THE EARTH’S CRUST: COMPOSITION
We have so far been able to penetrate to only very shallow depths beneath the surface of the earth. The deepest mine is only about 2 miles deep, and the deepest well about 5 miles deep.
But by using geophysical methods we can “x-ray” the earth. Careful tracing of earthquake waves shows that the earth has a distinctly layered structure. Studies of rock density and composition, heat flow, and magnetic and gravitational fields also aid in constructing an earth model of three layers: crust, mantle, and core. Estimates of the thickness of these layers, and suggested physical and chemical characteristics form an important part of modern theories of the earth (see here).
The crust of the earth is formed of many different kinds of rocks (see here), each of which is an aggregate of minerals, described here.

Grand Canyon of Colorado River, Arizona, is 1 mile deep, but exposes only a small part of upper portion of earth’s crust.
MINERALS are naturally occurring substances with a characteristic atomic structure and characteristic chemical and physical properties. Some minerals have a fixed chemical composition; others vary within certain limits. It is their atomic structure that distinguishes minerals from one another.
Some minerals consist of a single element, but most minerals are composed of two or more elements. A diamond, for instance, consists only of carbon atoms, but quartz is a compound of silica and oxygen. Of the 105 elements presently known, nine make up more than 99 percent of the minerals and rocks.

OXYGEN AND SILICON are the two most abundant elements in the earth’s crust. Their presence, in such enormous quantities, indicates that most of the minerals are silicates (compounds of metals with silicon and oxygen) or aluminosilicates. Their presence in rocks is also an indication of the abundance of quartz (SiO2, silicon dioxide) in sandstones and granites, as well as in quartz veins and geodes.

Smoky Quartz
The most striking feature of minerals is their crystal form, and this is a reflection of their atomic structure. The simplest example of this is rock salt, or halite (NaCl, sodium chloride), in which the positive ions (charged atoms) of sodium are linked with negatively charged chlorine ions by their unlike electrical charges. We can imagine these ions as spheres, with the spheres of sodium having about half the radius of the chlorine ions (.98 Å as against 1.8 Å; Å is an Ångstrom Unit, which is equivalent to one hundred millionth of a centimeter, written numerically as 0.00000001 cm or 10–8cm). The unit is named for Anders Angstrom, a Swedish physicist.
X-RAY STUDIES show that the internal arrangement of halite is a definite cubic pattern, in which ions of sodium alternate with those of chlorine. Each sodium ion is thus held in the center of and at equal distance from six symmetrically arranged chlorine ions, and vice versa. It is this basic atomic arrangement or crystalline structure that gives halite its distinctive cubic crystal form and its characteristic physical properties.
SODIUM ATOM joins CHLORINE ATOM


THE ATOMIC STRUCTURE of each mineral is distinctive but most minerals are more complicated than halite, some because they comprise more elements, others because the ions are linked together in more complex ways. A good example of this is the difference between diamond and graphite. Both have an identical chemical composition (they are both pure carbon) but they have very different physical properties. Diamond is the hardest mineral known, and graphite is one of the softest. Their different atomic structures reflect their different geologic modes of origin.
DIAMOND, the hardest natural substance known, consists of pure carbon atoms. Each carbon atom is linked with four others by electron-sharing. The four electrons in the outer shell are shared with four neighboring atoms. Each atom of carbon then has eight electrons in its outer shell. This provides a very strong bond. Its crystal form is a reflection of its structure and of the conditions under which it was formed. Diamond is usually pale yellow or colorless, but is found also in shades of red, orange, green, blue, brown, or black. Pure white or blue-white are best for gems.
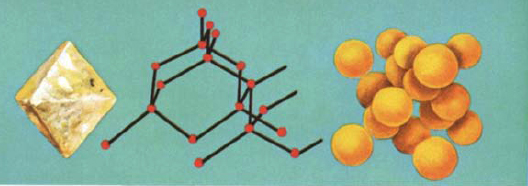
GRAPHITE, quite different from diamond, is soft and greasy, and widely used as an industrial lubricant. In graphite, carbon atoms are arranged in layers, giving the mineral its flaky form. The atoms within each layer have very strong bonds, but those that hold successive layers together are very weak. Some atoms between layers are held together so poorly that they move freely, giving the graphite its soft, slippery, lubricating properties. Because of its poor bonding, graphite is a good conductor of electricity. Its best-known use is in “lead” pencils.
CRYSTAL FORM of minerals is an important factor in their identification. Grown without obstruction, minerals develop a characteristic crystal form. The outer arrangement of plane surfaces reflects their internal structure. Perfect crystals are rare. Most minerals occur in irregular masses of small crystals because of restricted growth. Since all crystals are three-dimensional, they may be classified on the basis of the intersection of their axes. Axes are imaginary lines passing through the geometric center of a crystal from the middle of its faces and intercepting in a single point.
CUBIC CRYSTALS have three axes of equal length meeting at right angles, as in galena, garnet, pyrite, and halite.

Galena
TETRAGONAL CRYSTALS have three axes at right angles, two of equal length, as in zircon, rutile, and scapolite.

Zircon
HEXAGONAL CRYSTALS have three equal horizontal axes with 60° angles and one shorter or longer at right angles, as in quartz and tourmaline.

Quartz
ORTHORHOMBIC CRYSTALS have three axes at right angles, but each is of different length, as in barite and staurolite.

Staurolite
MONOCLINIC CRYSTALS have three unequal axes, two forming an oblique angle and one perpendicular, as in augite, orthoclase, and epidote.

Epidote
TRICLINIC CRYSTALS have three axes of unequal lengths, none forming a right angle with others, as in plagioclase feldspars.

Amazonite feldspar
MINERAL IDENTIFICATION involves the use of various chemical and physical tests to determine what minerals are present in rock. There are over 2,000 minerals known, and elaborate laboratory tests (such as X-ray diffraction) are required to identify some of them. But many of the common minerals can be recognized after a few simple tests. Six important physical properties of minerals (hardness, luster, color, specific gravity, cleavage, and fracture) are easily determined. A balance is needed to find specific gravity of crystals or mineral fragments. For the other tests, a hand lens, steel file, knife, and a few other common items are helpful. Specimens can sometimes be recognized by taste, tenacity, tarnish, transparency, iridescence, odor, or the color of their powder streak, especially when these observations are combined with tests for the other physical properties.

HARDNESS is the resistance of a mineral surface to scratching. Ten well-known minerals have been arranged in a scale of increasing hardness (Mohs’ scale). Other minerals are assigned comparable numbers from 1 to 10 to represent relative hardness. A mineral that scratches orthoclase (6) but is scratched by quartz (7) would be assigned a hardness value of 6.5.
LUSTER is the appearance of a mineral when light is reflected from its surface. Quartz is usually glassy; galena, metallic.

Galena crystals
SPECIFIC GRAVITY is the relative weight of a mineral compared with the weight of an equal volume of water. A balance is normally used to determine the two weights. Some minerals are similar superficially but differ in density. Barite may resemble quartz, but quartz has a specific gravity of 2.7; barite, 4.5.

COLOR varies in some minerals. Pigments or impurities may be the cause. Quartz occurs in many hues but is sometimes colorless. Among minerals with a constant color are galena (lead gray), sulfur (yellow), azurite (blue), and malachite (green). A fresh surface is used for identification, as weathering changes the true color.
CLEAVAGE is the tendency of some minerals to split along certain planes that are parallel to their crystal faces. A hammer blow or pressure with a knife blade can cleave a mineral. Galena and halite have cubic cleavage. Mica can be separated so easily that it is said to have perfect basal cleavage. Minerals without an orderly internal arrangement of atoms have no cleavage.

Cubic cleavage: galena

Basal cleavage: mica

Rhombohedral cleavage: calcite
FRACTURE is the way a mineral breaks other than by cleavage. Minerals with little or no cleavage are apt to show good fracture surfaces when shattered by a hammer blow. Quartz has a shell-like fracture surface. Copper has a rough, hackly surface; clay, an earthy fracture.

Earthy fracture: clay

Conchoidal fracture: obsidian

Uneven fracture: arsenopyrite
COMMON ROCK-FORMING MINERALS include carbonates, sulfates, and other compounds. Many minerals crystallize from molten rock material. A few form in hot springs and geysers, and some during metamorphism. Others are formed by precipitation, by the secretions of organisms, by evaporation of saline waters, and by the action of ground water.
MINERAL CARBONATES, SULFATES, AND OXIDES
LIMONITE is a group name for hydrated ferric oxide minerals, Fe2O3.H2O. It is an amorphous mineral that occurs in compact, smooth, rounded masses or in soft, earthy masses. No cleavage. Earthy fracture. Hardness (H) 5 to 5.5; Sp. Gr. 3.5 to 4.0. Rusty or blackish color. Dull, earthy luster gives a yellow-brown streak. Common weathering product of iron minerals.

Limonite
CALCITE is a calcium carbonate, CaCO3. It has dogtooth or flat hexagonal crystals with excellent cleavage. H. 3; Sp. Gr. 2.72. Colorless or white. Impurities show colors of yellow, orange, brown, and green. Transparent to opaque. Vitreous or dull luster. Major constituent of limestone. Common cave and vein deposit. Reacts strongly in dilute hydrochloric acid.

Calcite
GYPSUM is a hydrated calcium sulphate, CaSO4.2H2O. Tabular or fibrous monoclinic crystals, or massive. Good cleavage. H. 2. Sp. Gr. 2.3. Colorless or white. Vitreous to pearly luster. Streaks are white. Flexible but no elastic flakes. Sometimes fibrous. Found in sedimentary evaporites and as single crystals in black shales. The compact, massive form is known as alabaster.

QUARTZ is silicon dioxide, SiO2. Massive or prismatic. No cleavage. Conchoidal fracture. H. 7; Sp. Gr. 2.65. Commonly colorless or white. Vitreous to greasy luster. Transparent to opaque. Common in acid igneous, metamorphic, and clastic rocks, veins, and geodes. The most common of all minerals.

Quartz crystal

Flint
ROCK-FORMING SILICATE MINERALS
FELDSPARS are alumino-silicates of either potassium (KAlSi3O8 orthoclase, microcline, etc.) or sodium and calcium (plagioclase feldspars NaAlSi3O8, CaAl2Si2O8). Well-formed monoclinic or triclinic crystals, with good cleavage. H. 6 to 6.5; Sp. Gr. 2.5 to 2.7 Orthoclase feldspars are white, gray, or pink, vitreous to pearly luster, and lack surface striations. Plagioclase feldspars are white or gray, have two good cleavages, which produce fine parallel striations on cleavage surfaces. Common in igneous and metamorphic rocks, and arkosic sandstones.

MICAS are silicate minerals. White mica (muscovite) is a potassium alumino-silicate. Black mica (biotite) is a potassium, iron, magnesium alumino-silicate. Both occur in thin, monoclinic, pseudo-hexagonal, scalelike crystals. Superb cleavage gives thin, flexible flakes. Pearly to vitreous luster. Micas are common in igneous, metamorphic, and sedimentary rocks.

Biotite crystal

Biotite (black mica)
PYROXENES include a large group of silicates of calcium, magnesium, and iron. Augite, (CaMgFeAl)2•(AlSi)2O6, and hypersthene, (FeMg)SiO3, are the most common. Stubby, eight-sided prismatic, orthorhombic or monoclinic crystals, or massive. Two cleavages meet at 90° (compare amphiboles), but these are not always developed. Gray or green, grading into black. Vitreous to dull luster. H. 5 to 6. Sp. Gr. 3.2 to 3.6. Common in nearly all basic igneous and metamorphic rocks. Sometimes found in meteorites.

Augite
AMPHIBOLES are complex hydrated silicates of calcium, magnesium, iron, and aluminum. Hornblende, a common amphibole, has long, slender, prismatic, six-sided orthorhombic or monoclinic crystals; sometimes fibrous. Two good cleavages meeting at 56°. H. 5 to 6; Sp. Gr. 2.9 to 3.2. Black or dark green. Opaque with a vitreous luster. Common in basic igneous and metamorphic rocks. Asbestos is an amphibole.

Hornblende
OLIVINE is a magnesium-iron silicate, (FeMg)2SiO4. Small, glassy grains. Often found in large, granular masses. Crystals are relatively rare. Poor cleavage. Conchoidal fracture. H. 6.5 to 7; Sp. Gr. 3.2 to 3.6. Various shades of green; sometimes yellowish. Transparent or translucent. Vitreous luster. Common in basic igneous and metamorphic rocks. Olivine alters to a brown color.

GALENA is a lead sulphide, PbS. Heavy, brittle, granular masses of cubic crystals. Perfect cubic cleavage, H. 2.5; Sp. Gr. 7.3 to 7.6 Silver-gray. Metallic luster. Streaks are lead-gray. Important lead ore. Common vein mineral. Occurs with zinc, copper, and silver.

Galena
SPHALERITE is a zinc sulphide, ZnS. Cubic crystals or granular, compact. Six perfect cleavages at 60°. H. 3.5 to 4; Sp. Gr. 3.9 to 4.2. Usually brownish; sometimes yellow or black. Translucent to opaque. Resinous luster. Some specimens are fluorescent. Important zinc ore. Common vein mineral with galena.

Sphalerite
PYRITE is an iron sulphide, FeS2. Cubic, brassy crystals with striated faces. May be granular. No cleavage. Uneven fracture. H. 6 to 6.5; Sp. Gr. 4.9 to 5.2. Brassy yellow color. Metallic luster. Opaque and brittle. Also called fool’s gold. Common source of sulfur.

crystal

Pyrite

Miner in the lead mine Mesters Vig, Greenland