breast cancer in women has brought attention to the need to incorporate appropriate and sensitive methodologies with which to evaluate the mammary gland in animal models as applicable to hazard identification for humans. We now have a substantial number of genetically modified and spontaneously occurring mice and rat mammary cancer models to employ and various approaches to assess gland morphology. Evaluation of mammary tissue in studies with nonhuman primates can also serve as an important tool. Of the lessons learned, we know that hormonal perturbations, including chemicals that act as endocrine disruptors, pose a significant risk and that such risk may be increased when exposures occur during development in male and female research models. Given this information, animal studies designed for hazard identification should include exposures during development and include enhanced methods for histological and morphometric mammary gland evaluation, such as whole mount evaluations. In vitro screens also continue to serve as important tools for identifying potential hazards and supporting mechanistic understanding of effects. Indeed, discovering genetic and environmental causes of breast cancer go hand-in-hand with discoveries of therapies to treat breast cancer and strategies to prevent breast cancer.