A Conceptual Framework for Understanding Cell Signaling
If ligand binding to a receptor is to lead to a change in cellular function, the binding energy of the ligand-receptor interaction must be translated, or transduced, into a biochemical change in the cell’s behavior, location, or metabolism. Changes induced by ligand binding may include variations in the activity of transcription factors in that cell, which in turn cause changes in the expression of intracellular, membrane-bound, or secreted proteins; initiation of cellular programs leading to differentiation and division; alterations in the activity of proteasomes that induce destruction of particular proteins; fluctuations in the secretory or phagocytic activity of the target cell, and/or changes in the cell’s metabolic activity that ready it for division, differentiation, or even for death.
The process by which ligand binding to a cellular receptor is translated into a modification in cellular activity is referred to as signal transduction. Because the activity of the immune system is entirely dependent on ligand-induced alterations in immune cells, it should come as no surprise that many of the advances in our understanding of the biochemistry of signal transduction were made using the immune system as a model.
In the last decade or so, we have come to understand that common strategies, manifesting themselves as sequences of biochemical events, are shared across many signaling transduction pathways. For example, ligand binding will usually induce either an alteration in the conformation or in the polymerization status of the receptor molecule. As we discussed above in our description of B- and T-cell receptor binding, the primary receptor may be joined by coreceptors in strengthening the bonds between ligand and receptor, or between receptor- and ligand-bearing cells. We have also come to appreciate the vital role of tyrosine phosphorylation of the intracytoplasmic regions of either receptors or receptor-associated molecules in the passage of the signals from the exterior to the interior of the cell, as well as the function of adapter proteins that transmit the signal by bringing other proteins together without themselves being covalently altered.
Developing a grasp of these shared themes helps to provide a scaffold on which to hang the details of the specific signal transduction pathways that will be described in subsequent chapters. In this section, we therefore provide a conceptual framework for students to use when learning about the many signal transduction pathways they will encounter in their study of immunology and, more broadly, of cell biology. Where appropriate, examples will be drawn from particular immune receptor–ligand interactions (Overview Figure 3-24).
Note that the upstream components of a signaling path-way are those closest to the receptor; the downstream components are those closest to the effector molecules that determine the outcome of the pathway—for example, the transcription factors or enzymes whose activities are modified on receipt of the signal.
Ligand Binding Can Induce Dimerization or Multimerization of Receptors
The region of a receptor that makes molecular contact with a ligand is referred to as the receptor binding site. The binding site of most receptors represents only a small fraction of the size of the receptor molecule, and other parts of the receptor molecule are responsible for transmitting the signal induced by ligand binding across the cell membrane to the interior of the cell. To this end, binding of a ligand to a receptor binding site will often induce a conformational change in distal parts of the receptor molecule that alters its ability to bind to other receptor molecules. For example, in the case of some cytokine receptors, such as those that bind interferon molecules, interferon-induced receptor dimerization is the first step in the signal transduction cascade. For B- and T-cell receptors, binding of receptor to antigen facilitates the formation of clusters of receptor molecules on the cell surface.
Key Concept:
- Ligand binding can cause a conformational change in a receptor molecule that induces the receptor to bind to other receptors.
Ligand Binding Can Induce Phosphorylation of Tyrosine Residues in Receptors or Receptor-Associated Molecules
The cytoplasmic regions of some ligand receptors, such as c-Kit, which recognizes the stem cell factor critical to early lymphocyte development, have an intrinsic tyrosine kinase activity that is activated on ligand binding. Such receptors are known as receptor tyrosine kinases, or RTKs. In RTKs, ligand binding induces receptor dimerization, which brings the tyrosine residues of one receptor within range of the kinase activity of the partner molecule. This is followed by the reciprocal phosphorylation of the cytoplasmic regions of each of the receptor molecules by its dimerization partner.
Other cytokine receptors, such as those that recognize interferons, associate noncovalently with tyrosine kinase enzymes via the cytoplasmic regions of the receptor molecules. These tyrosine kinases, like the RTKs, are activated on ligand-receptor binding and phosphorylation; they then phosphorylate transcription factors that dimerize and enter the nucleus, allowing for transmission of the cytokine signal (Figure 3-25).

FIGURE 3-25 General model of signal transduction mediated by most class 1 and class 2 cytokine receptors. Binding of a cytokine induces dimerization of the receptor subunits, which leads to the activation of receptor subunit–associated JAK tyrosine kinases by reciprocal phosphorylation. Subsequently, the activated JAKs phosphorylate various tyrosine residues, resulting in the creation of docking sites for STATs on the receptor and the activation of the one or more STAT transcription factors. The phosphorylated STATs dimerize and translocate to the nucleus, where they activate transcription of specific genes. (Abbreviations: JAK = Janus kinase; STAT = signal transducer and activator of transcription. JAKs and STATs each exist in multiple isomeric forms, and each cytokine receptor signals through a pair of Janus kinases that may be either homo- or heterodimeric. A table illustrating which cytokine receptors use which JAK/STAT combinations is provided in Appendix II.)
A pair of alpha and beta chains is shown on the cell membrane, each of which is associated with a J A K family tyrosine kinase. A cytokine binds between the alpha and beta chains as they move closer together to form a Y shape, labeled, dimerization of receptor. This phosphorylates and activates the tyrosine kinases, which then phosphorylate the receptor at the bottom end. Two S T A T transcription factors are also shown being phosphorylated, which leads to dimerization of the two together, which then enter the nucleus to trigger specific gene transcription.
Receptors, such as the BCR and TCR, have intracytoplasmic regions that are too small to allow for direct association with kinase enzymes. Instead, ligand binding by TCRs or BCRs induces the receptors to associate noncovalently (oligomerize) on the membrane surface and then move into specialized regions of the lymphocyte membrane known as lipid rafts (Figure 3-26). These rafts are highly ordered, detergent-insoluble, cholesterol- and sphingolipid-rich membrane regions, populated by many molecules critical to receptor signaling. Moving receptors and coreceptors into the lipid rafts renders them susceptible to the action of enzymes associated with those rafts. For example, the raft-associated tyrosine kinase Lyn initiates the B-cell signaling cascade by phosphorylating the tyrosine residues on the ITAMs of the receptor-associated molecules Igα and Igβ (see Figure 3-14); Lck performs an analogous function in T cells by phosphorylating the ITAMs on CD3. These phosphorylation reactions then facilitate binding of downstream, signal-transducing molecules to the phosphorylated tyrosine residues in the receptor-associated molecules.
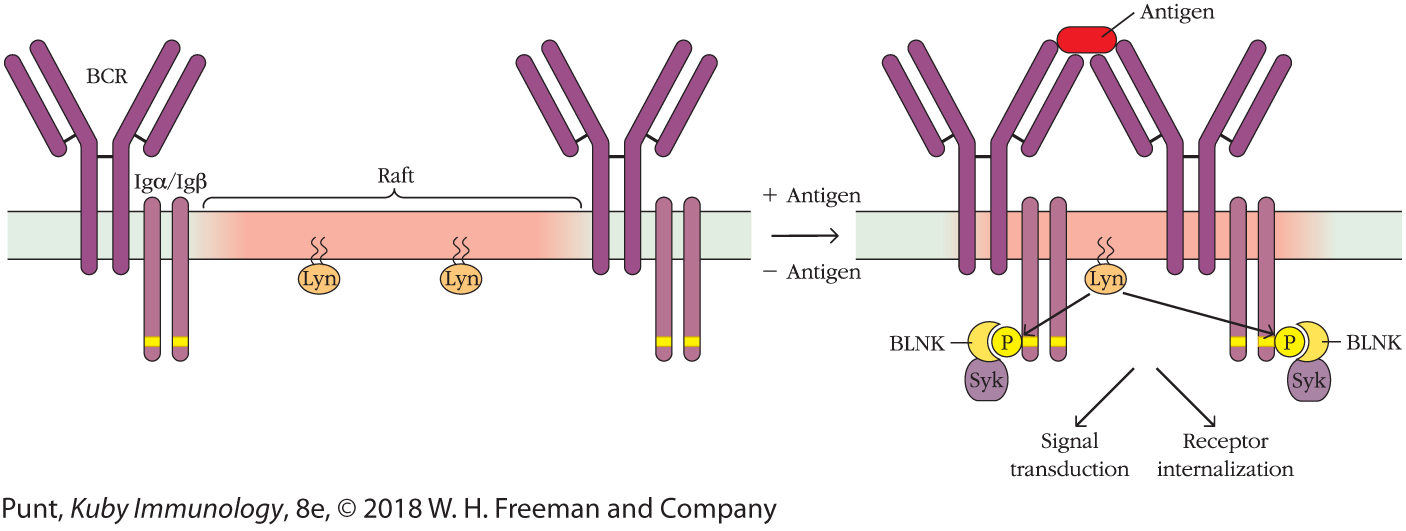
FIGURE 3-26 The role of lipid raft regions within membranes. In resting B cells, the B-cell receptor (BCR) is excluded from the lipid rafts, which are regions of the membrane high in cholesterol and rich in glycosphingolipids. The rafts are populated by tyrosine kinase signaling molecules, such as Lyn. Antigen binding induces the BCRs to oligomerize (form clusters), and increases their affinity for the lipid rafts. Movement of the BCRs into a lipid raft brings them into contact with the tyrosine kinase Lyn, which phosphorylates the receptor-associated proteins Igα,Igβ, thus initiating the activation cascade. A similar movement of TCRs into lipid rafts occurs on T-cell activation. Only two receptors are shown here for clarity.
The extracellular side of the cell membrane is labeled, plus antigen, and the cytoplasm side of the cell membrane is labeled, minus antigen. The region between two consecutive B-cell receptor pairs is labeled, raft. Two Lyn molecules are present in the raft region just inside the cytoplasm. Each B C R has a pair of Immunoglobulin alpha and beta chains associated with them
When the B-cell receptor pair binds to an antigen, the raft part of the cell membrane closes up, leaving one lyn molecule between them. This lyn molecule causes the phosphorylation of B L N K and S y k in the cytosol attached to each I g alpha I g beta group of the receptor proteins, leading to signal transduction and receptor internalization.
Key Concepts:
- Tyrosine kinase activation is a frequent early step in signal transduction. Phosphorylation may occur on the cytoplasmic domains of receptor proteins or on receptor-associated signaling proteins.
- Clustering of receptors at the cell membrane is an integral step in many signaling pathways. BCRs and TCRs, their coreceptors, and their accessory proteins cluster in lipid rafts, areas of the membrane that have a high concentration of cytosolic enzymes.
Src-Family Kinases Play Important Early Roles in the Activation of Many Immune Cells
A particular family of tyrosine kinases, the Src-family kinases, which includes the enzymes Lck and Lyn, plays an important early role in the activation of many immune cells. Since inadvertent activation of these enzymes can lead to uncontrolled proliferation—a precursor to tumor formation—it is not surprising that their activity is tightly regulated by phosphorylation in not one, but two different and interconnected ways.
Inactive Src-family tyrosine kinase enzymes exist in a closed conformation, in which a phosphorylated inhibitory tyrosine is tightly bound to an internal SH2 domain (Src homology 2 domain) (Figure 3-27). (SH2 domains in proteins bind to phosphorylated tyrosine residues.) In lymphocytes, the tyrosine kinase enzyme Csk is responsible for maintaining phosphorylation of the inhibitory tyrosine, Y508. On cell activation, a tyrosine phosphatase removes the phosphate and the Src-family kinase opens up into a partially active conformation. Lipid raft–associated phosphatase enzymes help to keep it open and unphosphorylated. Full activity is achieved when the Src kinase phosphorylates itself on a second activating tyrosine residue, Y397. Paradoxically, therefore, the first step in lymphocyte activation is not a tyrosine phosphorylation, but rather a tyrosine dephosphorylation reaction, from which many phosphorylation reactions then follow.

FIGURE 3-27 Activation of Src-family kinases. Src-family kinases are maintained in an inactive closed configuration by the binding of a phosphorylated inhibitory tyrosine residue (pY508 in this example) with an SH2 domain in the same protein. Dephosphorylation of this tyrosine opens up the molecule, allowing substrate access to the enzymatic site. Opening up the kinase also allows phosphorylation of a different internal tyrosine (pY397), which further stimulates the Src kinase activity.
The kinase in the inactive state has a S H 2 domain bonded to a phosphorylated p Y 508 tyrosine residue.
The kinase in the active state shows the p Y 397 tyrosine residue phosphorylated instead, and the rest of the kinase separated from the S H 2 domain.
Tyrosine phosphorylation typically results in one or both of two outcomes. It can induce a conformational change in the phosphorylated protein, turning its enzymatic activity on or off. Alternatively, tyrosine phosphorylation can permit other proteins to bind to the phosphorylated protein via their SH2 or PTB domains as described earlier.
Key Concept:
- The cytosolic domains of receptors may be phosphorylated by Src-family tyrosine kinases, which have dual mechanisms of regulation involving both a dephosphorylation and a subsequent, different autophosphorylation reaction.
Intracellular Adapter Proteins Gather Members of Signaling Pathways
The cytoplasm, far from being a random protein soup, is in fact an intricately organized environment in which three-dimensional arrays of proteins form and disperse as directed by cellular signaling events. Many of these reversible interactions between proteins are mediated by adapter proteins.
Adapter proteins have no intrinsic enzymatic or receptor function, nor do they act as transcription factors. They are characterized by having multiple surface domains, each of which possesses a precise binding specificity for a particular molecular structure, such as the phosphotyrosine residues mentioned above, or the proline-rich sequences recognized by SH3 domains. Their functions are to bind to specific motifs or domains on proteins or lipids and mediate a signal-induced redistribution of molecules within the cell. This redistribution may, for example, bring substrates within the range of enzymes or induce a conformational change in the bound protein that alters its activity, stabilizes it, or destabilizes it.
Note that multiple adapter proteins may participate in the formation of a protein scaffold (see Overview Figure 3-24) that provides a structural framework for the interaction of members of a signaling cascade. For example, during T-cell signaling, receptor-induced tyrosine phosphorylation of the adapter proteins LAT and SLP76 allows the formation of an extensive complex of at least five different adapter proteins. This complex then facilitates the clustering and activation of important intracellular enzymes including phospholipase Cg1 and protein kinase C, as well as the proteins of the MAP kinase cascade. Activation of all of these enzymes ultimately leads to alterations in the transcriptional program of the cell.
Key Concept:
- Adapter proteins bind multiple proteins, bringing receptors into physical proximity with downstream effectors.
Common Sequences of Downstream Effector Relays Pass the Signal to the Nucleus
Many of the downstream effector molecules in immune receptor signaling pathways will be familiar as components of general cellular signaling pathways. In Overview Figure 3-24 we show how three signal transduction pathways in lymphocytes pass a molecular signal from the antigen receptor to the nucleus, resulting in antigen-mediated alterations in the cellular transcriptional program.
Those enzymes that cleave membrane phospholipids into the glycerol and phosphorylated head group components are collectively termed phospholipases, and enzymes that cleave the specific membrane phospholipid phosphatidyl inositol bisphosphate are referred to as phospholipases C. There are multiple members of the phospholipase C family, but those important in lymphocyte activation belong to the phospholipase Cγ classification, with phospholipase Cγ1 being active in T cells and phospholipase Cγ2 assuming the same role in B cells.
Activation of phospholipase Cγ as described above will facilitate the breakdown of phosphatidyl inositol bisphosphate into the soluble inositol trisphosphate and the membrane-bound lipid, diacylglycerol. Diacylglycerol binds and activates the serine/threonine kinase, protein kinase C, causing it to phosphorylate IκB, the inhibitory component of the transcription factor NF-κB. Once phosphorylated, IκB releases the active NF-κB transcription factor, which is now able to move through to the nucleus with resultant activation of transcription.
Simultaneously, inositol trisphosphate binds to receptors on intracellular, calcium-containing membrane vesicles, resulting in the release of Ca2+ from intracellular stores. This induces the activation of calcium-dependent molecules such as calmodulin. Binding to calcium induces a dramatic conformational alteration in calmodulin, such that it is now able to bind and activate calcineurin, a phosphatase. Calcineurin dephosphorylates the transcription factor NFAT, which is normally unable to enter the nucleus from the cytoplasm because of the presence of its charged phosphate group. Dephosphorylation of NFAT by calcineurin therefore allows the nuclear migration of NFAT and activation of NFAT-controlled transcription. Hence, activation of phospholipase Cγ1 by upstream, antigen-induced signals leads to changes in the cell’s transcriptional program mediated by the two transcription factors, NF-κB and NFAT.
Receptor-mediated activation of the Ras/MAP kinase pathway is initiated in similar ways. Phosphorylation of receptor-associated molecules in B and T cells induces the binding of adapter molecules that, in turn, bind and activate the nucleotide exchange factor protein SOS. SOS binds to Ras, a small G protein in the lymphocyte membrane, inducing it to exchange GDP in its nucleotide-binding site for GTP. This exchange results in an activating conformational change in the Ras protein. The Ras-GTP complex can then bind and activate the cytoplasmic kinase Raf, which phosphorylates and activates MEK. MEK, in turn phosphorylates the kinase ERK. On phosphorylation, ERK is able to enter the nucleus, where it is responsible for phosphorylating and activating several other transcription factors including Fos, which is one of the components of an important transcription factor, AP-1. Activation of AP-1, NFAT, and NF-κB alter the transcriptional program of the cell.
However, if signaling of cells through a variety of receptors can give rise to increased activity of similar downstream signaling pathways, we must ask how different cells achieve distinct biological functions through intervention of similar enzyme cascades. For example, every time a cell increases the activity of phospholipase Cγ, is the biological end point of this up-regulation the same?
The answer to this question is most definitely no. First of all, many of these signal transduction pathway enzymes exist as multiple different isoforms that are differentially expressed in various cell types; they are subject to distinct means of regulation and act on different target populations.
Second, even when cells use shared pathways to mediate similar essential functions such as the induction of division or cell death, the transcription factors that specify such differentiated functions as antibody or cytokine secretion vary among discrete cell types. Furthermore, in addition to signaling for the production of mRNAs that will be translated into proteins, other alterations in transcriptional programming that occur on signaling may give rise to short, noncoding RNA sequences that vary from cell to cell, and that may have powerful regulatory effects on the differentiation of the activated cell.
Third, although when working in a laboratory environment one gets used to thinking of transcription as being either “on” or “off,” in the in vivo situation cells are capable of fine levels of modulation of gene activity. For example, T and B cells can both be activated to greater or lesser extents following ligand binding. Both the CD3 complex in T cells and the Igα,Igβ pair in B cells have multiple phosphorylatable tyrosine residues, and these can be less or more fully phosphorylated depending on the strength of the initial ligand-receptor binding event, leading to stronger or weaker signal transduction; this is known as “scalable signaling.” Scalable signaling allows for activation of different targets depending on the strength of the received signal.
Finally, each cell will be subject to interacting signal transduction cascades that begin with multiple ligand-receptor interactions. Recall that B cells, for example, express both a BCR and several Toll-like, innate immune receptors. A B cell whose receptor is specific for a gram-negative bacterium will thus have to integrate signals received through both its BCR and its TLR4 receptors, in addition to those received through coreceptors and receptors for modulating signals such as cytokines.
As you read about the activation of cells of the innate and adaptive immune systems in future chapters, you will learn where each of the strategies described above is used, separately and in combination, to induce immune responses in particular cell types. Overview Figure 3-24 illustrates how activation of the pathways discussed in this section, which are all signaled through the B- and T-cell receptors, combine to change the transcriptional program of the cell.
Key Concept:
- The components of a signaling pathway transduce a molecular signal from receptor-ligand binding at the cell surface to changes in enzyme and transcription factor activity. The specificity of the signal is generated by the specific combination of receptors, kinases, and downstream effector molecules activated by ligand binding.
Not All Ligand-Receptor Signals Result in Transcriptional Alterations
Although the majority of signaling events in an immune cell will result in alterations in the cell’s transcriptome, it is important to remember that some immune signals, particularly those that interact with cells of the innate immune system, give rise to immediate cellular responses that do not require new transcription. For example, in the Paneth cells of the gut, sensing of bacteria in the gut lumen triggers the release of preformed antimicrobial peptides from intracellular vesicles. Another example: signaling through neutrophil surface receptors can induce immediate activation of the enzymes involved in the production of superoxides. Interestingly, in this latter case, activation of a member of the phospholipase Cγ family by the neutrophil receptor is still implicated, demonstrating that a similar pathway can be used in both transcription-mediated and non–transcription-mediated signal transduction.
Other events that may be induced by immune signals include an increase in the destruction of particular proteins. For example, initiation of the apoptotic cascade is induced by proteolytic cleavage of procaspases to active caspases, whose activity leads ultimately to cellular destruction.
Finally, sometimes, immune signaling leads to modifications in the stability of mRNA that affects the levels of particular proteins in the cell. For example, the mRNA that encodes IL-2 is quite unstable in resting T cells. On T-cell activation, the IL-2 message is stabilized by the binding of proteins to the 3′ end of the mRNA, leading to an increase in translation and hence in the levels of secreted IL-2.
Key Concept:
- Signal transduction pathways can culminate in cellular functions such as the release of effector molecules from preformed vesicles, the destruction or modification of particular proteins, the alteration of mRNA stability, or the initiation of apoptosis.